AdRAP2.3, a Novel Ethylene Response Factor VII from Actinidia deliciosa, Enhances Waterlogging Resistance in Transgenic Tobacco through Improving Expression Levels of PDC and ADH Genes
Abstract
:1. Introduction
2. Results
2.1. Cloning and Sequence Analysis of AdRAP2.3
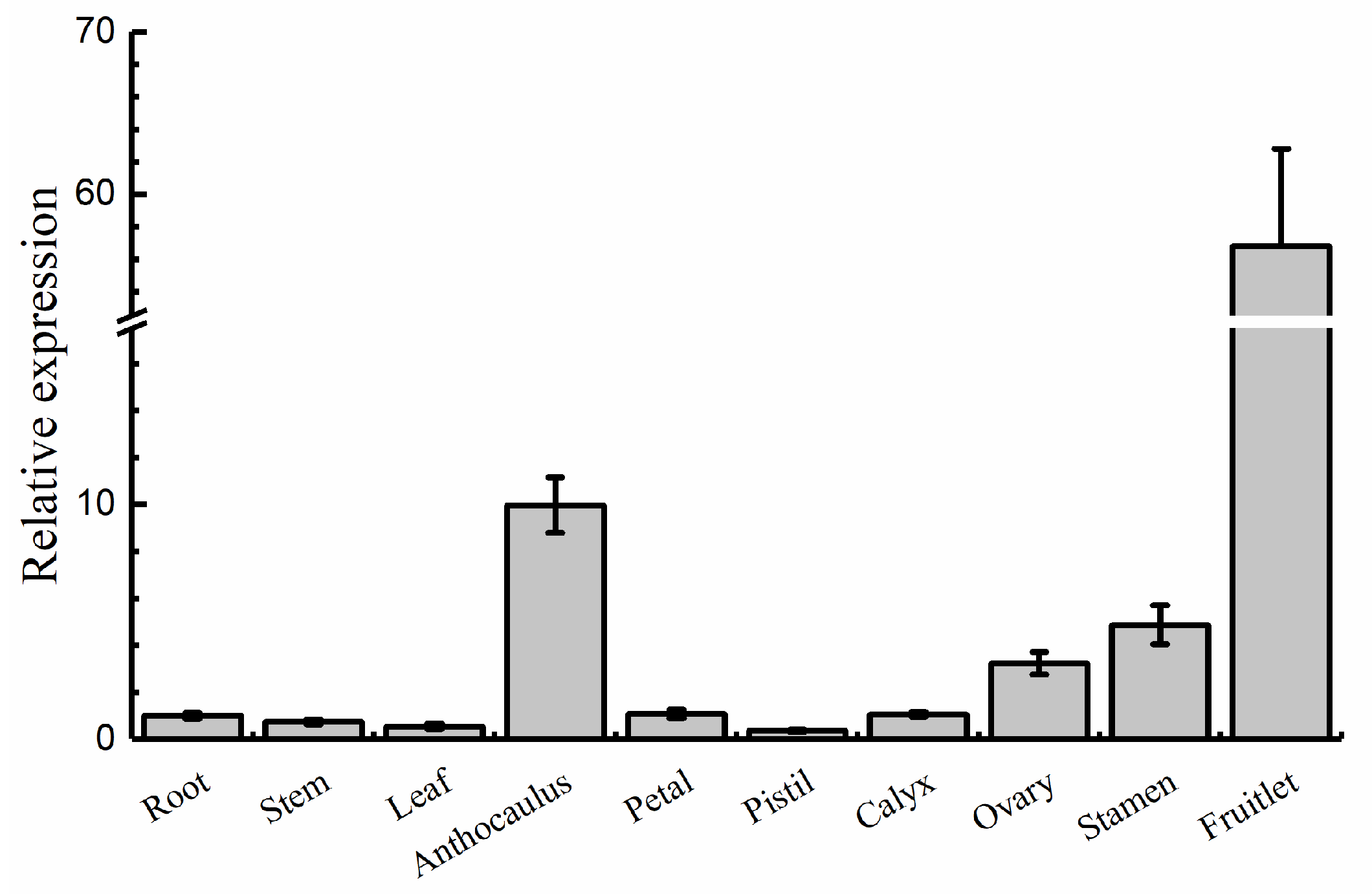
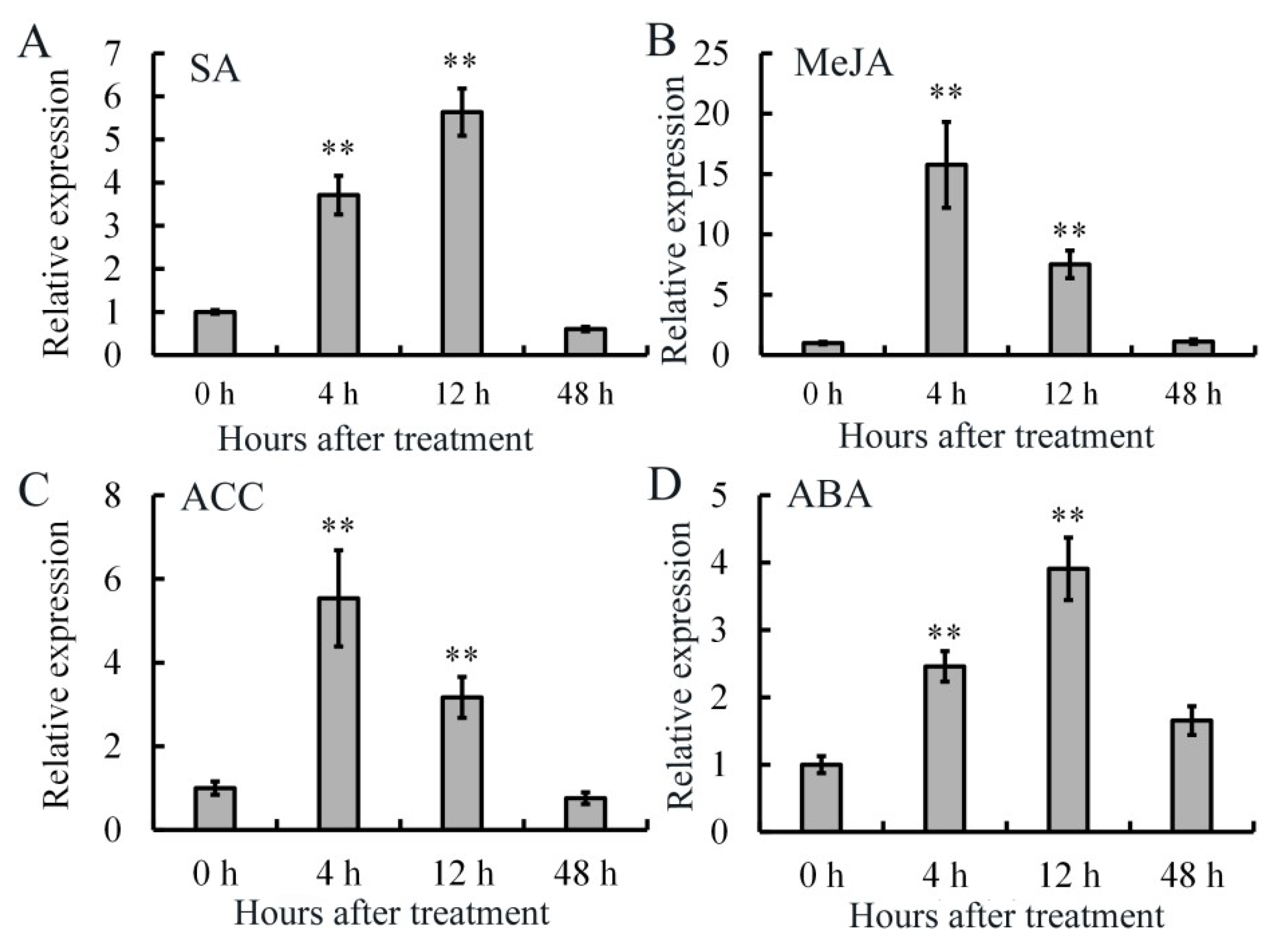
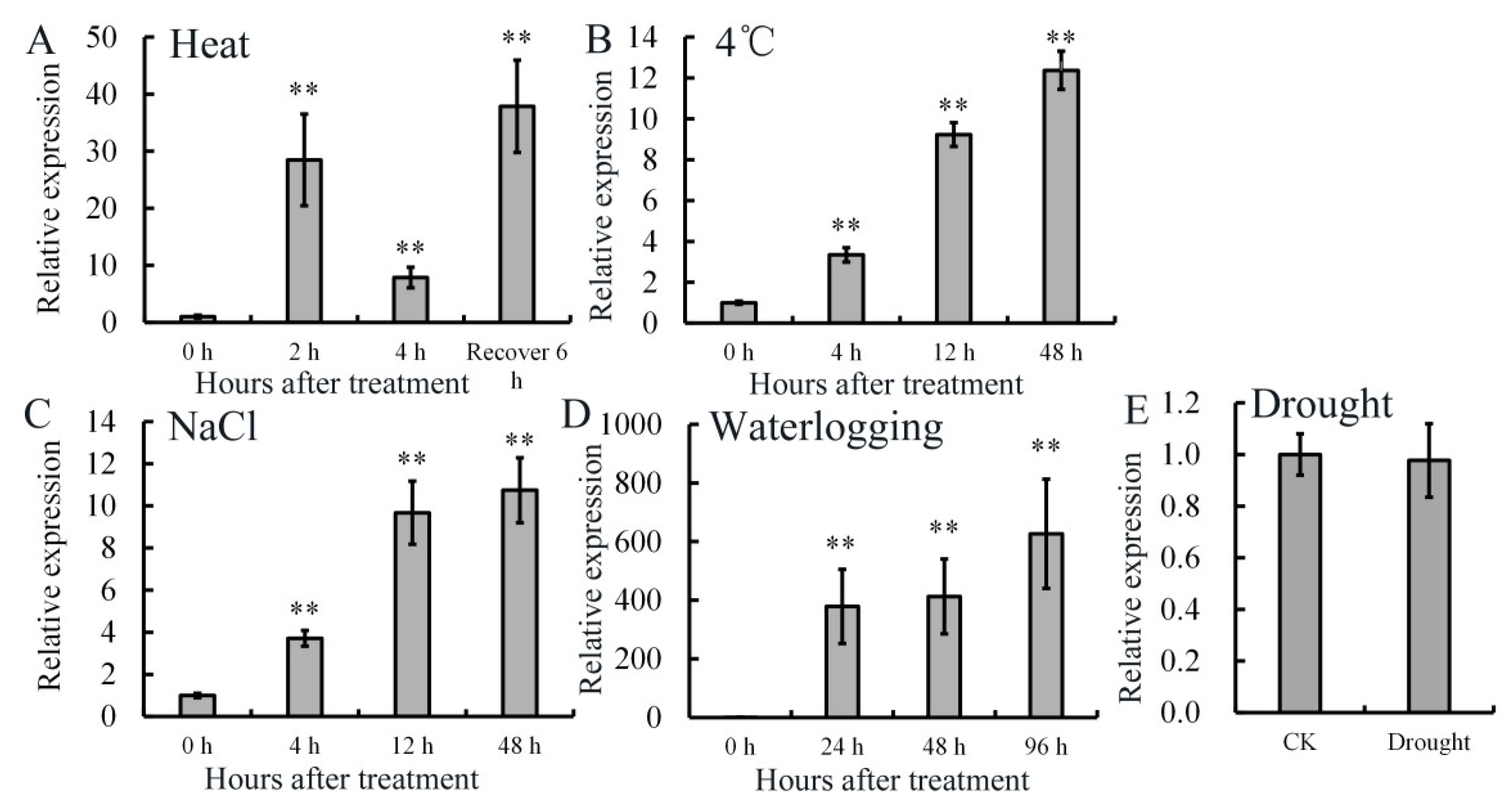
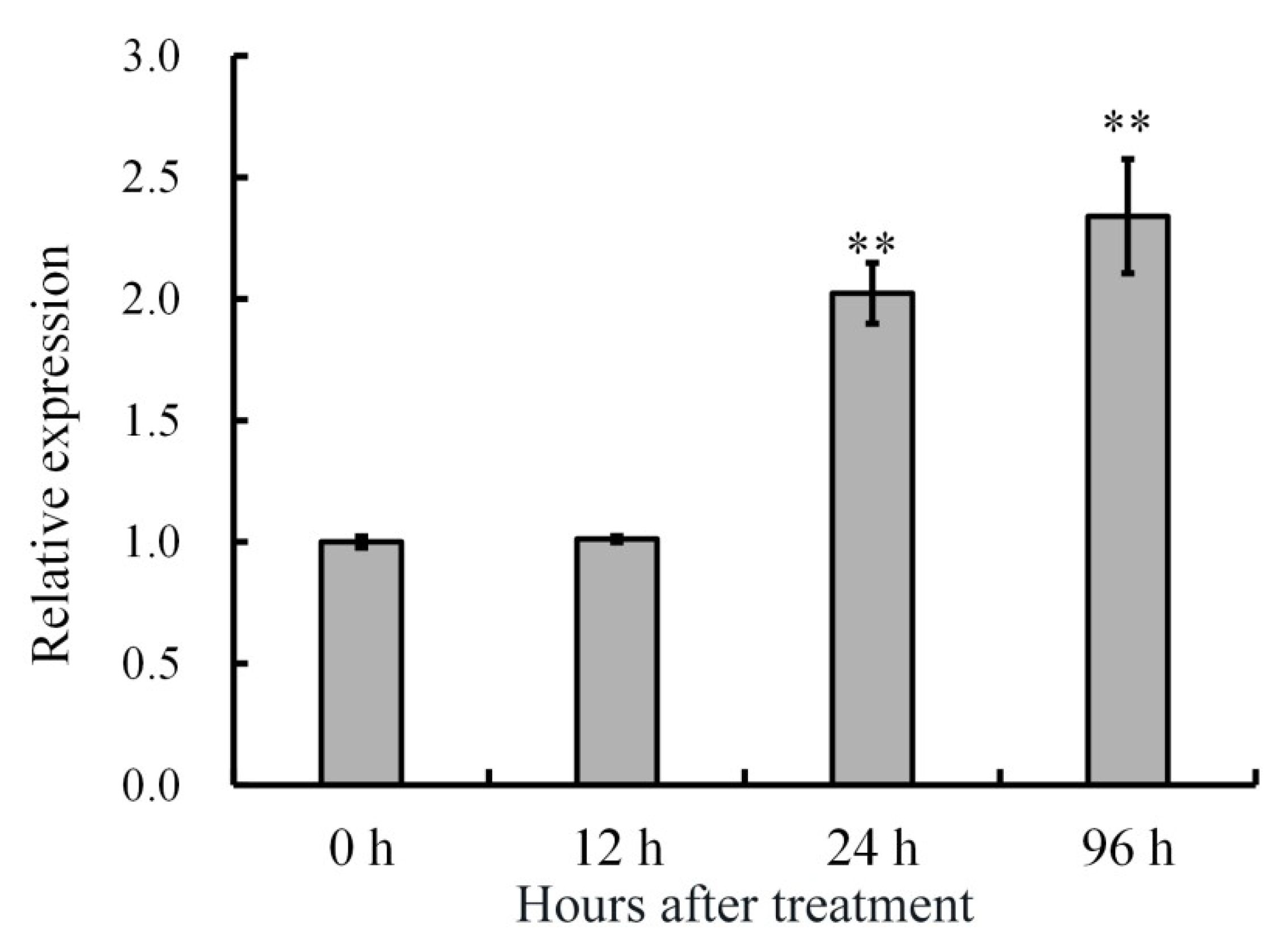
2.2. Expression Patterns of AdRAP2.3 in A. deliciosa
2.3. Overexpression of AdRAP2.3 Enhanced Waterlogging Tolerance in Transgenic Plants
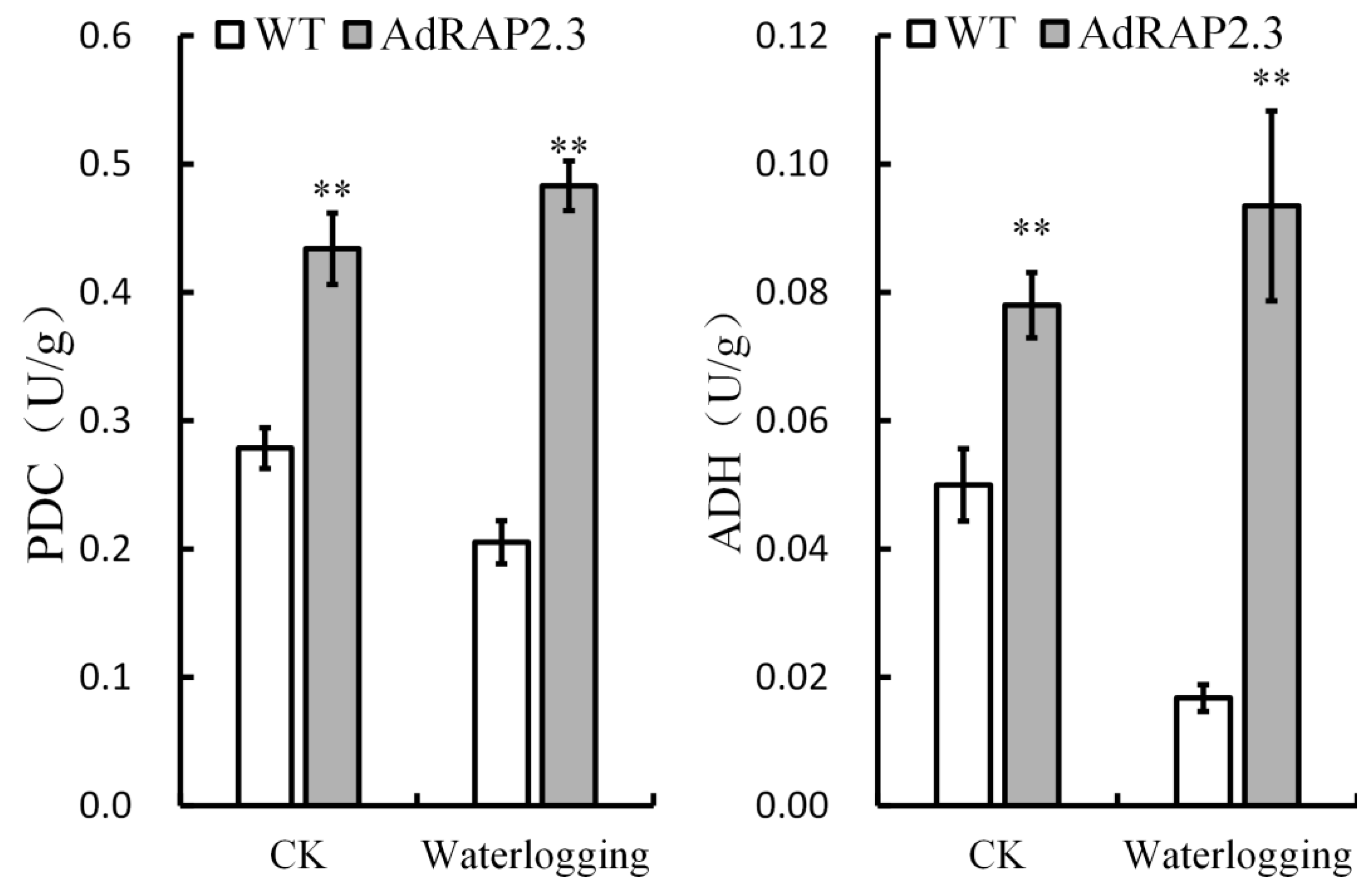
2.4. Physiological Changes in Transgenic Plants under Waterlogging Stress
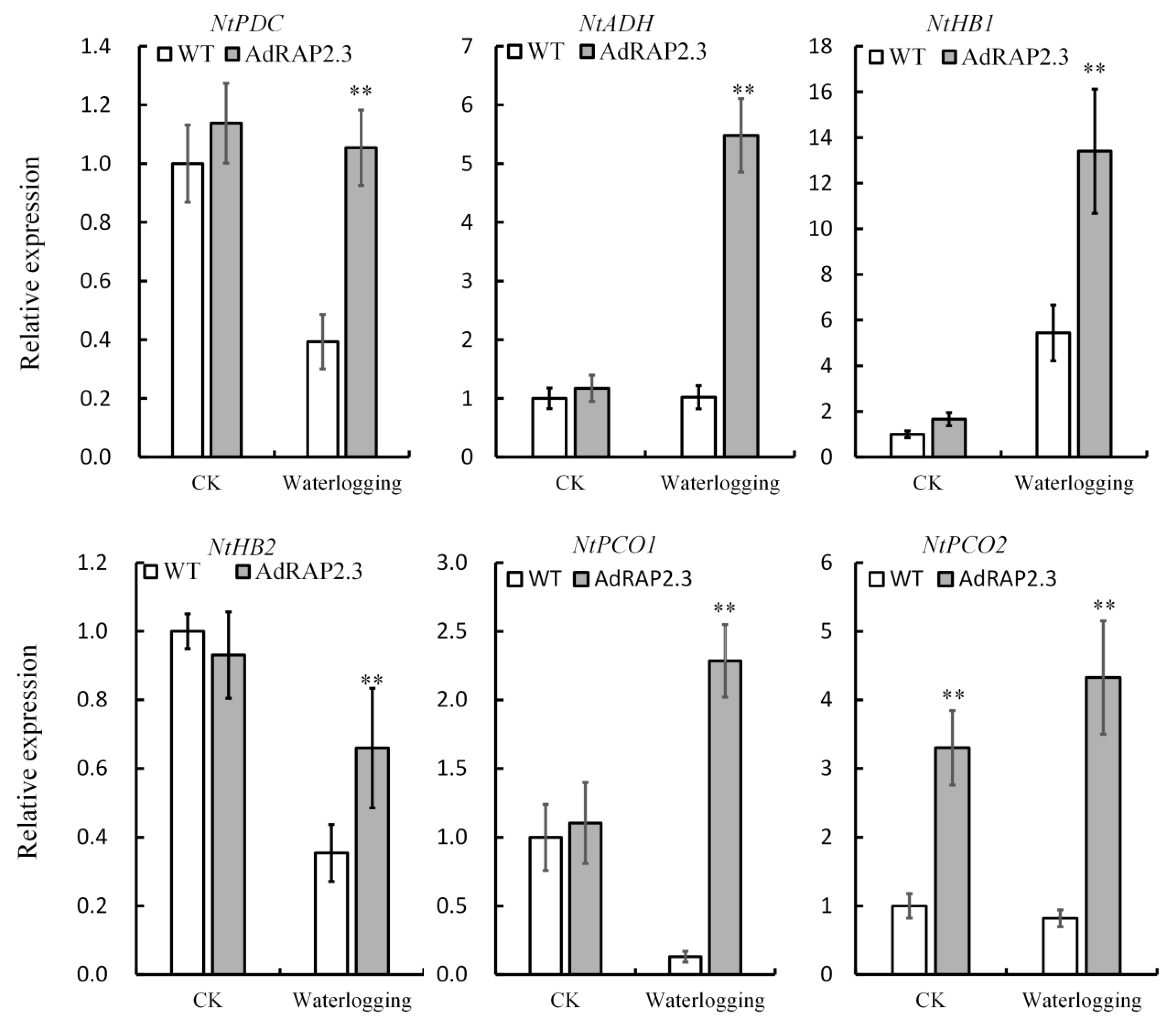
2.5. Waterlogging-Related Genes Changes in Transgenic Plants under Waterlogging Stress
3. Discussion
3.1. Kiwi Fruit AdRAP2.3 Plays a Key Role in Resistance to Waterlogging Stress
3.2. AdRAP2.3 Could Enhance Resistance to Waterlogging through Promoting Pneumatophore Production
3.3. Kiwi Fruit AdRAP2.3 Enhances Waterlogging Resistance in Transgenic Tobacco through Improving Expression Levels of PDC and ADH Genes
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Treatments
4.3. RNA Extraction and cDNA Synthesis
4.4. Gene Clone and Sequence Analysis
4.5. Gene Expression Analysis Using Quantitative Real-Time PCR
4.6. Construction Binary Vector and Transformation of Tobacco
4.7. Phenotype Analysis of Transgenic Tobacco under Waterlogging Resistance
4.8. Measurement of Anaerobic Respiration and ADH and PDC Activities
4.9. Expression Analysis of Downstream Genes
