Lack of Rhes Increases MDMA-Induced Neuroinflammation and Dopamine Neuron Degeneration: Role of Gender and Age
Abstract
:1. Introduction
2. Results
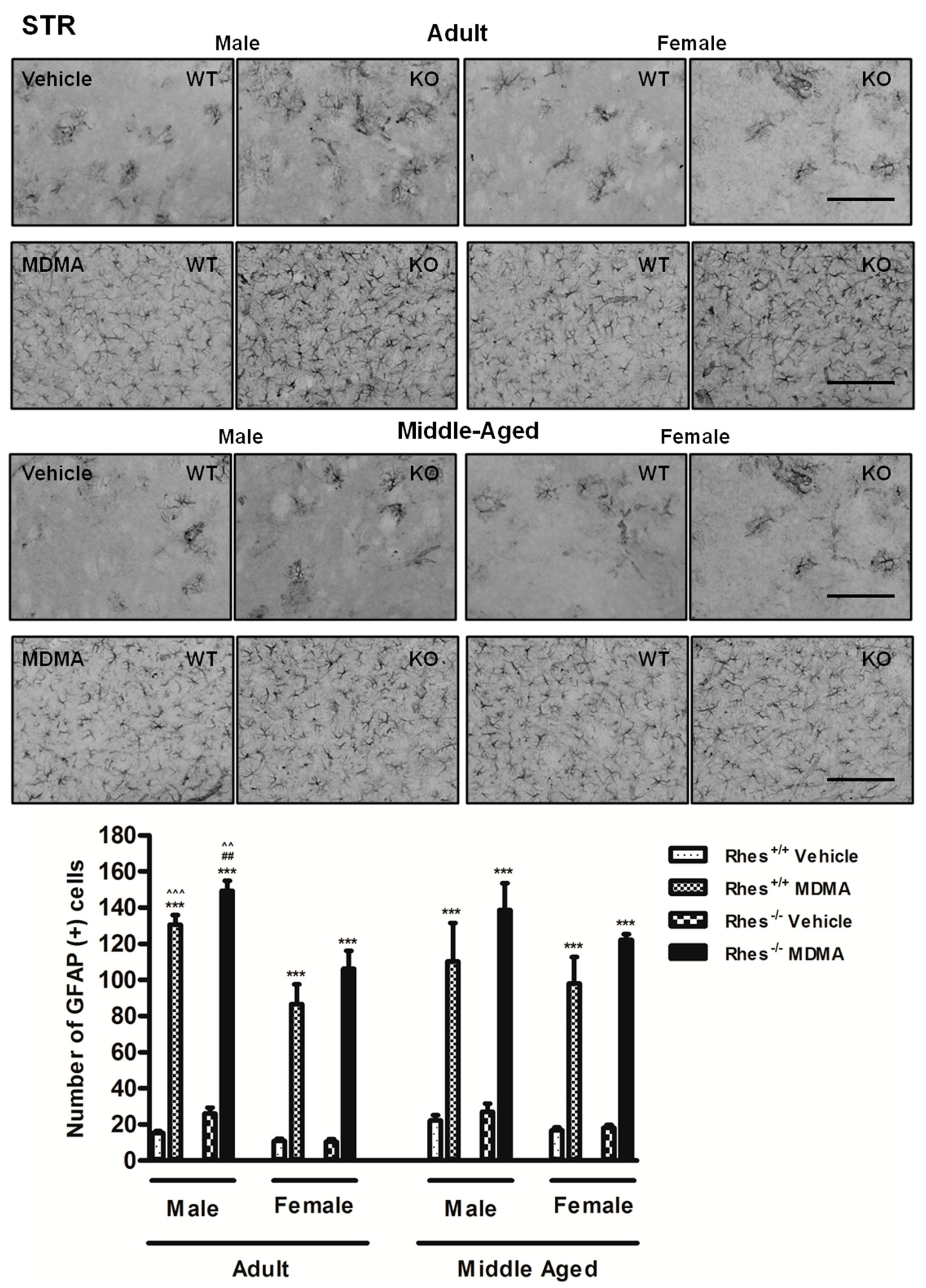
2.1. Effects of MDMA on GFAP Immunoreactivity
2.1.1. Adult Males and Females
2.1.2. Middle-Aged Males and Females
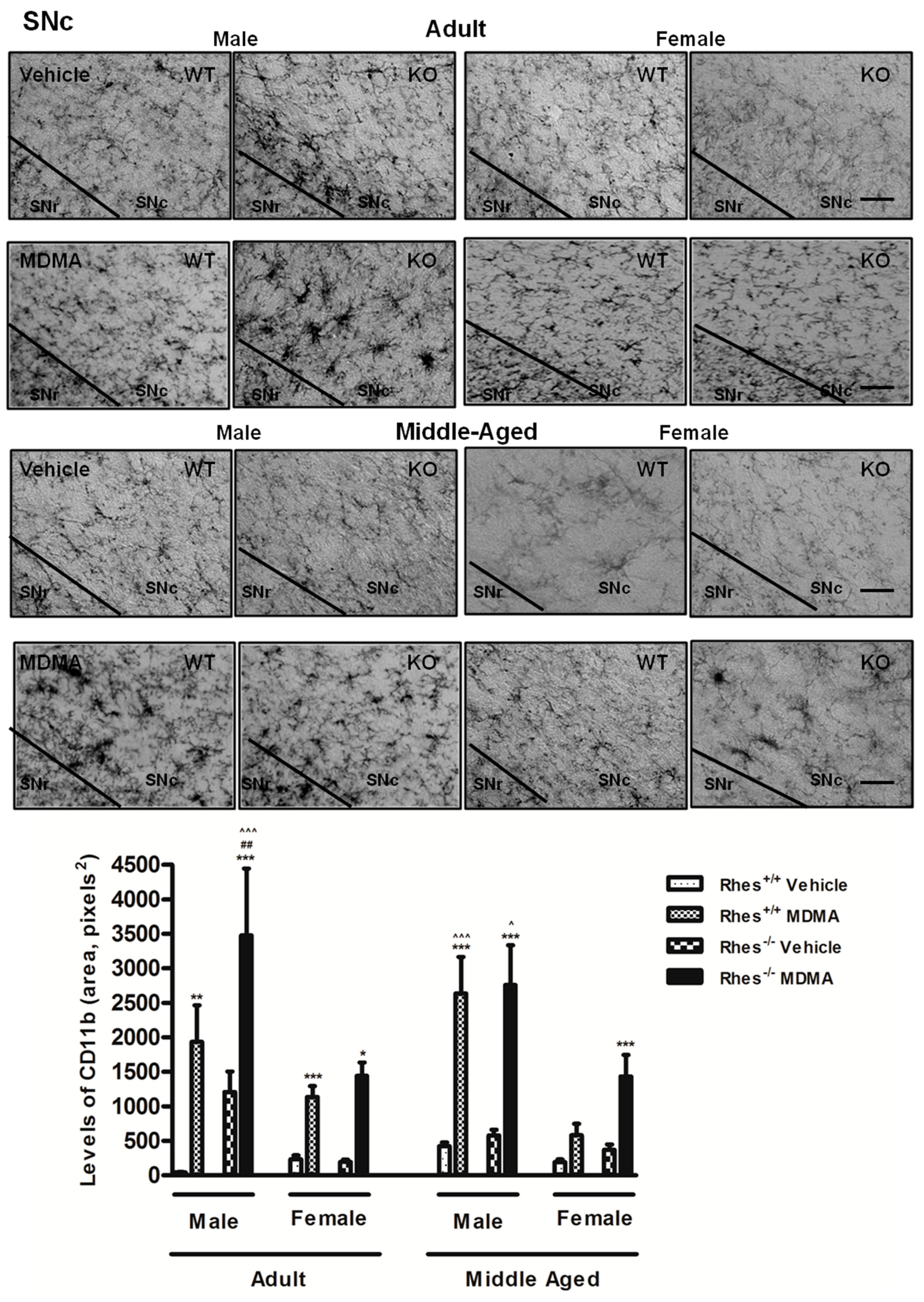
2.2. Effects of MDMA on CD11b Immunoreactivity
2.2.1. Adult Males and Females
2.2.2. Middle-Aged Males and Females
2.3. Effects of MDMA on TH Immunoreactivity
2.3.1. Adult Males and Females
2.3.2. Middle-Aged Males and Females
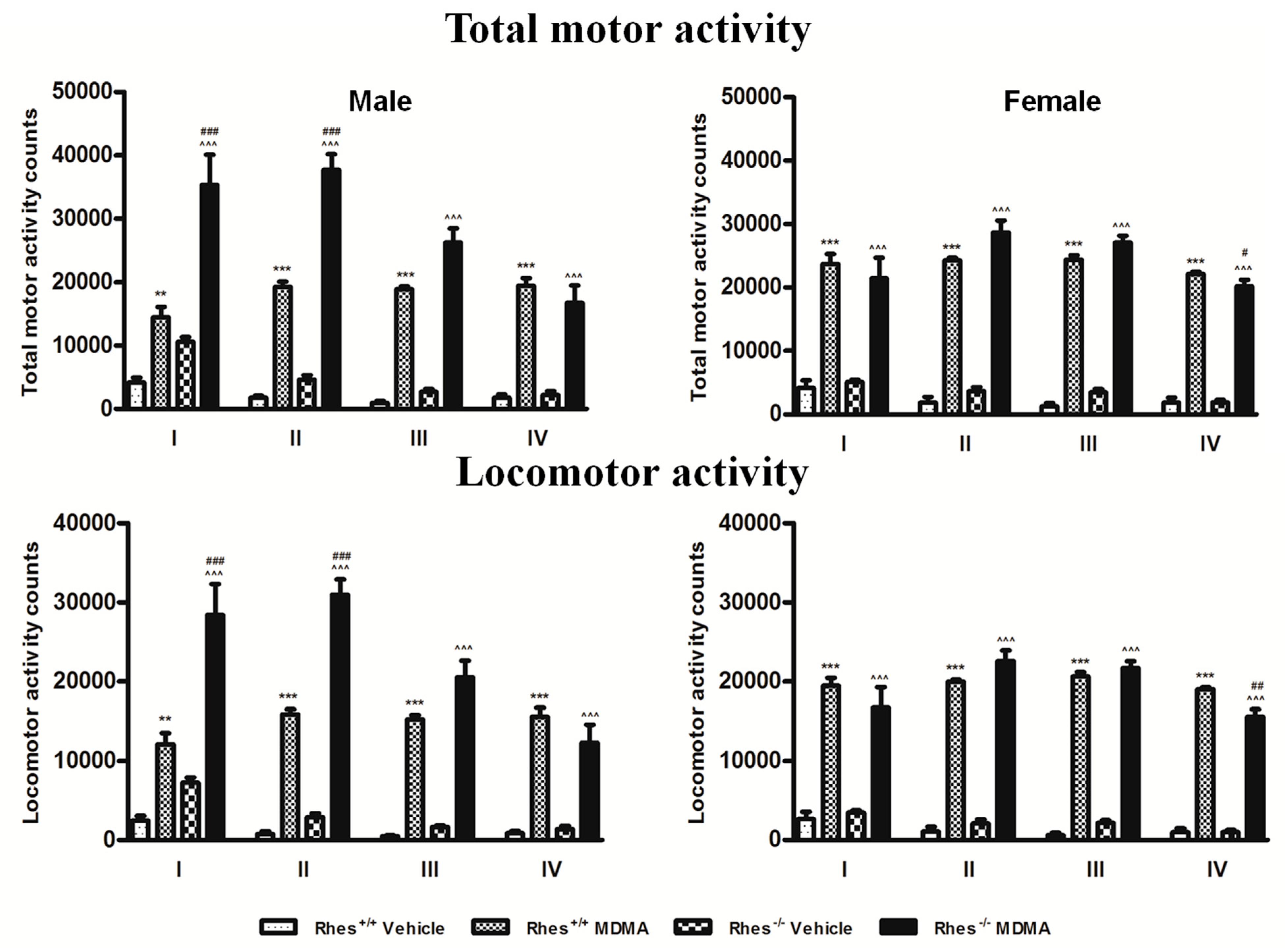
2.4. Motor Activity after MDMA in Adult Mice
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals and Treatments
4.3. Immunohistochemistry
4.4. Analysis of GFAP Immunoreactivity
4.5. Analysis of CD11b Immunoreactivity
4.6. Analysis of TH Immunoreactivity in the CTX, STR or NAc
4.7. Stereological Counting of TH-Immunoreactive Neurons in the SNc or VTA
4.8. Motor Activity Measurement
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | anteriority |
| ABC | avidin–biotin–peroxidase complex |
| CD11b | complement type 3 receptor |
| STR | striatum |
| CTX | motor cortex |
| DA | dopamine |
| DAergic | dopaminergic |
| GFAP | glial fibrillary acidic protein |
| KO | knockout |
| IgG | immunoglobulin G |
| i.p. | intraperitoneally |
| NAc | nucleus accumbens |
| PD | Parkinson’s disease |
| SNc | substantia nigra pars compacta |
| TH | tyrosine hydroxylase |
| VTA | ventral tegmental area |
| WT | wild-type |
References
- Harrison, L.M. Rhes: A GTP-binding protein integral to striatal physiology and pathology. Cell. Mol. Neurobiol. 2012, 32, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Errico, F.; Santini, E.; Migliarini, S.; Santini, E.; Migliarini, S.; Borgkvist, A.; Centonze, D.; Nasti, V.; Carta, M.; De Chiara, V.; et al. The GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neurons. Mol. Cell. Neurosci. 2008, 37, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Ghiglieri, V.; Napolitano, F.; Pelosi, B.; Schepisi, C.; Migliarini, S.; Di Maio, A.; Pendolino, V.; Mancini, M.; Sciamanna, G.; Vitucci, D.; et al. Rhes influences striatal cAMP/PKA-dependent signaling and synaptic plasticity in a gender-sensitive fashion. Sci. Rep. 2015, 5, 