Current Advances in Molecular Mechanisms and Physiological Basis of Panicle Degeneration in Rice
Abstract
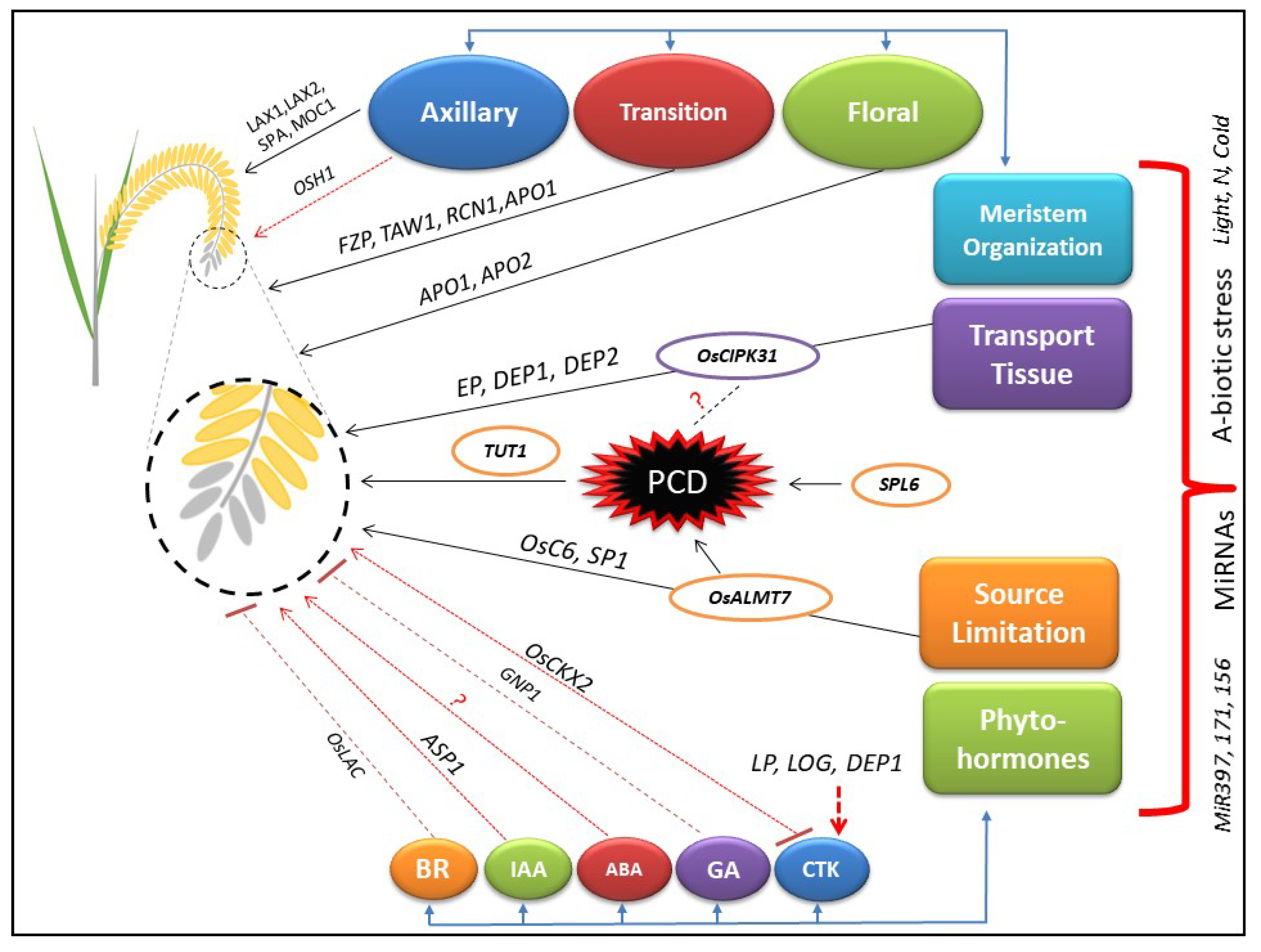
:1. Introduction
2. Control of Panicle Development by Meristems Organization
2.1. Axillary Meristem (Number/Size)
2.2. Meristem Transition Controlling Genes
2.3. Floral Meristems Controlling Genes
3. Regulation of Inflorescence Development by Phytohormones
3.1. Role of CTK in Panicle
3.2. Role of GA in Panicle
3.3. Role of BR in Panicle
3.4. Role of Auxin in Panicle
3.5. Role of ABA in Panicle
4. Inflorescence Degeneration Occurs Due to Limitation of Source Transportation
5. Role of Transporting Tissues
6. Role of Programmed Cell Death (PCD)
7. Role of Abiotic Stresses on Panicle Development
8. Role of miRNAs
9. Molecular and Physiological Mechanism of Panicle Development and Degeneration: A Discussion Based on Our Hypothesized Model
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takeda:, S.; Matsuoka, M. Genetic approaches to crop improvement: Responding to environmental and population changes. Nat. Rev. Genet. 2008, 9, 444–457. [Google Scholar] [CrossRef]
- Newton, A.C.; Johnson, S.N.; Gregory, P.J. Implications of climate change for diseases, crop yields and food security. Euphytica 2011, 179, 3–18. [Google Scholar] [CrossRef]
- Wang, D.; Pan, Y.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genom. 2011, 12, 149. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Wang, Z.; Yang, J. Polyamines and ethylene in rice young panicles in response to soil drought during panicle differentiation. Plant Growth Regul. 2017, 82, 491–503. [Google Scholar] [CrossRef]
- Peng, Y.; Hou, F.; Bai, Q.; Xu, P.; Liao, Y.; Zhang, H.; Gu, C.; Deng, X.; Wu, T.; Chen, X.; et al. Rice Calcineurin B-like Protein-interacting Protein Kinase 31 (OsCIPK31) is involved in the development of panicle apical spikelets. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.; Nonomura, K.; Ikeda, K.; Yamaki, S.; Inukai, Y.; Yamagishi, H.; Kitano, H.; Nagato, Y. Rice plant development: From zygote to spikelet. Plant Cell Physiol. 2005, 46, 23–47. [Google Scholar] [CrossRef]
- Ahmadi, A.; Baker, D. Effects of abscisic acid (ABA) on grain filling processes in wheat. Plant Growth Regul. 1999, 28, 187–197. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, X.; Wang, Q.; Zhang, J.; Chen, H.; Dong, G.; Zhu, L.; Zheng, H.; Xie, Q.; Nian, J.; et al. Rice TUTOU1 encodes a suppressor of cAMP Receptor-Like Protein that is important for actin organization and panicle development. Plant Physiol. 2015, 169, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Gallavotti, A.; Zhao, Q.; Kyozuka, J.; Meeley, R.B.; Ritter, M.; Doebley, J.; Pe, M.E.; Schmidt, R.J. The role of barren stalk1 in the architecture of maize. Nature 2004, 432, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, F.; Zhang, Y.; Lin, J.; Song, C.; Fang, X. Fine mapping and candidate gene analysis of a novel PANICLE AND SPIKELET DEGENERATION gene in rice. Euphytica 2015, 206, 793–803. [Google Scholar] [CrossRef]