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Tian, Y.S.; Xu, J.; Fu, X.Y.; Gao, J.J.; Wang, B.; Han, H.J.; Wang, L.J.; Peng, R.H.; Yao, Q.H. A tomato ERF transcription factor, SlERF84, confers enhanced tolerance to drought and salt stress but negatively regulates immunity against Pseudomonas syringae pv. tomato DC3000. Plant Physiol. Biochem. 2018, 132, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Meyerowitz, E.M. The AP2/EREBP family of plant transcription factors. Biol. Chem. 1998, 379, 633–646. [Google Scholar] [PubMed]
- Liang, Y.Q.; Li, X.S.; Zhang, D.Y.; Gao, B.; Yang, H.L.; Wang, Y.C.; Guan, K.Y.; Wood, A.J. ScDREB8, a novel A-5 type of DREB gene in the desert moss Syntrichia caninervis, confers salt tolerance to Arabidopsis. Plant Physiol. Biochem. 2017, 120, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Ji, J.; Wang, G.; Guan, C.F.; Jin, C. LchERF, a novel ethylene-responsive transcription factor from Lycium chinense, confers salt tolerance in transgenic tobacco. Plant Cell Rep. 2014, 33, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Zhu, X.Y.; Mao, J.; Zou, Y.; Fu, D.W.; Chen, W.X.; Lu, W.J. Isolation and characterization of ethylene response factor family genes during development, ethylene regulation and stress treatments in papaya fruit. Plant Physiol. Biochem. 2013, 70, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-Binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Lee, S.C.; Isa, N.M.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J.; et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Gasch, P.; Fundinger, M.; Müller, J.T.; Lee, T.; Bailey-serres, J.; Mustroph, A. Redundant ERF-VII transcription factors bind an evolutionarily-conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell 2015, 28, 160–180. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.; Voesenek, L.A.C.J.; Perata, P.; Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.V.; Iyer, S.; Amerhauser, C.; Lehmann, M.; Dongen, J.T.; Geigenberger, P. Oxygen sensing via the ethylene response transcription factor RAP2.12 affects plant metabolism and performance under both normoxia and hypoxia. Plant Physiol. 2016, 172, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Wilson, I.W.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. Arabidopsis RAP2.2: An ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar]
- Perata, P.; Voesenek, L.A.C.J. Submergence tolerance in rice requires Sub1A, an ethylene-response-factor-like gene. Trends Plant Sci. 2007, 12, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Li, J.Y.; Wang, H.N.; Fu, Z.D.; Liu, J.; Yu, Y.X. Identification and expression analysis of ERF transcription factor genes in petunia during flower senescence and in response to hormone treatments. J. Exp. Bot. 2011, 62, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Pan, D.L.; Wang, G.; Xuan, J.P.; Wang, T.; Guo, Z.R. Genome-wide analysis and expression pattern of the AP2/ERF gene family in kiwifruit under waterlogging stress treatment. Int. J. Environ. Agric. Res. 2017, 3, 83–90. [Google Scholar]
- Zhang, J.Y.; Huang, S.N.; Mo, Z.H.; Xuan, J.P.; Jia, X.D.; Wang, G.; Guo, Z.R. De novo transcriptome sequencing and comparative analysis of differentially expressed genes in kiwifruit under waterlogging stress. Mol. Breed. 2015, 35, 208. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Abscisic acid signaling system in plant innate immunity. In Plant Hormone Signaling Systems in Plant Innate Immunity. Signaling and Communication in Plants; Springer: Dordrecht, The Netherlands, 2015; Volume 2, pp. 245–309. [Google Scholar]