10933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciamanna, G.; Napolitano, F.; Pelosi, B.; Bonsi, P.; Vitucci, D.; Nuzzo, T.; Punzo, D.; Ghiglieri, V.; Ponterio, G.; Pasqualetti, M.; et al. Rhes regulates dopamine D2 receptor transmission in striatal cholinergic interneurons. Neurobiol. Dis. 2015, 78, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Vitucci, D.; Di Giorgio, A.; Napolitano, F.; Pelosi, B.; Blasi, G.; Errico, F.; Attrotto, M.T.; Gelao, B.; Fazio, L.; Taurisano, P.; et al. Rasd2 Modulates Prefronto-Striatal Phenotypes in Humans and ‘Schizophrenia-Like Behaviors’ in Mice. Neuropsychopharmacology 2015, 41, 916–917. [Google Scholar] [CrossRef]
- Napolitano, F.; D’Angelo, L.; de Girolamo, P.; Avallone, L.; de Lange, P.; Usiello, A. The Thyroid Hormone-target Gene Rhes a Novel Crossroad for Neurological and Psychiatric Disorders: New Insights from Animal Models. Neuroscience 2018, 384, 419–428. [Google Scholar] [CrossRef]
- Pinna, A.; Napolitano, F.; Pelosi, B.; Di Maio, A.; Wardas, J.; Casu, M.A.; Costa, G.; Migliarini, S.; Calabresi, P.; Pasqualetti, M.; et al. The Small GTP-Binding Protein Rhes Influences Nigrostriatal-Dependent Motor Behavior During Aging. Mov. Disord. 2016, 31, 583–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, F.; Booth Warren, E.; Migliarini, S.; Punzo, D.; Errico, F.; Li, Q.; Thiolat, M.L.; Vescovi, A.L.; Calabresi, P.; Bezard, E.; et al. Decreased Rhes mRNA levels in the brain of patients with Parkinson’s disease and MPTP-treated macaques. PLoS ONE 2017, 12, e0181677. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Napolitano, F.; Mealer, R.G.; Kim, S.; Errico, F.; Barrow, R.; Shahani, N.; Tyagi, R.; Snyder, S.H.; Usiello, A. Rhes, a striatal–enriched small G–protein, mediates mTOR signaling and L–DOPA–induced dyskinesia. Nat. Neurosci. 2012, 15, 191–193. [Google Scholar] [CrossRef]
- Costa, G.; Pinna, A.; Porceddu, P.F.; Casu, M.A.; Di Maio, A.; Napolitano, F.; Usiello, A.; Morelli, M. Rhes counteracts dopamine neuron degeneration and neuroinflammation depending on gender and age. Front. Aging Neurosci. 2018, 10, 163. [Google Scholar] [CrossRef]
- Pasternak, O.; Kubicki, M.; Shenton, M.E. In vivo imaging of neuroinflammation in schizophrenia. Schizophr. Res. 2016, 173, 200–212. [Google Scholar] [CrossRef]
- Réus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef]
