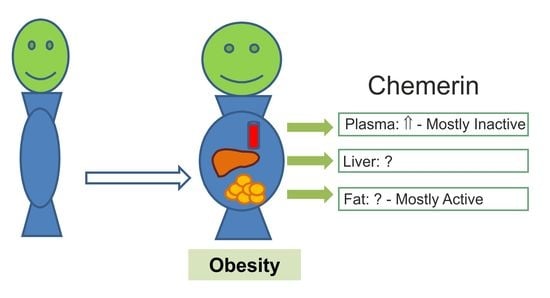
Chemerin Isoforms and Activity in Obesity
Abstract
1. Introduction
2. Expression of Chemerin and its Receptors in Different Tissues
2.1. Chemerin Expression in Adipose Tissues
2.1.1. Expression of Chemerin in Human Tissues
2.1.2. Expression of Chemerin in Experimental Models
2.2. Chemerin Receptor Expression in Adipose Tissues
2.2.1. Expression of Chemerin Receptors in Human Tissues
2.2.2. Expression of Chemerin Receptors in Experimental Models
2.3. Chemerin in the Liver
2.3.1. Expression of Chemerin in Human Tissue
2.3.2. Expression of Chemerin in Experimental Models and in vitro Systems
2.4. Chemerin Receptors in the Liver
2.5. Chemerin and its Receptors in Skeletal Muscle
3. Chemerin Isoforms and Activity in Adipose Tissue and Serum
3.1. C-terminal Processing and Activity of Chemerin Isoforms
3.2. Quantification of Chemerin Activity with the Tango Bioassay
3.3. Ex-vivo Analyzed Systemic Chemerin Activity in Human and Murine Obesity
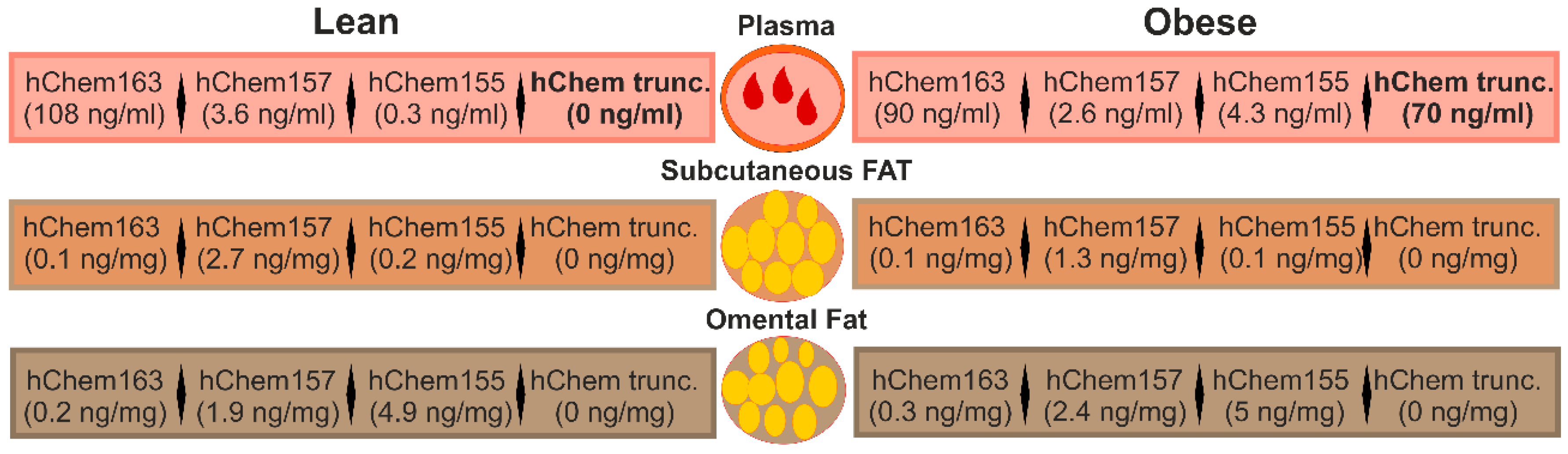
3.4. Chemerin Isoforms in Serum
3.5. Chemerin Isoforms in Adipose Tissues
4. Conclusions
Funding
Conflicts of Interest
References
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C. Chemerin in Liver Diseases. Endocrinol. Metab. Syndr. 2014, 3, 144. [Google Scholar]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Oppenheim, J.J. Chemerin reveals its chimeric nature. J. Exp. Med. 2008, 205, 2187–2190. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Kwitniewski, M.; Banas, M.; Zabieglo, K.; Murzyn, K.; Cichy, J. Chemerin regulation and role in host defense. Am. J. Clin. Exp. Immunol. 2014, 3, 1–19. [Google Scholar] [PubMed]
- Takahashi, M.; Okimura, Y.; Iguchi, G.; Nishizawa, H.; Yamamoto, M.; Suda, K.; Kitazawa, R.; Fujimoto, W.; Takahashi, K.; Zolotaryov, F.N.; et al. Chemerin regulates beta-cell function in mice. Sci. Rep. 2011, 1, 123. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.C.; Issa, M.; Goralski, K.B.; Sinal, C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 2010, 151, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Rabe, K.; Lebherz, C.; Zugwurst, J.; Goke, B.; Parhofer, K.G.; Lehrke, M.; Broedl, U.C. Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in LDL receptor knockout mice on high-fat diet. Diabetes 2010, 59, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Naruse, K.; Kobayashi, Y.; Miyabe, M.; Saiki, T.; Enomoto, A.; Takahashi, M.; Matsubara, T. Chemerin promotes angiogenesis in vivo. Physiol. Rep. 2018, 6, e13962. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.K.; Kaur, J.; Adya, R.; Miras, A.D.; Mattu, H.S.; Hattersley, J.G.; Kaltsas, G.; Tan, B.K.; Randeva, H.S. Chemerin induces endothelial cell inflammation: Activation of nuclear factor-kappa beta and monocyte-endothelial adhesion. Oncotarget 2018, 9, 16678–16690. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brezillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Oppenheim, J.J. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp. Cell Res. 2011, 317, 674–684. [Google Scholar] [CrossRef] [PubMed]