- Pál, M.; Szalai, G.; Kovács, V.; Gondor, O.K.; Janada, T. Salicylic acid-mediated abiotic stress tolerance. In Salicylic Acid; Hayat, S., Ahmad, A., Alyemeni, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 183–247. [Google Scholar]
- Janse, J.D.; Scortichini, M. Characterization of Pseudomonas Syringae pv. actinidiae, the causal agent of bacterial canker of kiwifruit by whole cell protein electrophoresis and fatty acid analysis. In Pseudomonas Syringae Pathovars and Related Pathogens. Developments in Plant Pathology; Rudolph, K., Burr, T.J., Mansfield, J.W., Stead, D., Vivian, A., von Kietzell, J., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 9, p. 499. [Google Scholar]
- Yin, D.M.; Chen, S.M.; Chen, F.D.; Guan, Z.Y.; Fang, W.M. Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ. Exp. Bot. 2010, 68, 122–130. [Google Scholar] [CrossRef]
- Ricard, B.; Couee, I.; Raymond, P.; Saglio, P.H.; Saintges, V.; Pradet, A. Plant metabolism under hypoxia and anoxia. Plant Physiol. Biochem. 1994, 32, 1–10. [Google Scholar]
- Tadege, M.; Dupuis, I.; Kuhlemeier, C. Ethanolic fermentation: New functions for an old pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A. Flooding stress: Acclimations and genetic diversity. Ann. Rev. Plant Boil. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Watanabe, K.; Fukazawa, A.; Mori, H.; Abe, F.; Kawaguchi, K.; Oyanagi, A.; Nakazono, M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J. Exp. Bot. 2014, 65, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Van