- Mondelli, V.; Vernon, A.C.; Turkheimer, F.; Dazzan, P.; Pariante, C.M. Brain microglia in psychiatric disorders. Lancet Psychiatry 2017, 4, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Barcia, C.; Hunot, S.; Guillemin, G.J.; Pitossi, F. Inflammation and Parkinson’s disease. Parkinsons Dis. 2011, 729054. [Google Scholar] [CrossRef]
- Phani, S.; Loike, J.D.; Przedborski, S. Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, S207–S209. [Google Scholar] [CrossRef]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.S.; Cocores, J. Neuropsychiatric manifestations following the use of 3,4-methylenedioxymethamphetamine (MDMA: “Ecstasy”). Prog. Neuropsychopharmacol. Biol. Psychiatry 1997, 21, 727–734. [Google Scholar] [CrossRef]
- McGuire, P.; Fahy, T. Chronic paranoid psychosis after misuse of MDMA (“ecstasy”). BMJ 1991, 302, 697. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.; Daya, G.N.; Brainch, N.; Kotapati, V.P.; Zaveri, D.; Ahmed, S. Persistent Psychosis due to Single Dose of Ecstasy. Cureus 2018, 10, e3058. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, R.; Khairnar, A.; Simola, N.; Granado, N.; García-Montes, J.R.; Porceddu, P.F.; Tizabi, Y.; Costa, G.; Morelli, M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog. Neurobiol. 2017, 155, 149–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iravani, M.M.; Asari, D.; Patel, J.; Wieczorek, W.J.; Kruk, Z.L. Direct effects of 3,4-methylenedioxymethamphetamine (MDMA) on serotonin or dopamine release and uptake in the caudate putamen, nucleus accumbens, substantia nigra pars reticulata, and the dorsal raphé nucleus slices. Synapse 2000, 36, 275–285. [Google Scholar] [CrossRef]
- Colado, M.I.; Camarero, J.; Mechan, A.O.; Sanchez, V.; Esteban, B.; Elliott, J.M.; Green, A.R. A study of the mechanisms involved in the neurotoxic action of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) on dopamine neurones in mouse brain. Br. J. Pharmacol. 2001, 134, 1711–1723. [Google Scholar] [CrossRef]
- Camarero, J.; Sanchez, V.; O’Shea, E.; Green, A.R.; Colado, M.I. Studies, using in vivo microdialysis, on the effect of the dopamine uptake inhibitor GBR 12909 on 3,4-methylenedioxymethamphetamine (‘ecstasy’)-induced dopamine release and free radical formation in the mouse striatum. J. Neurochem. 2002, 81, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Green, A.R.; Mechan, A.O.; Elliott, J.M.; O’Shea, E.; Colado, M.I. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’). Pharmacol. Rev. 2003, 55, 463–508. [Google Scholar] [CrossRef]
