N-Linked Glycosylation and Near-Infrared Spectroscopy in the Diagnosis of Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Tissue Characteristics
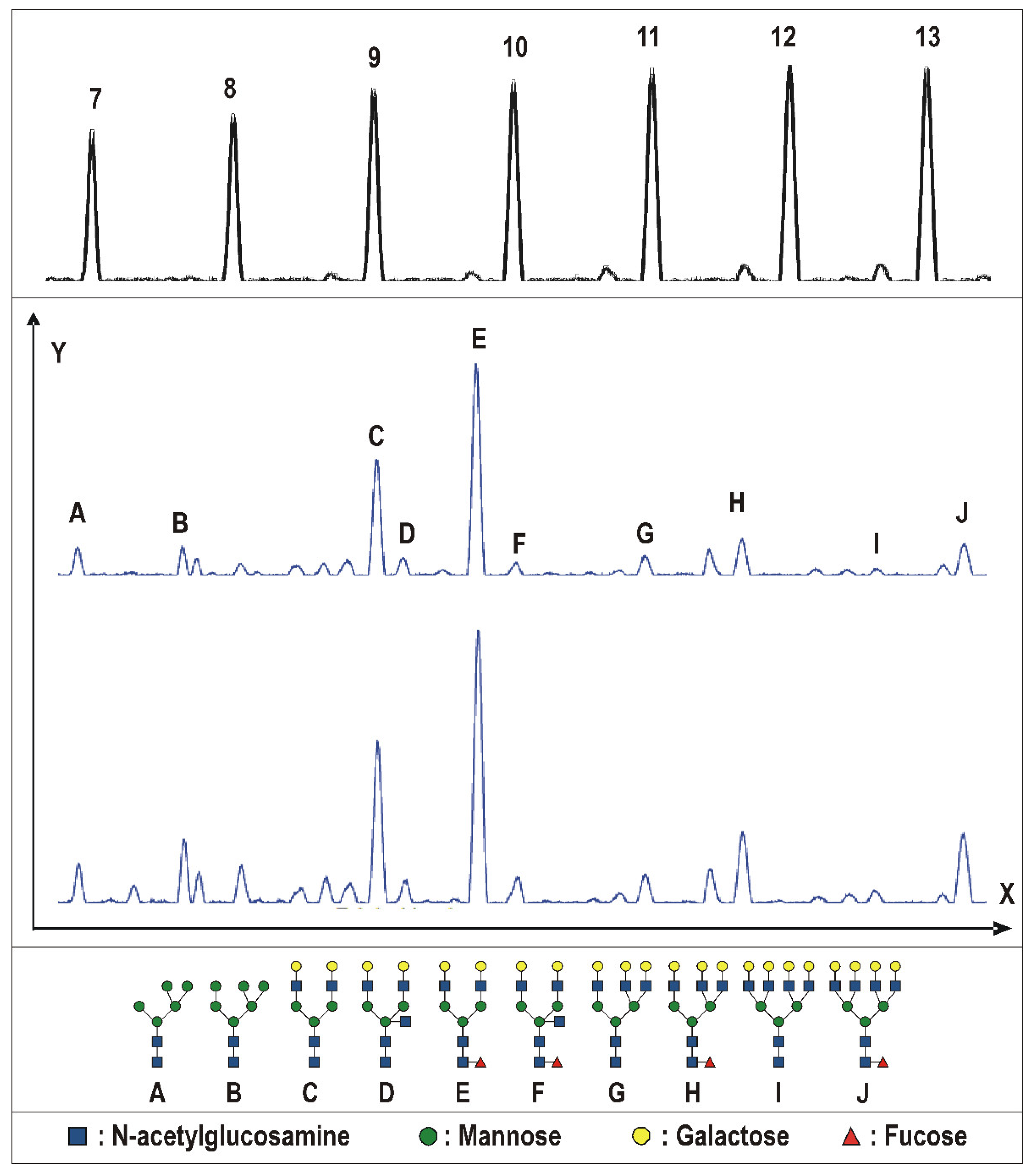
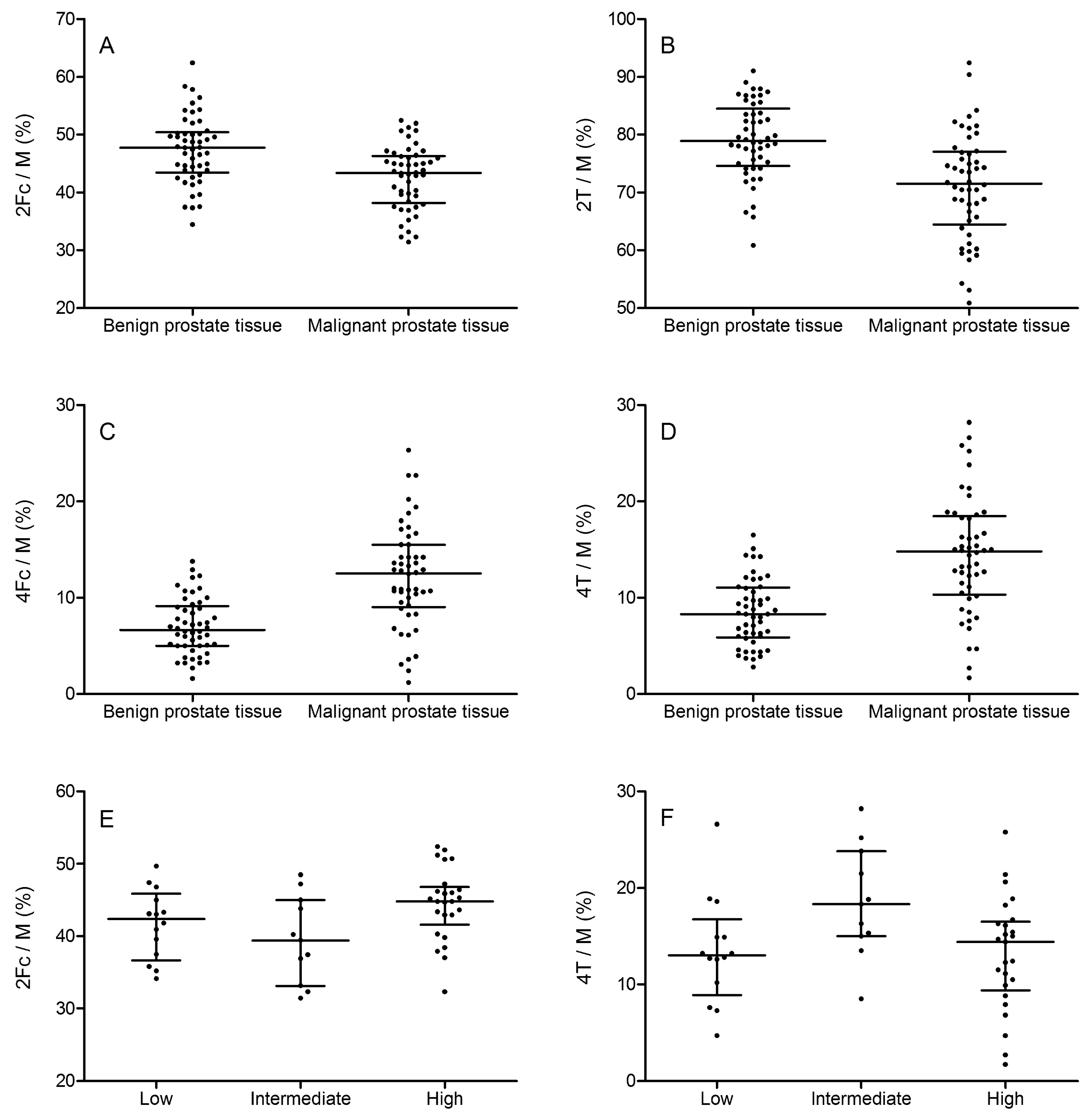
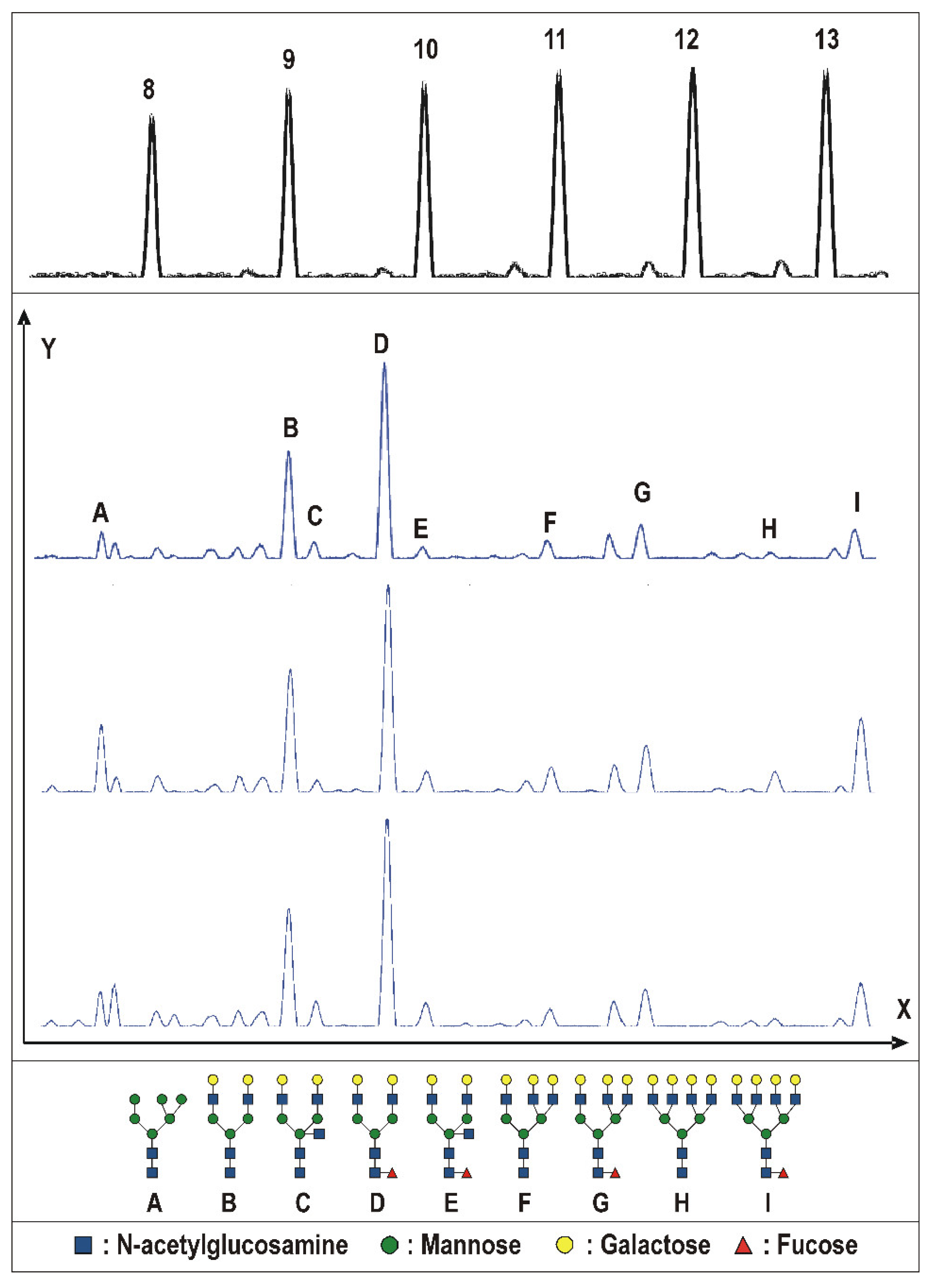
2.2. Tissue Prostate Protein N-Glycosylation
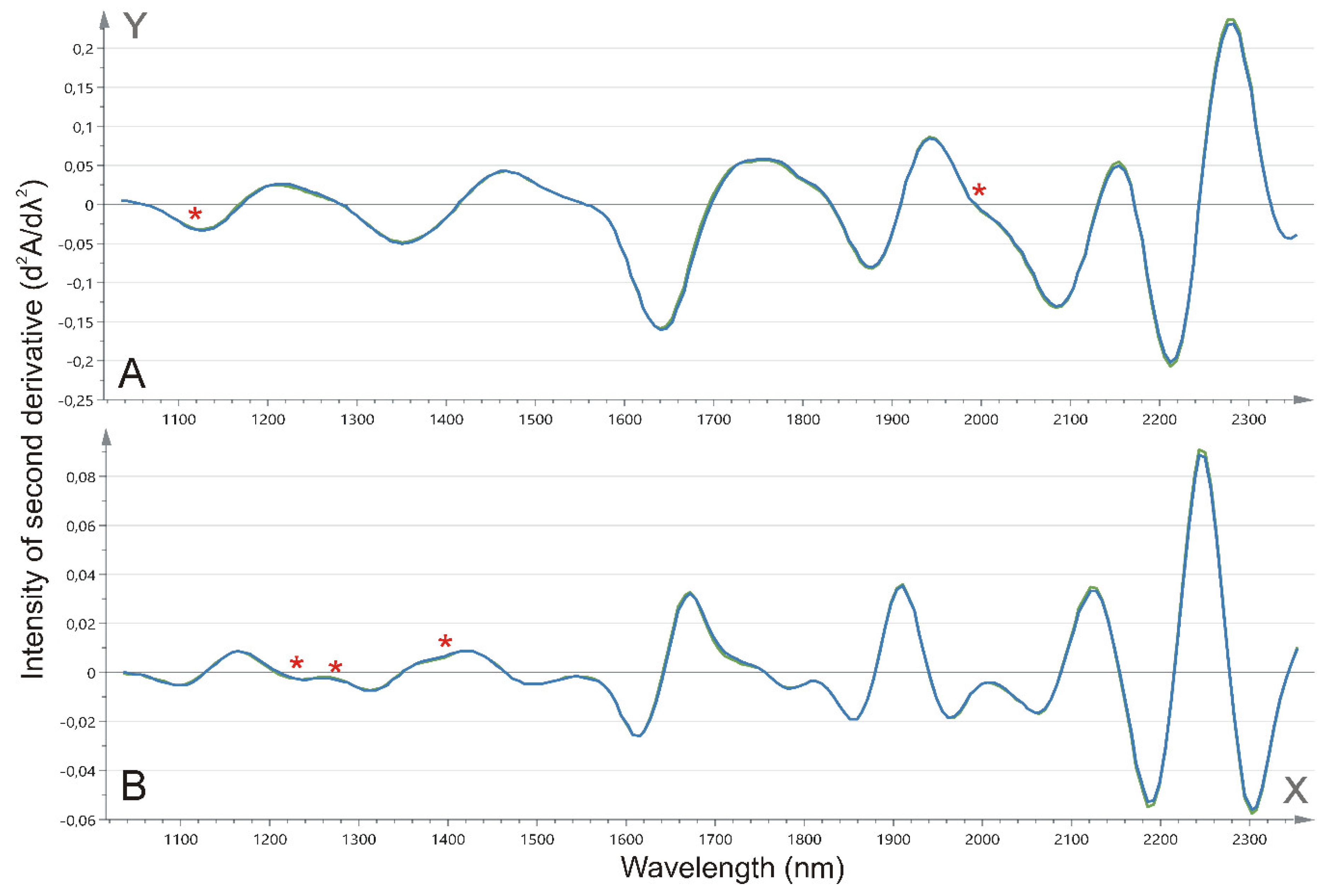
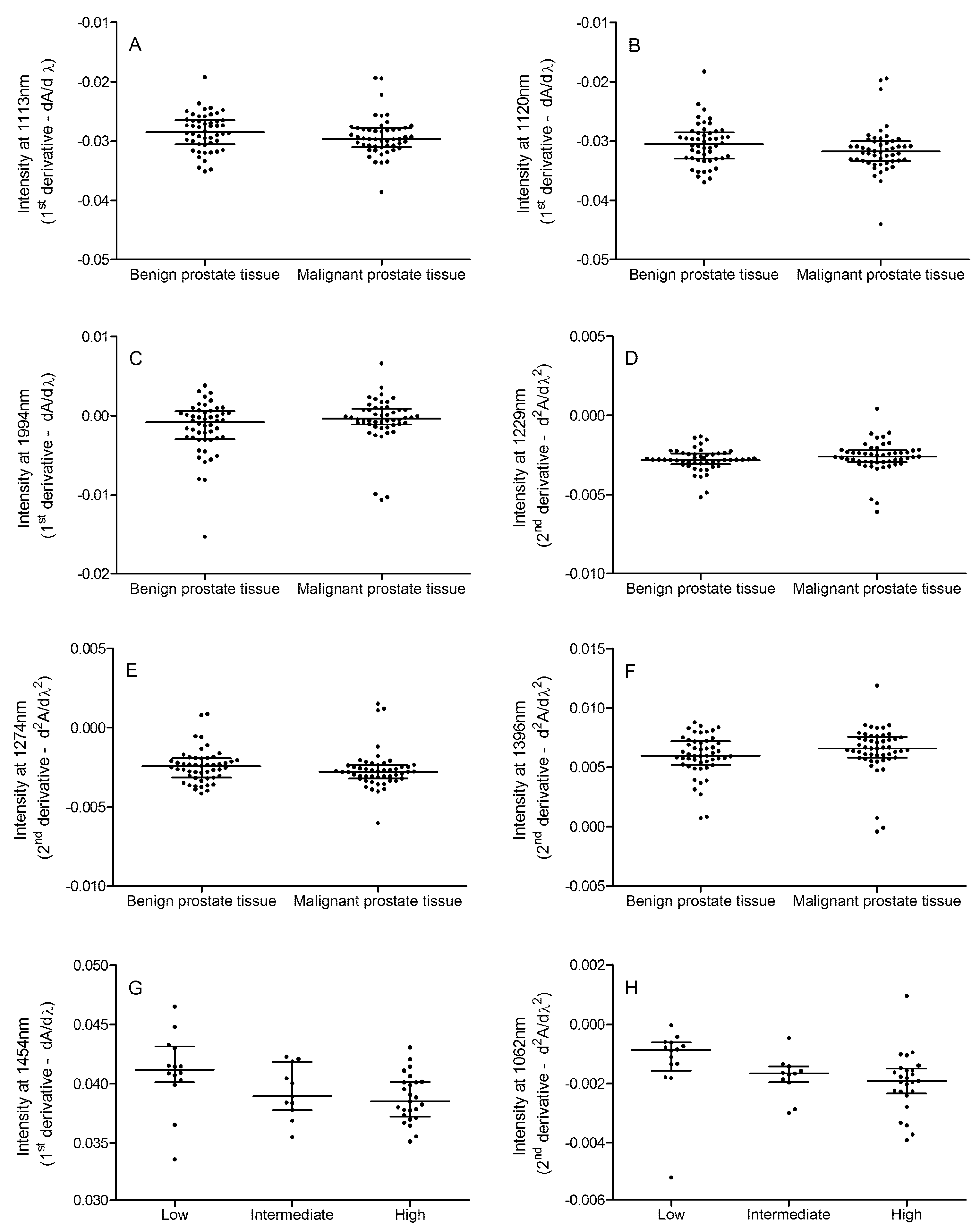
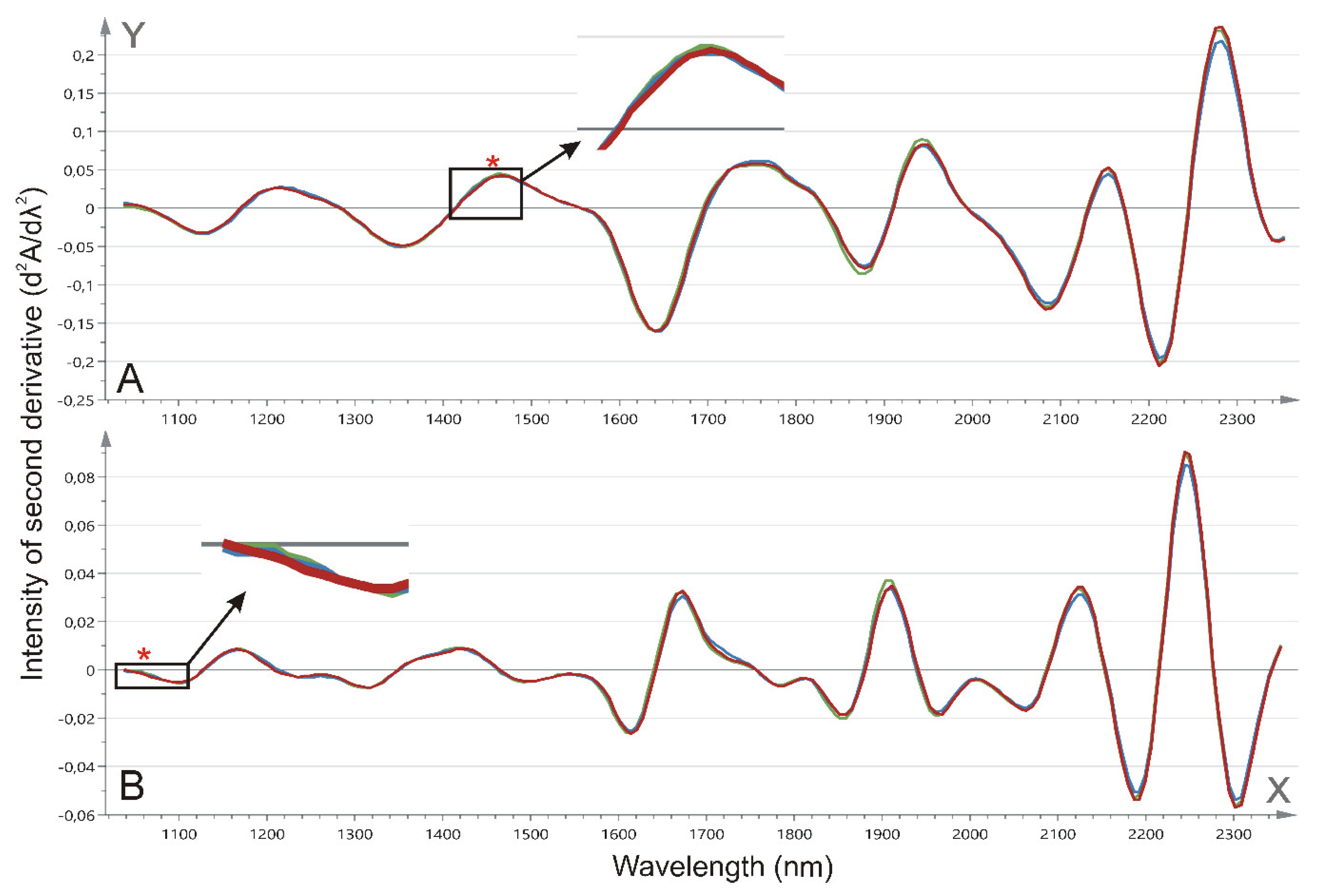
2.3. Tissue NIR Spectroscopy
3. Materials and Methods
3.1. Tissue Specimens
3.2. Biochemical Analysis
3.3. Tissue Prostate Protein N-Glycosylation Profile
3.4. Tissue NIR Spectroscopic Analysis
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2016, 7, 193–208. [Google Scholar] [CrossRef]
- Magee, J.A.; Piskounova, E.; Morrison, S.J. Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer Cell 2012, 21, 283–296. [Google Scholar] [CrossRef]
- Ward, A.M.; Catto, J.W.; Hamdy, F.C. Prostate specific antigen: Biology, biochemistry and available commercial assays. Ann. Clin. Biochem. 2001, 38, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Greene, K.L.; Albertsen, P.C.; Babaian, R.J.; Carter, H.B.; Gann, P.H.; Han, M.; Kuban, D.A.; Sartor, A.O.; Stanford, J.L.; Zietman, A.; et al. Prostate specific antigen best practice statement: 2009 update. J. Urol. 2013, 189 (Suppl. 