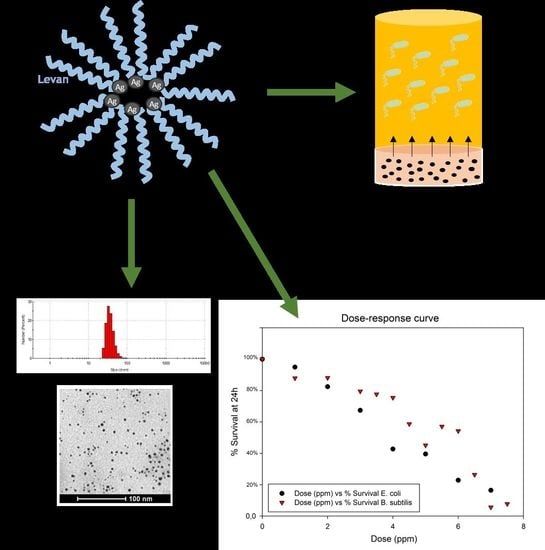
Levan-Capped Silver Nanoparticles for Bactericidal Formulations: Release and Activity Modelling
Abstract
:1. Introduction
2. Results
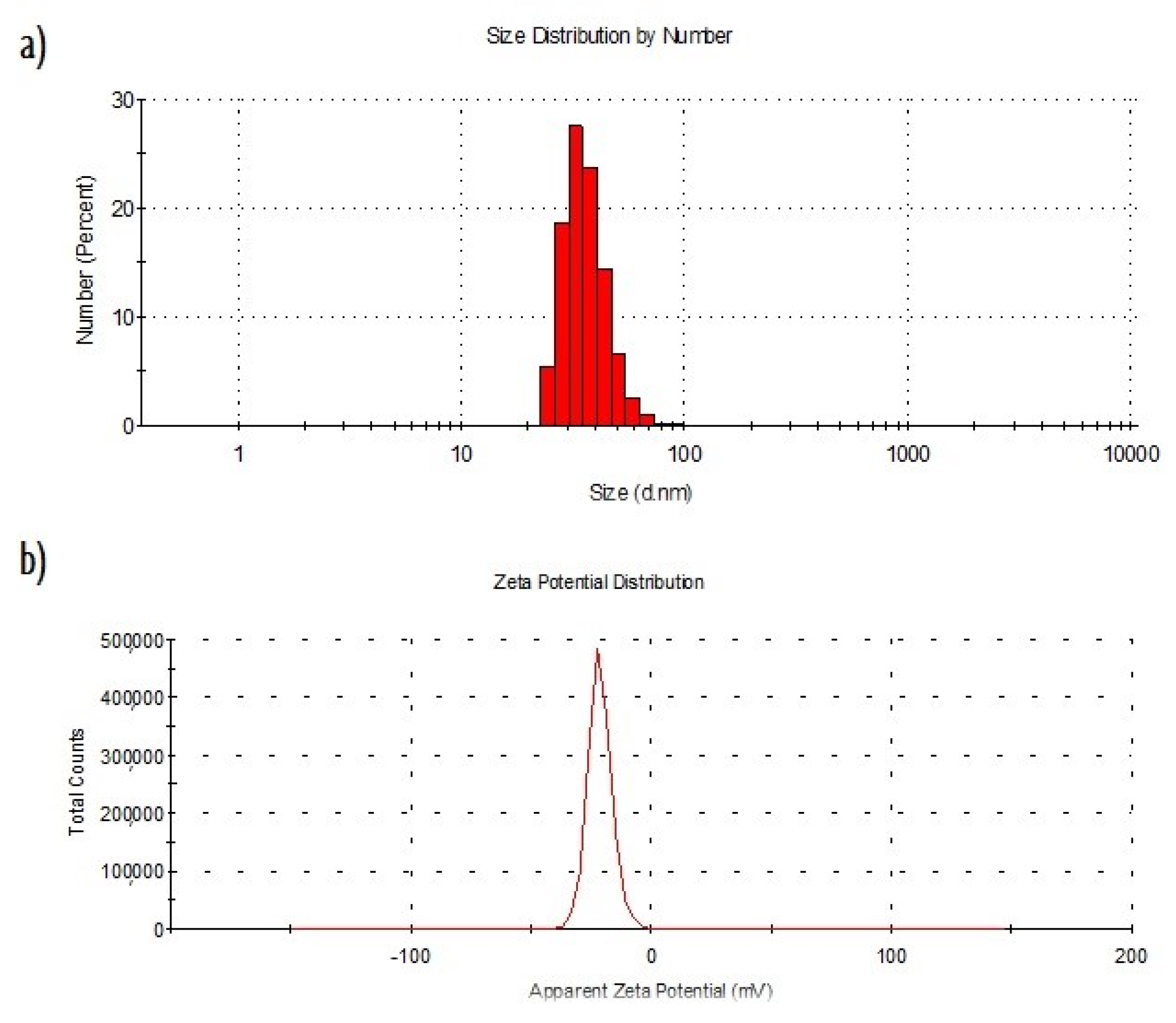
2.1. Characterization of Nanoparticles Obtained
2.2. Bacteria Survival
2.3. Parameters Estimated
2.4. Simulations from Model
3. Materials and Methods
3.1. Nanoparticle Synthesis and Characterization
3.2. Silver Determination in the Polymer-Capped Nanoparticles
3.3. Gel Formation
3.4. Bacteria Survival Assays
3.5. Model Formulation
3.5.1. Nanoparticles Effect on Bacteria, Survival Modelling
- Each cell has n targets
- Each target is inactivated by one nanoparticle
- The inactivation of 1 target is considered as “sub-lethal” event.