- Cadet, J.L.; Krasnova, I.N.; Jayanthi, S.; Lyles, J. Neurotoxicity of substituted amphetamines: Molecular and cellular mechanisms. Neurotox. Res. 2007, 11, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Halpin, L.E.; Collins, S.A.; Yamamoto, B.K. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 2014, 97, 37–44. [Google Scholar] [CrossRef]
- Colado, M.I.; O’Shea, E.; Green, A.R. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology 2004, 173, 249–263. [Google Scholar] [CrossRef]
- Costa, G.; Frau, L.; Wardas, J.; Pinna, A.; Plumitallo, A.; Morelli, M. MPTP-Induced Dopamine Neuron Degeneration and Glia Activation Is Potentiated in MDMA-Pretreated Mice. Mov. Disord. 2013, 28, 1957–1965. [Google Scholar] [CrossRef]
- Costa, G.; Morelli, M.; Simola, N. Progression and Persistence of Neurotoxicity Induced by MDMA in Dopaminergic Regions of the Mouse Brain and Association with Noradrenergic, GABAergic, and Serotonergic Damage. Neurotox. Res. 2017, 32, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Granado, N.; O’Shea, E.; Bove, J.; Vila, M.; Colado, M.I.; Moratalla, R. Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J. Neurochem. 2008, 107, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Frau, L.; Costa, G.; Porceddu, P.F.; Khairnar, A.; Castelli, M.P.; Ennas, M.G.; Madeddu, C.; Wardas, J.; Morelli, M. Influence of caffeine on 3,4-methylenedioxymethamphetamine-induced dopaminergic neuron degeneration and neuroinflammation is age-dependent. J. Neurochem. 2016, 136, 148–162. [Google Scholar] [CrossRef]
- Frau, L.; Simola, N.; Porceddu, P.F.; Morelli, M. Effect of crowding, temperature and age on glia activation and dopaminergic neurotoxicity induced by MDMA in the mouse brain. Neurotoxicology 2016, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Godlewski, T.; Karabenick, R.L.; Stephens, J.M.; Ullrich, T.; Rice, K.C.; Woods, J.H. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology 2003, 166, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Frau, L.; Simola, N.; Plumitallo, A.; Morelli, M. Microglial and astroglial activation by 3,4-methylenedioxymethamphetamine (MDMA) in mice depends on S(+) enantiomer and is associated with an increase in body temperature and motility. J. Neurochem. 