1), S2–S11. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Bjartell, A.; Montironi, R.; Berney, D.M.; Egevad, L. Tumour markers in prostate cancer II: Diagnostic and prognostic cellular biomarkers. Acta Oncol. (Stockholm, Sweden) 2011, 50 (Suppl. 1), 76–84. [Google Scholar] [CrossRef] [PubMed]
- Clinton, T.N.; Bagrodia, A.; Lotan, Y.; Margulis, V.; Raj, G.V.; Woldu, S.L. Tissue-based biomarkers in prostate cancer. Expert Rev. Precis. Med. Drug Dev. 2017, 2, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Dani, H.; Loeb, S. The role of prostate cancer biomarkers in undiagnosed men. Curr. Opin. Urol. 2017, 27, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Moschini, M.; Spahn, M.; Mattei, A.; Cheville, J.; Karnes, R.J. Incorporation of tissue-based genomic biomarkers into localized prostate cancer clinics. BMC Med. 2016, 14, 67. [Google Scholar] [CrossRef]
- Vermassen, T.; Speeckaert, M.M.; Lumen, N.; Rottey, S.; Delanghe, J.R. Glycosylation of prostate specific antigen and its potential diagnostic applications. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 413, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, T.; Van Praet, C.; Lumen, N.; Decaestecker, K.; Vanderschaeghe, D.; Callewaert, N.; Villeirs, G.; Hoebeke, P.; Van Belle, S.; Rottey, S.; et al. Urinary prostate protein glycosylation profiling as a diagnostic biomarker for prostate cancer. Prostate 2015, 75, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications. In Analytical Techniques in the Sciences; Ando, D.J., Ed.; Wiley: Chichester, UK, 2004. [Google Scholar]
- Orsonneau, J.L.; Douet, P.; Massoubre, C.; Lustenberger, P.; Bernard, S. An improved pyrogallol red-molybdate method for determining total urinary protein. Clin. Chem. 1989, 35, 2233–2236. [Google Scholar]
- Vermassen, T.; Van Praet, C.; Vanderschaeghe, D.; Maenhout, T.; Lumen, N.; Callewaert, N.; Hoebeke, P.; Van Belle, S.; Rottey, S.; Delanghe, J. Capillary electrophoresis of urinary prostate glycoproteins assists in the diagnosis of prostate cancer. Electrophoresis 2014, 35, 1017–1024. [Google Scholar] [CrossRef]
- De Bruyne, S.; Speeckaert, R.; Boelens, J.; Hayette, M.P.; Speeckaert, M.; Delanghe, J. Infrared spectroscopy as a novel tool to diagnose onychomycosis. Br. J. Dermatol. 2018, 180, 637–646. [Google Scholar] [CrossRef]