- All targets should be inactivated to kill the cell
3.5.2. Mass Transfer of Nanoparticles in Gel
3.5.3. Mass transfer of Nanoparticles in Liquid Culture Medium
3.6. Parameter Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Area for NPs release (m2), π·rw2 |
| a1 | Specific area for mass transfer gel to liquid (m2/m3) |
| a2 | Specific area for mass transfer liquid to bacteria surface (m2/m3) |
| Cbroth | Concentration of NPs in culture broth (mg/mL) |
| Cin | Average concentration of NPs inside gel (mg/mL) |
| Cint | Concentration of NPs in the interface liquid-gel (mg/mL) |
| CNPS | Concentration of NPS (mg/mL) |
| Cs | Concentration of nanoparticles in the surface of bacteria (mg/mL) |
| D | Silver dose (ppm) |
| D0 | Lethal dose (ppm) |
| Def | Effective diffusion coefficient in gel (m2/s) |
| Dif | Diffusion coefficient (m2/s) |
| Dw | Well diameter (m) |
| k | Kornsmeyer-Peppas constant 1 (s−n) |
| Kb | Boltzmann constant (J/K) |
| Kl | Mass transfer coefficient (m/s) |
| Min | Mass of nanoparticles in gel-solid medium (mg) |
| Mout | Mass of nanoparticles in liquid medium (mg) |
| Mtot | Total mass of nanoparticles in the well (mg) |
| n | Number of targets in bacteria |
| NNPS | Number of nanoparticles (mol) |
| p | Kornsmeyer-Peppas constant 2 |
| Re | Reynolds number, η/(ρliq· Dif) |
| rH | Hydrodynamic radious of nanoparticles (m) |
| rw | Well radious (m) |
| S | Survival percentage (%) |
| Sag | Agitation speed in orbital shaker (s−1) |
| Sc | Scmidtt number, ρliq·Sag·dw2/η |
| Sh | Sherwood number, dw·kl/Dif |
| T | Temperature (K) |
| t | Time (s) |
| vr | Release velocity (mg/mL·s) |
| µ | Fluid viscosity (kg/m·s) |
| ρgel | Gel density (kg/m3) |
| ρliq | Liquid-broth density (kg/m3) |
References
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver and silver nanoparticles materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Sadigui, A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv. Colloid. Interface Sci. 2013, 189–190, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Takkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biology synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dhayagude, A.C.; Newase, S.K.; Joshi, S.S.; Kapadnis, B.P.; Kapoor, S. Preparation of silver nanoparticles in the presence of polyoxometalates. Mater Sci. Eng. C 2019, 94, 437–444. [Google Scholar] [CrossRef]
- Fanta, G.F.; Kenar, J.A.; Felker, F.C.; Byars, J.A. Preparation of starch-stabilized nanoparticles from amylose-sodium palmitate inclusion complexes. Carbohyd. Polym. 2013, 92, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, M.; Attianese, I.; Buonocore, G.; Conte, A.; del Nobile, M.; Tescione, F.; Amendola, E. MMT-supported Ag nanoparticles for chitosan nanocomposites: Structural properties and antibacterial activity. Carbohyd. Polym. 2014, 102, 385–392. [Google Scholar] [CrossRef]
- Valodkar, M.; Badhoria, A.; Pohnerkar, J.; Mohan, M.; Thakore, S. Morphology and antibacterial activity of carbohydrate-stabilized silver nanoparticles. Carbohyd. Res. 2010, 345, 1767–1773. [Google Scholar] [CrossRef]
- González-Garcinuño, A.; Tabernero, A.; Domínguez, A.; Galán, M.A.; del Valle, E.M.M. Levan and levansucroses: Polymer, enzyme, microorganisms and biomedical applications. Biocatal. Biotransform. 2017, 36, 233–244. [Google Scholar]
- Srikanth, R.; Reddy, H.S.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohyd. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Tabernero, A.; González-Garcinuño, A.; Sánchez-Álvarez, J.M.; Galán, M.A.; del Valle, E.M.M. Development of a nanoparticle system based on a fructose polymer: Stability and drug release studies. Carbohyd. Polym. 2017, 160, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomater. Sci. 2018, 10, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Chung, C.B.; Kim, Y.H.; Kim, K.S.; Hang, C.S.; Kim, C.H. Cosmeceutical properties of levan produced by Zymomonas mobilis. J. Cosmet. Sci. 2005, 56, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.A.; Kalla, D.; Uppuluri, K.B.; Anbazhagan, V. Green synthesis of silver and gold nanoparticles employing levan, a biopolymer from Acetobacter xylinum NCIM 2526, as a reducing agent and capping agent. Carbohyd. Polym. 2014, 112, 539–545. [Google Scholar] [CrossRef]