- Akter, M.B.; Piao, R.; Kim, B.; Lee, Y.; Koh, E.; Koh, H.-J. Fine mapping and candidate gene analysis of a new mutant gene for panicle apical abortion in rice. Euphytica 2014, 197, 387–398. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Mao, B.G.; Gao, S.W.; Zhang, L.; Wang, J.L.; Lei, C.L.; Zhang, X.; Wu, F.Q.; Guo, X.P.; Wan, J. Fine mapping of qPAA8, a gene controlling panicle apical development in rice. J Integr. Plant Biol. 2011, 53, 710–718. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Yang, J. Physiological mechanism underlying spikelet degeneration in rice. J. Integr. Agric. 2018, 17, 1475–1481. [Google Scholar] [CrossRef]
- Kamoi, T.; Kenzo, T.; Kuraji, K.; Momose, K. Abortion of reproductive organs as an adaptation to fluctuating daily carbohydrate production. Oecologia 2008, 154, 663–677. [Google Scholar] [CrossRef]
- Ishimaru, T.; Hirose, T.; Matsuda, T.; Goto, A.; Takahashi, K.; Sasaki, H.; Terao, T.; Ishii, R.-i.; Ohsugi, R.; Yamagishi, T. Expression patterns of genes encoding carbohydrate-metabolizing enzymes and their relationship to grain filling in rice (Oryza sativa L.): Comparison of caryopses located at different positions in a panicle. Plant Cell Physiol. 2005, 46, 620–628. [Google Scholar] [CrossRef]
- Skazhennik, M.; Vorob’yov, N.; Sheudzhen, A.K.; Kovalyov, V. Causes of increased panicle spikelet sterility in rice. Russ. Agric. Sci. 2015, 41, 309–310. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, G.; Liu, L.; Wang, Z.; Wang, X.; Zhang, H.; Yang, J.; Zhang, J. Mid-season nitrogen application strategies for rice varieties differing in panicle size. Field Crops Res. 2013, 150, 9–18. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Naik, P.K.; Patel, R. Ethylene inhibitors improve dry matter partitioning and development of late flowering spikelets on rice panicles. Funct. Plant Physiol. 2000, 27, 311–323. [Google Scholar] [CrossRef]
- Ganeshaiah, K.; Uma Shaanker, R. Seed and fruit abortion as a process of self organization among developing sinks. Physiol. Plant. 1994, 91, 81–89. [Google Scholar] [CrossRef]
- Smith, C.W. Crop Production: Evolution, History, and Technology; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Zhu, Q.; Upadhyaya, N.M.; Gubler, F.; Helliwell, C.A. Over-expression of miR172 causes loss of spikelet determinacy and floral organ abnormalities in rice (Oryza sativa). BMC Plant Biol. 2009, 9, 149. [Google Scholar] [CrossRef]
- Huijser, P.; Schmid, M. The control of developmental phase transitions in plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef] [Green Version]
- Amasino, R.M. Seasonal and developmental timing of flowering. Plant J. 2010, 61, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, W.; Yin, C.; Zong, J.; Gu, F.; Zhang, D. OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol. 2010, 154, 149–162. [Google Scholar] [CrossRef]
- Zhang, D.; Wilson, Z.A. Stamen specification and anther development in rice. Chin. Sci. Bull. 2009, 54, 2342–2353. [Google Scholar] [CrossRef]