- Monteyne, T.; Coopman, R.; Kishabongo, A.S.; Himpe, J.; Lapauw, B.; Shadid, S.; Van Aken, E.H.; Berenson, D.; Speeckaert, M.M.; De Beer, T.; et al. Analysis of protein glycation in human fingernail clippings with near-infrared (NIR) spectroscopy as an alternative technique for the diagnosis of diabetes mellitus. Clin. Chem. Lab. Med. 2018, 56, 1551–1558. [Google Scholar] [CrossRef]
- Drake, R.R.; Jones, E.E.; Powers, T.W.; Nyalwidhe, J.O. Altered glycosylation in prostate cancer. Adv. Cancer Res. 2015, 126, 345–382. [Google Scholar] [PubMed]
- Gala, F.A.; Morrison, S.L. The role of constant region carbohydrate in the assembly and secretion of human IgD and IgA1. J. Biol. Chem. 2002, 277, 29005–29011. [Google Scholar] [CrossRef]
- Gazi, E.; Dwyer, J.; Gardner, P.; Ghanbari-Siahkali, A.; Wade, A.P.; Miyan, J.; Lockyer, N.P.; Vickerman, J.C.; Clarke, N.W.; Shanks, J.H.; et al. Applications of Fourier transform infrared microspectroscopy in studies of benign prostate and prostate cancer. A pilot study. J. Pathol. 