2013, 124, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Wnorowski, A.; Kaszubska, K.; Biala, G.; Kruk-Słomka, M.; Kurzepa, J.; Boguszewska-Czubara, A. Acute MDMA and Nicotine Co-administration: Behavioral Effects and Oxidative Stress Processes in Mice. Front. Behav. Neurosci. 2018, 12, 149. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Jochnowitz, N.D.; Zeevalk, G.D.; Oostveen, J.A.; Hall, E.D. Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res. 1996, 738, 172–175. [Google Scholar] [CrossRef]
- Granado, N.; Ares-Santos, S.; Oliva, I.; O’Shea, E.; Martin, E.D.; Colado, M.; Moratalla, R. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol. Dis. 2011, 42, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Jansen, K.L. Ecstasy (MDMA) dependence. Drug Alcohol Depend. 1999, 53, 121–124. [Google Scholar] [CrossRef]
- Ares-Santos, S.; Granado, N.; Espadas, I.; Martinez-Murillo, R.; Moratalla, R. Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining. Neuropsychopharmacology 2014, 39, 1066–1080. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Liu, Y.L.; Fann, C.S.; Liu, C.M.; Chen, W.J.; Wu, J.Y.; Hung, S.I.; Chen, C.H.; Jou, Y.S.; Liu, S.K.; Hwang, T.J.; et al. RASD2, MYH9, and CACNG2 genes at chromosome 22q12 associated with the subgroup of schizophrenia with non-deficit in sustained attention and executive function. Biol. Psychiatry 2008, 64, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Duan, X.; Liu, C.Y.; Jang, M.H.; Guo, J.U.; Pow-anpongkul, N.; Kang, E.; Song, H.; Ming, G.L. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 2009, 63, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Hong, J.S.; Zhang, W.; Liu, B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: Relevance to the etiology of Parkinson’s disease. J. Neurosci. 2003, 23, 1228–1236. [Google Scholar] [CrossRef]
- Maia, S.; Arlicot, N.; Vierron, E.; Bodard, S.; Vergote, J.; Guilloteau, D.; Chalon, S. Longitudinal and parallel monitoring of neuroinflammation and neurodegeneration in a 6-hydroxydopamine rat model of Parkinson’s disease. Synapse 2012, 66, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Kim, J.H.; Greenamyre, J.T. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci. Lett. 2003, 341, 87–90. [Google Scholar] [CrossRef]