- Ingram, G.C.; Goodrich, J.; Wilkinson, M.D.; Simon, R.; Haughn, G.W.; Coen, E. Parallels between UNUSUAL FLORAL ORGANS and FIMBRIATA, genes controlling flower development in Arabidopsis and Antirrhinum. Plant Cell 1995, 7, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Maekawa, M.; Shimamoto, K.; Kyozuka, J. The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Dev. Biol. 2001, 231, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, H.; Zhang, Y.; Hattori, S.; Omae, M.; Shimizusato, S.; Oikawa, T.; Qian, Q.; Nishimura, M.; Kitano, H.; Xie, H. LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell 2011, 23, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Maekawa, M.; Ujiie, S.; Satake, Y.; Furutani, I.; Okamoto, H.; Shimamoto, K.; Kyozuka, J. LAX and SPA: Major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. USA 2003, 100, 11765–11770. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Hiroshi, F. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar] [CrossRef]
- Sinha, N.; Williams, R.E.; Hake, S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Gene Dev. 1993, 7, 787–795. [Google Scholar] [CrossRef]
- Matsuoka, M.; Ichikawa, H.; Saito, A.; Tada, Y.; Fujimura, T.; Kanomurakami, Y. Expression of a rice homeobox gene causes altered morphology of transgenic plants. Plant Cell 1993, 5, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Ohno, C.; Smith, Z.R.; Meyerowitz, E.M. TOPLESS regulates apical embryonic fate in Arabidopsis. Science 2006, 312, 1520–1523. [Google Scholar] [CrossRef]
- Yoshida, A.; Ohmori, Y.; Kitano, H.; Taguchishiobara, F.; Hirano, H. ABERRANT SPIKELET AND PANICLE1, encoding a TOPLESS-related transcriptional co-repressor, is involved in the regulation of meristem fate in rice. Plant J. 2012, 70, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Chuck, G.; Muszynski, M.G.; Kellogg, E.A.; Hake, S.; Schmidt, R.J. The control of spikelet meristem identity by the BRANCHED SILKLESS1 gene in maize. Science 2002, 298, 1238–1241. [Google Scholar] [CrossRef]
- Komatsu, M.; Chujo, A.; Nagato, Y.; Shimamoto, K.; Kyozuka, J. FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 2003, 130, 3841–3850. [Google Scholar] [CrossRef]
- Colombo, L.; Marziani, G.; Masiero, S.; Wittich, P.E.; Schmidt, R.J.; Gorla, M.S.; Pe, M.E. Branched silkless mediates the transition from spikelet to floral meristem during Zea mays ear development. Plant J. 1998, 16, 355–363. [Google Scholar] [CrossRef]
- Yoshida, A.; Sasao, M.; Yasuno, N.; Takagi, K.; Daimon, Y.; Chen, R.; Yamazaki, R.; Tokunaga, H.; Kitaguchi, Y.; Sato, Y. TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc. Natl. Acad. Sci. USA 2013, 110, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, W.; Wang, L.; Lin, C.; Cong, B.; Sun, C.; Luo, D. TFL1/CEN-like genes control intercalary meristem activity and phase transition in rice. Plant Sci. 2005, 168, 1393–1408. [Google Scholar] [CrossRef]
- Nakagawa, M.; Shimamoto, K.; Kyozuka, J. Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J. 2002, 29, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Nagasawa, N.; Nagato, Y. ABERRANT PANICLE ORGANIZATION 1 temporally regulates meristem identity in rice. Dev. Biol. 2005, 282, 349–360. [Google Scholar] [CrossRef]