2003, 201, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gazi, E.; Baker, M.; Dwyer, J.; Lockyer, N.P.; Gardner, P.; Shanks, J.H.; Reeve, R.S.; Hart, C.A.; Clarke, N.W.; Brown, M.D. A correlation of FTIR spectra derived from prostate cancer biopsies with gleason grade and tumour stage. Eur. Urol. 2006, 50, 750–760. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.S.; Heston, W.D.; Guo, H.; Wang, B.C.; Basilion, J.P. Development of targeted near-infrared imaging agents for prostate cancer. Mol. Cancer Ther. 2014, 13, 2595–2606. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, X.; Yan, F.; Wang, F.; Qin, W.; Wu, G.; Yang, X.; Shao, C.; Chung, L.W. Nearinfrared fluorescence imaging of prostate cancer using heptamethine carbocyanine dyes. Mol. Med. Rep. 2015, 11, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Khajehpour, M.; Dashnau, J.L.; Vanderkooi, J.M. Infrared spectroscopy used to evaluate glycosylation of proteins. Anal. Biochem. 2006, 348, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.H.; Wang, W.B.; Zevallos, M.; Alfano, R.R. Near infrared spectroscopy and imaging to probe differences in water content in normal and cancer human prostate tissues. Technol. Cancer Res. Treat. 2004, 3, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Gazi, E.; Brown, M.D.; Shanks, J.H.; Clarke, N.W.; Gardner, P. Investigating FTIR based histopathology for the diagnosis of prostate cancer. J. Biophotonics 2009, 2, 104–113. [Google Scholar] [CrossRef]