- De Henau, O.; Degroot, G.N.; Imbault, V.; Robert, V.; De Poorter, C.; McHeik, S.; Gales, C.; Parmentier, M.; Springael, J.Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. CMKLR1 and GPR1 mediate chemerin signaling through the RhoA/ROCK pathway. Mol. Cell. Endocrinol. 2015, 417, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2010, 391, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Laurencikiene, J.; Taube, A.; Eckardt, K.; Cramer, A.; Horrighs, A.; Arner, P.; Eckel, J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 2009, 58, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yin, H.K.; Guan, D.X.; Zhao, J.S.; Feng, Y.X.; Deng, Y.Z.; Wang, X.; Li, N.; Wang, X.F.; Cheng, S.Q.; et al. Chemerin suppresses hepatocellular carcinoma metastasis through CMKLR1-PTEN-Akt axis. Br. J. Cancer 2018, 118, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Liao, D.; Zhang, S.; Cheng, N.; He, H.Q.; Ye, R.D. Chemerin C9 peptide induces receptor internalization through a clathrin-independent pathway. Acta Pharmacol. Sin. 2014, 35, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Dranse, H.J.; Muruganandan, S.; Fawcett, J.P.; Sinal, C.J. Adipocyte-secreted chemerin is processed to a variety of isoforms and influences MMP3 and chemokine secretion through an NFkB-dependent mechanism. Mol. Cell. Endocrinol. 2016, 436, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Segal, D.; Shields, K.A.; Cummings, N.; Curran, J.E.; Comuzzie, A.G.; Mahaney, M.C.; Rainwater, D.L.; VandeBerg, J.L.; MacCluer, J.W.; et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J. Clin. Endocrinol. Metab. 2009, 94, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Wanninger, J.; Eisinger, K.; Hader, Y.; Beck, M.; Kopp, A.; Schmid, A.; Weiss, T.S.; Dorn, C.; Buechler, C. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp. Mol. Pathol. 2013, 95, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Brandao Proenca, J.; Santos-Silva, A.; Neuparth, M.J. Adiponectin, leptin, and chemerin in elderly patients with type 2 diabetes mellitus: A close linkage with obesity and length of the disease. BioMed Res. Int. 2014, 2014, 701915. [Google Scholar] [CrossRef] [PubMed]
- Kukla, M.; Zwirska-Korczala, K.; Hartleb, M.; Waluga, M.; Chwist, A.; Kajor, M.; Ciupinska-Kajor, M.; Berdowska, A.; Wozniak-Grygiel, E.; Buldak, R. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2010, 45, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, D.; Karpisek, M.; Hanulova, Z.; Svestak, M. Chemerin Is an Independent Marker of the Metabolic Syndrome in a Caucasian Population—A Pilot Study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2008, 152, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Wiest, R.; Farkas, S.; Scherer, M.N.; Schaffler, A.; Aslanidis, C.; et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin. Endocrinol. 2010, 72, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.H.; Sun, F.D.; Chen, S.H.; Liu, Y.T.; Liu, W.; Li, X.B.; Pan, Z.; Lou, N.J. Circulating chemerin levels are increased in first-degree relatives of type 2 diabetic patients. Clin. Lab. 2014, 60, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Curran, J.E.; Stocker, C.J.; Zaibi, M.S.; Segal, D.; Konstantopoulos, N.; Morrison, S.; Carless, M.; Dyer, T.D.; Cole, S.A.; et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010, 95, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Er, L.K.; Wu, S.; Hsu, L.A.; Teng, M.S.; Sun, Y.C.; Ko, Y.L. Pleiotropic Associations of RARRES2 Gene Variants and Circulating Chemerin Levels: Potential Roles of Chemerin Involved in the Metabolic and Inflammation-Related Diseases. Mediat. Inflamm. 2018, 2018, 4670521. [Google Scholar] [CrossRef] [PubMed]