- Yusuf, A.; Brophy, A.; Gorey, B.; Casey, A. Liposomal encapsulation of silver nanoparticles enhances cytotoxicity and causes induction of reactive oxygen species-independent apoptosis. J. Appl. Toxicol. 2018, 38, 616–627. [Google Scholar] [CrossRef]
- Barani, H.; Montazer, M.; Samadi, N.; Toliyat, T. Nano silver entrapped in phospholipids membrane: Synthesis, characteristics and antibacterial kinetics. Mol. Membr. Biol. 2011, 28, 206–215. [Google Scholar] [CrossRef]
- Guldiren, D.; Aydin, S. Antimicrobial property of silver, silver-zinc and silver-copper incorporated soda lime glass prepared by ion exchange. Mater. Sci. Eng. C 2017, 78, 826–832. [Google Scholar] [CrossRef]
- Vale, A.C.; Pereira, P.R.; Barbosa, A.M.; Torrado, E.; Alves, N.M. Optimization of silver-containing bioglass nanoparticles envisaging biomedical applications. Mater Sci. Eng. C 2019, 94, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rescignano, N.; Hernández, R.; López, L.D.; Calvillo, I.; Kenny, J.M.; Mijangos, C. Preparation of alginate hydrogels containing silver nanoparticles: a facile approach for antibacterial applications. Polym. Int. 2016, 65, 921–926. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Obradovic, B.; Stojkovska, J.; Jovanovic, Z.; Miskovic-Stankovic, V. Novel alginate based nanocomposite hydrogels with incorporated silver nanoparticles. J. Mater. Sci. Mater. Med. 2012, 23, 99–107. [Google Scholar] [CrossRef]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial effect of silver nanoparticles and the modelling of bacterial growth kinetics using a modified Gompertz model. Biochim. Biophys. Acta 2015, 1850, 299–306. [Google Scholar] [CrossRef]
- Kostic, D.; Vidovic, S.; Obradovic, B. Silver release from nanocomposite Ag/alginate hydrogels in the presence of chloride ions: experimental results and mathematical modelling. J. Nanopart. Res. 2016, 18, 76. [Google Scholar] [CrossRef]
- Lawler, D.F.; Mikelonis, A.M.; Kim, I.; Lau, B.T.; Youn, S. Silver nanoparticle removal from drinking water: flocculation/sedimentation or filtration? Water Supply 2013, 13, 1181–1187. [Google Scholar] [CrossRef]
- Saeb, A.T.M.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of Silver Nanoparticles with Strong and Stable Antimicrobial Activity against Highly Pathogenic and Multidrug Resistant Bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Grube, M.; Bekers, M.; Upite, D.; Kaminska, E. Infrared spectra of some fructans. Spectroscopy 2002, 16, 289–296. [Google Scholar] [CrossRef]
- Rai, V.N.; Srivastava, A.K.; Mukherjee, C.; Deb, S.K. Localized surface plasmon resonance and refractive index sensitivity of vacuum-evaporated nanostructured gold thin films. Ind. J. Phys. 2016, 90, 107–116. [Google Scholar] [CrossRef]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 2000, 16, 452–455. [Google Scholar] [CrossRef]
- Taglietti, A.; Fernández, Y.A.D.; Amato, E.; Cucca, L.; Dacamo, G.; Grisoli, P.; Necchi, V.; Pallavicini, P.; Pasotti, L.; Patrini, M. Antibacterial Activity of Glutathione-coated Silver nanoparticles against Gram positive and Gram negative Bacteria. Langmuir 2012, 28, 8140–8148. [Google Scholar] [CrossRef]
- Panàcek, A.; Kvítek, L.; Smékalovà, M.; Vecerová, R.; Kolar, M.; Röderová, M.; Dycka, F.; Sebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotech. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Blandino, A.; Macías, M.; Cantero, D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 1999, 88, 686–689. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biometr. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Yu, T.; Xiong, Z.; Chen, S.; Tu, G. The use of models in “target” theory to evaluate the survival curves of human ovarian carcinoma cell line exposure to Adriamycin combined with ultrasound. Ultrason Sonochem. 2005, 12, 345–348. [Google Scholar] [CrossRef]
- Peppas, N.; Narasimhan, B. Mathematical models in drug delivery: how modelling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Doig, S.D.; Pickering, S.C.R.; Lye, G.J.; Baganz, F. Modelling surface aeration rates in shaken microtitre plates using dimensionless groups. Chem. Eng. Sci. 2005, 60, 2741–2750. [Google Scholar] [CrossRef]
- gPROMS Advanced User Guide; Process System Enterprise, Ltd.: London, UK, 2004; p. 37.