- Yasuda, Y.; Shinagawa, R.; Yamada, M.; Mori, T.; Tateishi, N.; Fujita, S. Long-lasting reactive changes observed in microglia in the striatal and substantia nigral of mice after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2007, 1138, 196–202. [Google Scholar] [CrossRef]
- Torres-Rojas, C.; Jones, B.C. Sex Differences in Neurotoxicogenetics. Front. Genet. 2018, 9, 196. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, F.A.; Baiamonte, B.A.; Spano, D.; Lahoste, G.J.; Soignier, R.D.; Harrison, L.M. Mice lacking rhes show altered morphine analgesia, tolerance, and dependence. Neurosci. Lett. 2011, 489, 182–186. [Google Scholar] [CrossRef]
- Mealer, R.G.; Murray, A.J.; Shahani, N.; Subramaniam, S.; Snyder, S.H. Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. J. Biol. Chem. 2014, 289, 3547–3554. [Google Scholar] [CrossRef]
- Thapliyal, A.; Bannister, R.A.; Hanks, C.; Adams, B.A. The monomeric G proteins AGS1 and Rhes selectively influence Galphai-dependent signaling to modulate N-type (CaV2.2) calcium channels. Am. J. Physiol. Cell Physiol. 2008, 295, C1417–C1426. [Google Scholar] [CrossRef]
- Shahani, N.; Swarnkar, S.; Giovinazzo, V.; Morgenweck, J.; Bohn, L.M.; Scharager-Tapia, C.; Pascal, B.; Martinez-Acedo, P.; Khare, K.; Subramaniam, S. RasGRP1 promotes amphetamine-induced motor behavior through a Rhes interaction network (“Rhesactome”) in the striatum. Sci. Signal. 2016, 15. [Google Scholar] [CrossRef]
- Quintero, G.C.; Spano, D. Exploration of sex differences in Rhes effects in dopamine mediated behaviors. Neuropsychiatr. Dis. Treat. 2011, 7, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, U.D.; Ricaurte, G.A. Caveat emptor: Editors beware. Neuropsychopharmacology 2001, 24, 333–336. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Costa, G.; Morelli, M.; Simola, N. Repeated Administration of 3,4-Methylenedioxymethamphetamine (MDMA) Elevates the Levels of Neuronal Nitric Oxide Synthase in the Nigrostriatal System: Possible Relevance to Neurotoxicity. Neurotox. Res. 2018, 34, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Simola, N.; Morelli, M. MDMA administration during adolescence exacerbates MPTP-induced cognitive impairment and neuroinflammation in the hippocampus and prefrontal cortex. Psychopharmacology 2014, 231, 4007–4018, Erratum in 2015, 232, 315–316, doi:10.1007/s00213-014-3774-0. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Serra, M.; Pintori, N.; Casu, M.A.; Zanda, M.T.; Murtas, D.; De Luca, M.A.; Simola, N.; Fattore, L. The novel psychoactive substance methoxetamine induces persistent behavioral abnormalities and neurotoxicity in rats. Neuropharmacology 2019, 144, 219–232. [Google Scholar] [CrossRef]