- Samach, A.; Klenz, J.E.; Kohalmi, S.E.; Risseeuw, E.; Haughn, G.W.; Crosby, W.L. The UNUSUAL FLORAL ORGANS gene of Arabidopsis thaliana is an F-box protein required for normal patterning and growth in the floral meristem. Plant J. 1999, 20, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ito, M.; Nagasawa, N.; Kyozuka, J.; Nagato, Y. Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. Plant J. 2007, 51, 1030–1040. [Google Scholar] [CrossRef]
- Ikedakawakatsu, K.; Maekawa, M.; Izawa, T.; Itoh, J.I.; Nagato, Y. ABERRANT PANICLE ORGANIZATION 2/RFL, the rice ortholog of Arabidopsis LEAFY, suppresses the transition from inflorescence meristem to floral meristem through interaction with APO1. Plant J. 2012, 69, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Calderonvillalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lur, H.S. Ethylene may be involved in abortion of the maize caryopsis. Physiol. Plant. 1996, 98, 245–252. [Google Scholar] [CrossRef]
- Kyozuka, J. Control of shoot and root meristem function by cytokinin. Curr. Opin. Plant Biol. 2007, 10, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. The role of local biosynthesis of auxin and cytokinin in plant development. Curr. Opin. Plant Biol. 2008, 11, 16–22. [Google Scholar] [CrossRef]
- Li, M.; Tang, D.; Wang, K.; Wu, X.; Lu, L.; Yu, H.; Gu, M.; Yan, C.; Cheng, Z. Mutations in the F-box gene LARGER PANICLE improve the panicle architecture and enhance the grain yield in rice. Plant Biotechnol. J. 2011, 9, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, J.; Li, Z.; Yi, C.; Liu, J.; Zhang, H.; Tang, S.; Gu, M.; Liang, G. Deletion in a quantitative trait gene qPE9-1 associated with panicle erectness improves plant architecture during rice domestication. Genetics 2009, 183, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.; Ueguchitanaka, M.; Kondo, M.; Hamada, K.; Yano, K.; Nishimura, M.; Matsuoka, M. Gibberellin Modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 2009, 21, 1453–1472. [Google Scholar] [CrossRef]
- Yokoyama, C.; Tsuda, M.; Hirai, Y. Effects of plant growth regulators on number of spikelets per panicle in Rice (Oryza sativa L.) under Saline Flooding Conditions (Crop Physiology and Cell Biology). Jpn. J. Crop. Sci. 2002, 71, 376–382. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Mi, X.; Shan, J.; Li, X.; Xu, J.; Lin, H. The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet. 2016, 12, e1006386. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-B.; Huang, H.-Y.; Hu, Y.-W.; Zhu, S.-W.; Wang, Z.-Y.; Lin, W.-H. Brassinosteroid regulates seed size and shape in Arabidopsis. Plant Physiol. 2013, 162, 1965–1977. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Wang, C.; Li, Z.; Liu, Q.; Xu, J.; Liao, J.; Wang, X.; Qu, L.; Chen, F. Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat. Biotechnol. 2013, 31, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y. Mechanism underlying water and nitrogen regulating spikelet development and grain filling of rice. Ph. D. Thesis, Yangzhou University, Yangzhou, China, 2018. [Google Scholar]
- Zhang, C.; Feng, B.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Mockaitis, K.; Estelle, M. Auxin receptors and plant development: A new signaling paradigm. Annu. Rev. Cell Dev. Biol. 2008, 24, 55–80. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.; Stieger, P.A.; Mandel, T.; Baltensperger, K.; Bennett, M.J.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef]