Dongen, J.T.; Giuntoli, B.; Novi, G.; Santaniello, A.; Geigenberger, P.; Perata, P. HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J. 2010, 62, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Bacardit, J.; Bachmair, A.; Holdsworth, M.J. The eukaryotic N-end rule pathway: Conserved mechanisms and diverse functions. Trends Cell Biol. 2014, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Pucciariello, C.; Perata, P. New role for an old rule: N-end rule-mediated degradation of ethylene responsive factor proteins governs low oxygen response in plants. J. Integr. Plant Boil. 2013, 55, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rasheed-Depardieu, C.; Parelle, J.; Tatin-Froux, F.; Parent, C.; Capelli, N. Short-term response to waterlogging in Quercus petraea and Quercus robur: A study of the root hydraulic responses and the transcriptional pattern of aquaporins. Plant Physiol. Biochem. 2015, 97, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zhao, S.J.; Cui, M.X.; Han, G.X.; Wen, P. Vulnerability of photosynthesis and photosystem I in Jerusalem artichoke (Helianthus tuberosus L.) exposed to waterlogging. Plant Physiol. Biochem. 2018, 125, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Purnobasuki, H.; Nurhidayati, T.; Hariyanto, S.; Jadid, N. Data of root anatomical responses to periodic waterlogging stress of tobacco (Nicotiana tabacum) varieties. Data Brief 2018, 20, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Chen, S.M.; Chen, F.D.; Jiang, J.F. Ethylene promotes induction of aerenchyma formation and ethanolic; fermentation in waterlogged roots of Dendranthema spp. Mol. Biol. Rep. 2013, 40, 4581–4590. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Y.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Hu, H.L.; Zhou, M.X. A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theor. Appl. Genet. 2017, 130, 1559–1568. [Google Scholar] [CrossRef]