- Baker, M.J.; Gazi, E.; Brown, M.D.; Shanks, J.H.; Gardner, P.; Clarke, N.W. FTIR-based spectroscopic analysis in the identification of clinically aggressive prostate cancer. Br. J. Cancer 2008, 99, 1859–1866. [Google Scholar] [CrossRef]
- Loeb, S.; Folkvaljon, Y.; Robinson, D.; Lissbrant, I.F.; Egevad, L.; Stattin, P. Evaluation of the 2015 Gleason Grade Groups in a Nationwide Population-based Cohort. Eur. Urol. 2016, 69, 1135–1141. [Google Scholar] [CrossRef]
- Kim, S.B.; Temiyasathit, C.; Bensalah, K.; Tuncel, A.; Cadeddu, J.; Kabbani, W.; Mathker, A.V.; Liu, H. An effective classification procedure for diagnosis of prostate cancer in near infrared spectra. Expert Syst. Appl. 2010, 37, 3863–3869. [Google Scholar] [CrossRef]
- Partsvania, B.; Petriashvili, G.; Fonjavidze, N. Possibility of using near infrared irradiation for early cancer diagnosis. Electromagn. Biol. Med. 2014, 33, 18–20. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Ansari, M.A.; Garmarudi, A.B.; Hassanzadeh, G.; Garoosi, G. Cancer diagnosis by discrimination between normal and malignant human blood samples using attenuated total reflectance-Fourier transform infrared spectroscopy. Cancer Investig. 2007, 25, 397–404. [Google Scholar] [CrossRef] [PubMed]