| Strain | AgLeNPs Concentration (µg/mL) | k (s−n) | p | Weight Residuals | χ2 | Number of NLP Iterations | Time for Estimation (s) |
|---|---|---|---|---|---|---|---|
| E. coli | 50 | 0.135 | 0.266 | 7.99 | 11.07 | 57 | 17 |
| 80 | 0.205 | 0.181 | 6.99 | 9.49 | 64 | 21 | |
| 110 | 0.328 | 0.116 | 3.11 | 11.10 | 36 | 10 | |
| B. subtilis | 50 | 0.715 | 0.203 | 0.82 | 12.59 | 20 | 7 |
| 80 | 0.900 | 0.129 | 0.51 | 11.07 | 27 | 8 | |
| 110 | 0.156 | 0.262 | 2.25 | 12.56 | 27 | 8 |
| Parameter | Value | Unit | Reference/Source |
|---|---|---|---|
| dp | 0.017 | m | Measurement (microwell plate) |
| Dif | 1.23 × 10−11 | m2/s | Calculated from Stokes-Einstein equation |
| a1 | 3 × 106 | m−1 | Calculated from gel geometry |
| a2 | 2.27 × 103 | m−1 | Calculated from bacteria diameter |
| Sag | 15.7 | s−1 | Set experimental (150 rpm) |
| ρliq | 1000 | kg/m3 | Tabulated value for water |
| ρgel | 1064 | kg/m3 | Determined experimentally |
| Vgel | 0.1 | mL | Set experimental |
| Vwell | 0.9 | mL | Set experimental |
| µ | 0.001 | kg/m·s | Tabulated value for water |
| T | 298 | K | Set experimental |
| rH | 36 | nm | Determined experimentally (DLS) |
| kb | 1.38 × 10−23 | J/K | Constant |
| Initial Conditions | |||
| Cbroth | 0 | µg/mL | |
| Cs | 0 | µg/mL | |
| Mout | 0 | µg | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Garcinuño, Á.; Masa, R.; Hernández, M.; Domínguez, Á.; Tabernero, A.; del Valle, E.M. Levan-Capped Silver Nanoparticles for Bactericidal Formulations: Release and Activity Modelling. Int. J. Mol. Sci. 2019, 20, 1502. https://doi.org/10.3390/ijms20061502
González-Garcinuño Á, Masa R, Hernández M, Domínguez Á, Tabernero A, del Valle EM. Levan-Capped Silver Nanoparticles for Bactericidal Formulations: Release and Activity Modelling. International Journal of Molecular Sciences. 2019; 20(6):1502. https://doi.org/10.3390/ijms20061502
Chicago/Turabian StyleGonzález-Garcinuño, Álvaro, Rubén Masa, María Hernández, Ángel Domínguez, Antonio Tabernero, and Eva Martín del Valle. 2019. "Levan-Capped Silver Nanoparticles for Bactericidal Formulations: Release and Activity Modelling" International Journal of Molecular Sciences 20, no. 6: 1502. https://doi.org/10.3390/ijms20061502
APA StyleGonzález-Garcinuño, Á., Masa, R., Hernández, M., Domínguez, Á., Tabernero, A., & del Valle, E. M. (2019). Levan-Capped Silver Nanoparticles for Bactericidal Formulations: Release and Activity Modelling. International Journal of Molecular Sciences, 20(6), 1502. https://doi.org/10.3390/ijms20061502