- Ponte, N.H.T.; Santos, R.I.; Filho, W.R.; Cunha, R.L.; Magalhaes, M.M.; Pinheiro, H.A. Morphological assessments evidence that higher number of pneumatophores improves tolerance to long-term waterlogging in oil palm (Elaeis guineensis) seedlings. Flora 2019, 250, 52–58. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, F.S.; Zheng, Y.Z.; Mei, H.X. Comparison of morphology, physiology and mineral element contents among genotypes of sesame with different tolerance to waterlogging under anaerobic condition. Chin. J. Appl. Ecol. 2002, 13, 421–424. [Google Scholar]
- Zhou, C.P.; Bai, T.; Wang, Y.; Wu, T.; Zhang, X.Z.; Xu, X.F.; Han, Z.H. Morpholoical and enzymatic responses to waterlogging in three Prunus species. Sci. Hortic. 2017, 221, 62–67. [Google Scholar] [CrossRef]
- An, Y.Y.; Qi, L.; Wang, L.J. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS ONE 2016, 11, e0147202. [Google Scholar] [CrossRef] [PubMed]
- Bahmaniar, M.A. The influence of continuous rice cultivation and different waterlogging periods on the morphology, clay mineralogy, Eh, pH and K in paddy soils. Eurasian Soil Sci. 2008, 41, 87–92. [Google Scholar] [CrossRef]
- Qi, X.H.; Xu, X.W.; Lin, X.J.; Zhang, W.J.; Chen, X.H. Identification of differentially expressed genes in cucumber (Cucumis sativus L.) root under waterlogging stress by digital gene expression profile. Genomics 2012, 99, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.Y.; Yang, Y.; Yin, Y.L.; Xu, M.; Li, H.G. De novo sequencing, assembly, and analysis of the Taxodium ‘Zhongshansa’ roots and shoots transcriptome in response to short-term waterlogging. BMC Plant Biol. 2014, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.W.; Chen, M.Y.; Ji, J.; Xu, J.; Qi, X.H.; Chen, X.H. Comparative RNA-seq based transcriptome profiling of waterlogging response in cucumber hypocotyls reveals novel insights into the de novo adventitious root primordia initiation. BMC Plant Biol. 2017, 17, 129. [Google Scholar] [CrossRef]
- Zhang, P.; Lyu, D.G.; Jia, L.T.; He, J.L.; Qin, S.J. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Huang, S.N.; Wang, G.; Xuan, J.P.; Guo, Z.R. Overexpression of Actinidia deliciosa pyruvate decarboxylase 1 gene enhances waterlogging stress in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2016, 106, 244–252. [Google Scholar] [CrossRef]
- Luo, H.T.; Zhang, J.Y.; Wang, G.; Jia, Z.H.; Huang, S.N.; Wang, T.; Guo, Z.R. Functional characterization of waterlogging and heat stresses tolerance gene Pyruvate decarboxylase 2 from Actinidia deliciosa. Int. J. Mol. Sci. 2017, 18, 2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Huang, S.N.; Chen, Y.H.; Wang, G.; Guo, Z.R. Identification and characterization of two waterlogging responsive alcohol dehydrogenase genes (AdADH1 and AdADH2) in Actinidia deliciosa. Mol. Breed. 2017, 37, 52. [Google Scholar] [CrossRef]
- Weits, D.A.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Hubberten, H.M.; Riegler, H.; Hoefgen, R.; Perata, P.; Dongen, J.T.; Licausi, F. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 2014, 5, 3425. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, G.; Jia, Z.H.; Pan, D.L.; Zhang, J.Y.; Guo, Z.R. Transcriptome analysis of kiwifruit in response to Pseudomonas syringae pv. actinidiae infection. Int. J. Mol. Sci. 2018, 19, 373. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.H.; Zhang, J.Y.; Gao, Z.H.; Qu, S.C.; Tong, Z.G.; Mi, L.; Qiao, Y.S.; Zhang, Z. An improved method for isolation of total RNA from the leaves of Fragaria spp. Jiangsu J. Agric. Sci. 2008, 24, 875–877. [Google Scholar]
- Yin, X.R.; Allan, A.C.; Xu, Q.; Burdon, J.; Dejnoprat, S.; Chen, K.S.; Fergusom, L.B. Differential expression of kiwifruit genes in response to postharvest abiotic stress. Postharvest Biol. Technol. 2012, 66, 1–7. [Google Scholar] [CrossRef]
- Horsch, R.B. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, D.-L.; Wang, G.; Wang, T.; Jia, Z.-H.; Guo, Z.-R.; Zhang, J.-Y. AdRAP2.3, a Novel Ethylene Response Factor VII from Actinidia deliciosa, Enhances Waterlogging Resistance in Transgenic Tobacco through Improving Expression Levels of PDC and ADH Genes. Int. J. Mol. Sci. 2019, 20, 1189. https://doi.org/10.3390/ijms20051189
Pan D-L, Wang G, Wang T, Jia Z-H, Guo Z-R, Zhang J-Y. AdRAP2.3, a Novel Ethylene Response Factor VII from Actinidia deliciosa, Enhances Waterlogging Resistance in Transgenic Tobacco through Improving Expression Levels of PDC and ADH Genes. International Journal of Molecular Sciences. 2019; 20(5):1189. https://doi.org/10.3390/ijms20051189
Chicago/Turabian StylePan, De-Lin, Gang Wang, Tao Wang, Zhan-Hui Jia, Zhong-Ren Guo, and Ji-Yu Zhang. 2019. "AdRAP2.3, a Novel Ethylene Response Factor VII from Actinidia deliciosa, Enhances Waterlogging Resistance in Transgenic Tobacco through Improving Expression Levels of PDC and ADH Genes" International Journal of Molecular Sciences 20, no. 5: 1189. https://doi.org/10.3390/ijms20051189