| GFAP | |||||
|---|---|---|---|---|---|
| Adult Male | Adult Female | Middle-Aged Male | Middle-Aged Female | ||
| VTA | Rhes+/+ Vehicle | 41.31 ± 3.06 | 28.25 ± 1.18 | 32.12 ± 1.48 | 28.62 ± 0.80 |
| Rhes+/+ MDMA | 42.62 ± 5.28 | 28.63 ± 1.19 | 38.12 ± 1.93 | 30.56 ± 2.03 | |
| Rhes−/− Vehicle | 43.44 ± 1.25 | 31.19 ± 1.57 | 32.75 ± 1.46 | 31.56 ± 2.13 | |
| Rhes−/− MDMA | 46.62 ± 3.44 ^ | 30.06 ± 2.66 | 39.44 ± 2.32 | 30.62 ± 2.08 | |
| NAc | Rhes+/+ Vehicle | 5.37 ± 0.55 | 3.92 ± 0.35 | 4.08 ± 0.26 | 3.79 ± 0.34 |
| Rhes+/+ MDMA | 4.96 ± 0.48 | 4.08 ± 0.25 | 4.62 ± 0.26 | 3.83 ± 0.43 | |
| Rhes−/− Vehicle | 4.50 ± 0.26 | 4.54 ± 0.51 | 5.08 ± 0.26 | 3.71 ± 0.38 | |
| Rhes−/− MDMA | 4.60 ± 0.65 | 4.52 ± 0.33 | 4.96 ± 0.42 | 3.37 ± 0.16 | |
| CTX | Rhes+/+ Vehicle | 29.46 ± 0.99 | 25.73 ± 3.61 | 39 ± 1.61 | 30.04 ± 1.01 |
| Rhes+/+ MDMA | 33.33 ± 0.67 | 26.13 ± 1.29 | 48.62 ± 2.22 | 31.87 ± 0.31 | |
| Rhes−/− Vehicle | 30.29 ± 3.43 | 21.67 ± 0.83 | 37.54 ± 2.22 | 31.37 ± 1.86 | |
| Rhes−/− MDMA | 35.87 ± 3.13 | 21.69 ± 1.53 | 47.92 ± 5.32 | 32.37 ± 1.90 | |
| CD11b | |||||
|---|---|---|---|---|---|
| Adult Male | Adult Female | Middle-Aged Male | Middle-Aged Female | ||
| VTA | Rhes+/+ Vehicle | 43.25 ± 0.46 | 46.19 ± 1.71 | 47 ± 0.46 | 45.5 ± 1.57 |
| Rhes+/+ MDMA | 51.56 ± 0.46 * | 50.63 ± 0.78 | 54.94 ± 0.92 * | 53.31 ± 0.97 * | |
| Rhes−/− Vehicle | 49.37 ± 1.29 | 49.19 ± 0.22 | 50.94 ± 0.26 | 49.5 ± 0.57 | |
| Rhes−/− MDMA | 56.44 ± 1.17 * | 53.19 ± 0.28 | 60.5 ± 1.09 ** | 58.25 ± 1.75 ** | |
| NAc | Rhes+/+ Vehicle | 47.33 ± 1.94 | 50.06 ± 2.95 | 65.62 ± 1.20 $$ | 58.58 ± 1.96 |
| Rhes+/+ MDMA | 63.66 ± 1.86 * | 59.25 ± 0.49 | 73.87 ± 2.99 | 67.42 ± 0.78 | |
| Rhes−/− Vehicle | 52.64 ± 0.58 | 57.42 ± 1.45 | 66.92 ± 0.79 $ | 58.13 ± 1.85 | |
| Rhes−/− MDMA | 62.12 ± 1.07 | 59.66 ± 0.73 | 78 ± 5.73 $ | 66.42 ± 0.33 | |
| CTX | Rhes+/+ Vehicle | 50.66 ± 0.66 | 54.29 ± 0.25 | 54.12 ± 0.85 | 50.54 ± 0.41 |
| Rhes+/+ MDMA | 56.16 ± 0.77 * | 61.69 ± 0.43 ** | 63.29 ± 0.89 ***$$ | 56.92 ± 0.35 ** | |