- Gallavotti, A.; Yang, Y.; Schmidt, R.J.; Jackson, D. The relationship between auxin transport and maize branching. Plant Physiol. 2008, 147, 1913–1923. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Pandey, G.K. Mechanism of Plant Hormone Signaling Under Stress, 2 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1. [Google Scholar]
- Bangerth, F. Dominance among fruits/sinks and the search for a correlative signal. Physiol. Plant. 1989, 76, 608–614. [Google Scholar] [CrossRef]
- Li, S.; Qian, Q.; Fu, Z.; Zeng, D.; Meng, X.; Kyozuka, J.; Maekawa, M.; Zhu, X.; Zhang, J.; Li, J.; et al. SHORT PANICLE1 encodes a putative PTR family transporter and determines rice panicle size. Plant J. 2009, 58, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Abscisic acid and ethylene interact in rice spikelets in response to water stress during meiosis. J. Plant Growth Regul. 2007, 26, 318–328. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, N.; Yang, J.; Peng, X.; Zhang, J. Regulation of expression of starch synthesis genes by ethylene and ABA in relation to the development of rice inferior and superior spikelets. J. Exp. Bot. 2011, 62, 3907–3916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, J.; Wang, Z.; Liu, K.; Wang, P.Y. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 2006, 57, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kobayasi, K.; Yamane, K.; Imaki, T. Effects of non-structural carbohydrates on spikelet differentiation in Rice. Plant Prod. Sci. 2001, 4, 9–14. [Google Scholar] [CrossRef]
- Chiang, C.; Stacey, G.; Tsay, Y. Mechanisms and functional properties of two peptide transporters, AtPTR2 and fPTR2. J. Biol. Chem. 2004, 279, 30150–30157. [Google Scholar] [CrossRef]
- Heng, Y.; Wu, C.; Long, Y.; Luo, S.; Ma, J.; Chen, J.; Liu, J.; Zhang, H.; Ren, Y.; Wang, M. OsALMT7 maintains panicle size and grain yield in rice by mediating malate transport. Plant Cell 2018, 30, 889–906. [Google Scholar] [CrossRef]
- Xie, R.; Deng, L.; Jing, L.; He, S.; Ma, Y.T.; Yi, S.; Zheng, Y.; Zheng, L. Recent advances in molecular events of fruit abscission. Biol. Plant. 2013, 57, 201–209. [Google Scholar] [CrossRef]
- Afza, R.; Shen, M.; Zapataarias, F.J.; Xie, J.; Fundi, H.K.; Lee, K.; Bobadillamucino, E.; Kodym, A. Effect of spikelet position on rice anther culture efficiency. Plant Sci. 2000, 153, 155–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, P.; Wang, L.; Tan, C.; Hu, Z.; Zhu, Y.; Zhu, L. Identification of quantitative trait loci (QTLs) for the characters of vascular bundles in peduncle related to indica-japonica differentiation in rice (Oryza sativa L.). Euphytica 2002, 128, 279–284. [Google Scholar] [CrossRef]