- Mussig, K.; Staiger, H.; Machicao, F.; Thamer, C.; Machann, J.; Schick, F.; Claussen, C.D.; Stefan, N.; Fritsche, A.; Haring, H.U. RARRES2, encoding the novel adipokine chemerin, is a genetic determinant of disproportionate regional body fat distribution: A comparative magnetic resonance imaging study. Metabolism 2009, 58, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Tonjes, A.; Scholz, M.; Breitfeld, J.; Marzi, C.; Grallert, H.; Gross, A.; Ladenvall, C.; Schleinitz, D.; Krause, K.; Kirsten, H.; et al. Genome wide meta-analysis highlights the role of genetic variation in RARRES2 in the regulation of circulating serum chemerin. PLoS Genet. 2014, 10, e1004854. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C. Chemerin, a novel player in inflammatory bowel disease. Cell. Mol. Immunol. 2014, 11, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.S.; Rehman, R.; Baig, M.; Khan, T.A. New roles of the multidimensional adipokine: Chemerin. Peptides 2014, 62, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Roncucci, L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm. Res. 2015, 64, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.J.; Pachynski, R.K. Chemerin modulation of tumor growth: Potential clinical applications in cancer. Discov. Med. 2018, 26, 31–37. [Google Scholar] [PubMed]
- Farsam, V.; Basu, A.; Gatzka, M.; Treiber, N.; Schneider, L.A.; Mulaw, M.A.; Lucas, T.; Kochanek, S.; Dummer, R.; Levesque, M.P.; et al. Senescent fibroblast-derived Chemerin promotes squamous cell carcinoma migration. Oncotarget 2016, 7, 83554–83569. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Liu, W.; Li, B.; Yin, W.; Shi, Y.; He, R. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene 2017, 36, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.; Greaves, D.R. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Luangsay, S.; Wittamer, V.; Bondue, B.; De Henau, O.; Rouger, L.; Brait, M.; Franssen, J.D.; de Nadai, P.; Huaux, F.; Parmentier, M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009, 183, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Yue, W.; Xu, X.; Li, B.; Zou, L.; He, R. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell. Mol. Immunol. 2014, 11, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Chakaroun, R.; Raschpichler, M.; Kloting, N.; Oberbach, A.; Flehmig, G.; Kern, M.; Schon, M.R.; Shang, E.; Lohmann, T.; Dressler, M.; et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 2012, 61, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Wanninger, J.; Schmidhofer, S.; Weigert, J.; Neumeier, M.; Dorn, C.; Hellerbrand, C.; Zimara, N.; Schaffler, A.; Aslanidis, C.; et al. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces Chemerin in hypertrophic adipocytes. Endocrinology 2011, 152, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Catalan, V.; Gomez-Ambrosi, J.; Rodriguez, A.; Ramirez, B.; Rotellar, F.; Valenti, V.; Silva, C.; Gil, M.J.; Salvador, J.; Fruhbeck, G. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: Tumor necrosis factor-α stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg. Obes. Relat. Dis. 2013, 9, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Parlee, S.D.; Ernst, M.C.; Muruganandan, S.; Sinal, C.J.; Goralski, K.B. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-α. Endocrinology 2010, 151, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M.; Chishti, M.A.; Moustafa, A.S.; Fatma, S.; Alomaim, W.S.; Al-Naami, M.Y.; Bassas, A.F.; Chrousos, G.P.; Jo, H. Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: Adipose depot specificity and gender dimorphism. Mol. Cells 2012, 33, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Wolfs, M.G.; Gruben, N.; Rensen, S.S.; Verdam, F.J.; Greve, J.W.; Driessen, A.; Wijmenga, C.; Buurman, W.A.; Franke, L.; Scheja, L.; et al. Determining the association between adipokine expression in multiple tissues and phenotypic features of non-alcoholic fatty liver disease in obesity. Nutr. Diabetes 2015, 5, e146. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rebordelo, E.; Cunarro, J.; Perez-Sieira, S.; Seoane, L.M.; Dieguez, C.; Nogueiras, R.; Tovar, S. Regulation of Chemerin and CMKLR1 Expression by Nutritional Status, Postnatal Development, and Gender. Int. J. Mol. Sci. 2018, 19, 2905. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J. The long road to leptin. J. Clin. Investig. 2016, 126, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Wanninger, J.; Neumeier, M. Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 2011, 17, 2801–2811. [Google Scholar] [PubMed]
- Sjoholm, A.; Nystrom, T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab. Res. Rev. 2006, 22, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Hong, Y.H.; Song, S.H.; Ardiyanti, A.; Kato, D.; So, K.H.; Katoh, K.; Roh, S.G. The Regulation of Chemerin and CMKLR1 Genes Expression by TNF-α, Adiponectin, and Chemerin Analog in Bovine Differentiated Adipocytes. Asian-Australas. J. Anim. Sci. 2012, 25, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Muruganandan, S.; Dranse, H.J.; McMullen, N.M.; Sinal, C.J. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J. Endocrinol. 2014, 222, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Docke, S.; Lock, J.F.; Birkenfeld, A.L.; Hoppe, S.; Lieske, S.; Rieger, A.; Raschzok, N.; Sauer, I.M.; Florian, S.; Osterhoff, M.A.; et al. Elevated hepatic chemerin gene expression in progressed human non-alcoholic fatty liver disease. Eur. J. Endocrinol. 2013, 169, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kajor, M.; Kukla, M.; Waluga, M.; Liszka, L.; Dyaczynski, M.; Kowalski, G.; Zadlo, D.; Berdowska, A.; Chapula, M.; Kostrzab-Zdebel, A.; et al. Hepatic chemerin mRNA in morbidly obese patients with nonalcoholic fatty liver disease. Pol. J. Pathol. 2017, 68, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Pohl, R.; Haberl, E.M.; Rein-Fischboeck, L.; Zimny, S.; Neumann, M.; Aslanidis, C.; Schacherer, D.; Krautbauer, S.; Eisinger, K.; Weiss, T.S.; et al. Hepatic chemerin mRNA expression is reduced in human nonalcoholic steatohepatitis. Eur. J. Clin. Investig. 2017, 47, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, H.; Lu, Y.; Liu, S.; Zhang, Q.; Huang, J.; Zhu, R.; Yang, J.; Zhang, R.; Zhang, D.; et al. Identification of Chemerin as a Novel FXR Target Gene Down-Regulated in the Progression of Nonalcoholic Steatohepatitis. Endocrinology 2013, 154, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, J.; Bauer, S.; Eisinger, K.; Weiss, T.S.; Walter, R.; Hellerbrand, C.; Schaffler, A.; Higuchi, A.; Walsh, K.; Buechler, C. Adiponectin upregulates hepatocyte CMKLR1 which is reduced in human fatty liver. Mol. Cell. Endocrinol. 2012, 349, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Meier, E.M.; Rein-Fischboeck, L.; Krautbauer, S.; Eisinger, K.; Aslanidis, C.; Pohl, R.; Weiss, T.S.; Buechler, C. Chemokine-Like Receptor 1 mRNA Weakly Correlates with Non-Alcoholic Steatohepatitis Score in Male but Not Female Individuals. Int. J. Mol. Sci. 2016, 17, 1335. [Google Scholar] [CrossRef] [PubMed]