- German, M.J.; Hammiche, A.; Ragavan, N.; Tobin, M.J.; Cooper, L.J.; Matanhelia, S.S.; Hindley, A.C.; Nicholson, C.M.; Fullwood, N.J.; Pollock, H.M.; et al. Infrared spectroscopy with multivariate analysis potentially facilitates the segregation of different types of prostate cell. Biophys. J. 2006, 90, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
| (A) Benign Versus Malignant | |||||
| Characteristic | Benign Tissue | Malignant Tissue | p-value | ||
| N | 50 | 50 | |||
| Total prostate weight (g) | 55.5 (14.8–146.0) | 49.0 (20.7–118.0) | 0.5757 | ||
| Tissue prostate weight (mg) | 28.1 (10.4–106.2) | 24.4 (11.4–61.5) | 0.9917 | ||
| Total protein (g/L) | 0.29 (0.07–0.97) | 0.25 (0.09–0.94) | 0.7669 | ||
| PSA ratio (mg/ g protein) | 3.4 (0.2–24.0) | 4.5 (0.1–21.5) | 0.4179 | ||
| (B) Between Different Gleason Scores * | |||||
| Characteristic | Low | Intermediate | High | p-value | |
| N | 14 | 11 | 21 | ||
| Total prostate weight (g) | 46.5 (34.0–95.0) | 48.0 (28.5–86.0) | 39.5 (20.7–118.0) | 0.6953 | |