| Rhes−/− Vehicle | 51.12 ± 1.50 | 54.21 ± 1.05 | 61.25 ± 1.27 ##$$$^^^ | 53.12 ± 0.96 | |
| Rhes−/− MDMA | 57.75 ± 0.70 * | 59.67 ± 0.37 * | 69.33 ± 0.50 ***#$$$^^^ | 58.29 ± 0.18 | |
| TH | |||||
|---|---|---|---|---|---|
| Adult Male | Adult Female | Middle-Aged Male | Middle-Aged Female | ||
| VTA | Rhes+/+ Vehicle | 128.06 ± 29.15 | 102.81 ± 16.52 | 139.25 ± 6.83 | 91.94 ± 6.23 |
| Rhes+/+ MDMA | 141.09 ± 34.01 | 94.38 ± 25.92 | 145 ± 10.43 | 86.13 ± 3.42 | |
| Rhes-/- Vehicle | 174.13 ± 44.64 | 75.81 ± 17.33 | 151.06 ± 10.48 | 75.63 ± 5.01 | |
| Rhes-/- MDMA | 169.84 ± 41.75 | 75.81 ± 17.33 | 153 ± 8.94 | 75.25 ± 2.36 | |
| NAc | Rhes+/+ Vehicle | 266.01 ± 53.59 | 209.35 ± 31.81 | 218.23 ± 0.78 | 213.96 ± 2.58 |
| Rhes+/+ MDMA | 261.96 ± 47.94 | 201.50 ± 28.37 | 210.12 ± 2.40 | 206.39 ± 2.02 | |
| Rhes-/- Vehicle | 236.73 ± 38.47 | 173.80 ± 20.16 | 218.67 ± 4.16 | 183.89 ± 7.65 | |
| Rhes-/- MDMA | 247.15 ± 42.99 | 213.33 ± 29.32 | 206.60 ± 8.46 | 196.54 ± 4.54 | |
| CTX | Rhes+/+ Vehicle | 33.66 ± 1 | 25.25 ± 2.13 | 30.73 ± 1.46 | 25 ± 0.77 |
| Rhes+/+ MDMA | 37.48 ± 0.72 | 27.71 ± 2.68 | 31.08 ± 0.64 | 24.33 ± 0.34 | |
| Rhes-/- Vehicle | 35.12 ± 1.51 | 26.54 ± 1.62 | 32.83 ± 1.65 ^ | 20.37 ± 1.63 | |
| Rhes-/- MDMA | 37.87 ± 1.19 | 28.75 ± 2.20 | 31.81 ± 0.50 | 22.58 ± 0.62 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, G.; Porceddu, P.F.; Serra, M.; Casu, M.A.; Schiano, V.; Napolitano, F.; Pinna, A.; Usiello, A.; Morelli, M. Lack of Rhes Increases MDMA-Induced Neuroinflammation and Dopamine Neuron Degeneration: Role of Gender and Age. Int. J. Mol. Sci. 2019, 20, 1556. https://doi.org/10.3390/ijms20071556
Costa G, Porceddu PF, Serra M, Casu MA, Schiano V, Napolitano F, Pinna A, Usiello A, Morelli M. Lack of Rhes Increases MDMA-Induced Neuroinflammation and Dopamine Neuron Degeneration: Role of Gender and Age. International Journal of Molecular Sciences. 2019; 20(7):1556. https://doi.org/10.3390/ijms20071556
Chicago/Turabian StyleCosta, Giulia, Pier Francesca Porceddu, Marcello Serra, Maria Antonietta Casu, Valentina Schiano, Francesco Napolitano, Annalisa Pinna, Alessandro Usiello, and Micaela Morelli. 2019. "Lack of Rhes Increases MDMA-Induced Neuroinflammation and Dopamine Neuron Degeneration: Role of Gender and Age" International Journal of Molecular Sciences 20, no. 7: 1556. https://doi.org/10.3390/ijms20071556