- Zimny, S.; Pohl, R.; Rein-Fischboeck, L.; Haberl, E.M.; Krautbauer, S.; Weiss, T.S.; Buechler, C. Chemokine (CC-motif) receptor-like 2 mRNA is expressed in hepatic stellate cells and is positively associated with characteristics of non-alcoholic steatohepatitis in mice and men. Exp. Mol. Pathol. 2017, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Wu, M.; Zhu, X.; Wang, T.; He, K.; Li, P.; Wu, X. PI3K inhibition protects mice from NAFLD by down-regulating CMKLR1 and NLRP3 in Kupffer cells. J. Physiol. Biochem. 2017, 73, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Herova, M.; Schmid, M.; Gemperle, C.; Hersberger, M. ChemR23, the Receptor for Chemerin and Resolvin E1, Is Expressed and Functional on M1 but Not on M2 Macrophages. J. Immunol. 2015, 194, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yamaguchi, Y.; Shen, W.J.; Morser, J.; Leung, L.L.K. Dynamic and tissue-specific proteolytic processing of chemerin in obese mice. PLoS ONE 2018, 13, e0202780. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Zabel, B.A.; Myles, T.; Allen, S.J.; Handel, T.M.; Lee, P.P.; Butcher, E.C.; Leung, L.L. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J. Biol. Chem. 2009, 284, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kulig, P.; Kantyka, T.; Zabel, B.A.; Banas, M.; Chyra, A.; Stefanska, A.; Tu, H.; Allen, S.J.; Handel, T.M.; Kozik, A.; et al. Regulation of chemerin chemoattractant and antibacterial activity by human cysteine cathepsins. J. Immunol. 2011, 187, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.; Saalbach, A.; Heiker, J.T.; Meier, R.; Zellmann, T.; Simon, J.C.; Beck-Sickinger, A.G. Proteolytic activation of prochemerin by kallikrein 7 breaks an ionic linkage and results in C-terminal rearrangement. Biochem. J. 2013, 452, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yamaguchi, Y.; Ge, X.; Robinson, W.H.; Morser, J.; Leung, L.L.K. Chemerin 156F, generated by chymase cleavage of prochemerin, is elevated in joint fluids of arthritis patients. Arthritis Res. Ther. 2018, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Guillabert, A.; Wittamer, V.; Bondue, B.; Godot, V.; Imbault, V.; Parmentier, M.; Communi, D. Role of neutrophil proteinase 3 and mast cell chymase in chemerin proteolytic regulation. J. Leukoc. Biol. 2008, 84, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yamaguchi, Y.; Zhao, L.; Bury, L.; Gresele, P.; Berube, C.; Leung, L.L.; Morser, J. Prochemerin cleavage by factor XIa links coagulation and inflammation. Blood 2018, 131, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Coates, D. The angiotensin converting enzyme (ACE). Int. J. Biochem. Cell Biol. 2003, 35, 769–773. [Google Scholar] [CrossRef]
- John, H.; Hierer, J.; Haas, O.; Forssmann, W.G. Quantification of angiotensin-converting-enzyme-mediated degradation of human chemerin 145-154 in plasma by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Anal. Biochem. 2007, 362, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, H.; Kazama, K.; Takai, M.; Oda, M.; Okada, M.; Yamawaki, H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1017–H1028. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, G.; Dong, J.; Liu, Y.; Zong, H.; Liu, H.; Boden, G.; Li, L. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J. Investig. Med. 2010, 58, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Zylla, S.; Pietzner, M.; Kuhn, J.P.; Volzke, H.; Dorr, M.; Nauck, M.; Friedrich, N. Serum chemerin is associated with inflammatory and metabolic parameters-results of a population-based study. Obesity 2017, 25, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Darios, E.S.; Mullick, A.E.; Garver, H.; Saunders, T.L.; Hughes, E.D.; Filipiak, W.E.; Zeidler, M.G.; McMullen, N.; Sinal, C.J.; et al. The chemerin knockout rat reveals chemerin dependence in female, but not male, experimental hypertension. FASEB J. 2018, 32, 3596–6614. [Google Scholar] [CrossRef] [PubMed]