| Tissue prostate weight (mg) | 24.7 (14.3–45.7) | 30.7 (11.4–54.1) | 23.5 (13.5–61.5) | 0.7644 | |
| Total protein (g/L) | 0.27 (0.18–0.85) | 0.32 (0.09–0.73) | 0.24 (0.10–0.94) | 0.5110 | |
| PSA ratio (mg/g protein) | 6.6 (1.1–20.2) | 5.3 (0.8–21.5) | 2.9 (0.1–7.0) | 0.0235 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermassen, T.; De Bruyne, S.; Himpe, J.; Lumen, N.; Callewaert, N.; Rottey, S.; Delanghe, J. N-Linked Glycosylation and Near-Infrared Spectroscopy in the Diagnosis of Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1592. https://doi.org/10.3390/ijms20071592
Vermassen T, De Bruyne S, Himpe J, Lumen N, Callewaert N, Rottey S, Delanghe J. N-Linked Glycosylation and Near-Infrared Spectroscopy in the Diagnosis of Prostate Cancer. International Journal of Molecular Sciences. 2019; 20(7):1592. https://doi.org/10.3390/ijms20071592
Chicago/Turabian StyleVermassen, Tijl, Sander De Bruyne, Jonas Himpe, Nicolaas Lumen, Nico Callewaert, Sylvie Rottey, and Joris Delanghe. 2019. "N-Linked Glycosylation and Near-Infrared Spectroscopy in the Diagnosis of Prostate Cancer" International Journal of Molecular Sciences 20, no. 7: 1592. https://doi.org/10.3390/ijms20071592