- Ferland, D.J.; Seitz, B.; Darios, E.S.; Thompson, J.M.; Yeh, S.T.; Mullick, A.E.; Watts, S.W. Whole-Body but Not Hepatic Knockdown of Chemerin by Antisense Oligonucleotide Decreases Blood Pressure in Rats. J. Pharmacol. Exp. Ther. 2018, 365, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Du, X.Y.; Zhao, L.; Morser, J.; Leung, L.L. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J. Biol. Chem. 2011, 286, 39510–39519. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Eisenberg, D.; Zhao, L.; Adams, C.; Leib, R.; Morser, J.; Leung, L. Chemerin activation in human obesity. Obesisty 2016, 24, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Sona, C.; Kumar, A.; Yadav, P.N. Tango assay for ligand-induced GPCR-beta-arrestin2 interaction: Application in drug discovery. Methods Cell Biol. 2016, 132, 233–254. [Google Scholar] [PubMed]
- Haberl, E.M.; Pohl, R.; Rein-Fischboeck, L.; Feder, S.; Eisinger, K.; Krautbauer, S.; Sinal, C.J.; Buechler, C. Ex vivo analysis of serum chemerin activity in murine models of obesity. Cytokine 2018, 104, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Toulany, J.; Parlee, S.D.; Sinal, C.J.; Slayter, K.; McNeil, S.; Goralski, K.B. CMKLR1 activation ex vivo does not increase proportionally to serum total chemerin in obese humans. Endocr. Connect. 2016, 5, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor α: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Bala, M.; Kopp, A.; Eisinger, K.; Schmid, A.; Schneider, S.; Neumeier, M.; Buechler, C. Adipocyte chemerin release is induced by insulin without being translated to higher levels in vivo. Eur. J. Clin. Investig. 2012, 42, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Pappas, C.; Kandaraki, E.A.; Tsirona, S.; Kountouras, D.; Kassi, G.; Diamanti-Kandarakis, E. Postprandial dysmetabolism: Too early or too late? Hormones 2016, 15, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Divoux, A.; Moutel, S.; Poitou, C.; Lacasa, D.; Veyrie, N.; Aissat, A.; Arock, M.; Guerre-Millo, M.; Clement, K. Mast cells in human adipose tissue: Link with morbid obesity, inflammatory status, and diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1677–E1685. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; Christian, A.R.; Greaves, D.R. Chemerin peptides promote phagocytosis in a ChemR23- and Syk-dependent manner. J. Immunol. 2010, 184, 5315–5324. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. https://doi.org/10.3390/ijms20051128
Buechler C, Feder S, Haberl EM, Aslanidis C. Chemerin Isoforms and Activity in Obesity. International Journal of Molecular Sciences. 2019; 20(5):1128. https://doi.org/10.3390/ijms20051128
Chicago/Turabian StyleBuechler, Christa, Susanne Feder, Elisabeth M. Haberl, and Charalampos Aslanidis. 2019. "Chemerin Isoforms and Activity in Obesity" International Journal of Molecular Sciences 20, no. 5: 1128. https://doi.org/10.3390/ijms20051128
APA StyleBuechler, C., Feder, S., Haberl, E. M., & Aslanidis, C. (2019). Chemerin Isoforms and Activity in Obesity. International Journal of Molecular Sciences, 20(5), 1128. https://doi.org/10.3390/ijms20051128