- Zhu, K.; Tang, D.; Yan, C.; Chi, Z.; Yu, H.; Chen, J.; Liang, J.; Gu, M.; Cheng, Z. ERECT PANICLE2 encodes a novel protein that regulates panicle erectness in indica rice. Genetics 2010, 184, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qian, Q.; Liu, Z.; Sun, H.; He, S.; Luo, D.; Xia, G.; Chu, C.; Li, J.; Fu, X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 2009, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, W.; Tang, J.; Chen, J.; Tong, H.; Hu, B.; Li, C.; Fang, J.; Chen, M.; Chu, C. Rice DENSE AND ERECT PANICLE 2 is essential for determining panicle outgrowth and elongation. Cell Res. 2010, 20, 838–849. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, T.; Bi, W.; Wang, Y.; Xu, H.; Tang, L.; Sun, J.; Xu, Z. Different effects of DEP1 on vascular bundle- and panicle-related traits under indica and japonica genetic backgrounds. Mol. Breed. 2015, 35, 173. [Google Scholar] [CrossRef]
- Lockshin, R.A.; Zakeri, Z. Apoptosis, autophagy, and more. Int. J. Biochem. Cell Biol. 2004, 36, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.B.; Green, D.R. Suicidal tendencies: Apoptotic cell death by caspase family proteinases. J. Biol. Chem. 1999, 274, 20049–20052. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Beers, E.P.; Dangl, J.L.; Franklintong, V.E.; Gallois, P.; Haranishimura, I.; Jones, A.M.; Kawaiyamada, M.; Lam, E.; Mundy, J. Morphological classification of plant cell deaths. Cell Death Differ. 2011, 18, 1241–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Sun, A.; Chen, S.; Chen, L.; Guo, F. SPL6 represses signalling outputs of ER stress in control of panicle cell death in rice. Nat. Plants 2018, 4, 280–288. [Google Scholar] [CrossRef]
- Beers, E.P.; Mcdowell, J.M. Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr. Opin. Plant Biol. 2001, 4, 561–567. [Google Scholar] [CrossRef]
- Sanchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Krishnan, P.; Nayak, M.; Ramakrishnan, B. High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ. Exp. Bot. 2014, 101, 36–46. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Longevity and temperature response of pollen as affected by elevated growth temperature and carbon dioxide in peanut and grain sorghum. Environ. Exp. Bot. 2011, 70, 51–57. [Google Scholar] [CrossRef]
- Rang, Z.W.; Jagadish, S.V.K.; Zhou, Q.M.; Craufurd, P.Q.; Heuer, S. Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ. Exp. Bot. 2011, 70, 58–65. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Koti, S.; Wallace, T.P.; Prasad, P.V.V.; Reddy, V.R.; Zhao, D. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann. Bot. 2005, 96, 59–67. [Google Scholar] [CrossRef]
- Yang, L.-C.; Liu, K.; Zhang, S.-F.; Wang, X.-M.; Wang, Z.-Q.; Liu, L.-J. Hormones in rice spikelets in responses to water stress during meiosis. Acta Agron. Sin. 2008, 34, 111–118. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Kobayashi, K.; Zhu, J.; Chen, C.P.; Yang, K.; Tang, H.; Wang, Y. Investigations on spikelet formation in hybrid rice as affected by elevated tropospheric ozone concentration in China. Agric. Ecosyst. Environ. 2012, 150, 63–71. [Google Scholar] [CrossRef]
- Ding, C.; You, J.; Chen, L.; Wang, S.; Ding, Y. Nitrogen fertilizer increases spikelet number per panicle by enhancing cytokinin synthesis in rice. Plant Cell Rep. 2014, 33, 363–371. [Google Scholar] [CrossRef]
- Liang, T.; Hong, G.; Yoshihiro, H.; Koki, H.; Tetsuya, N.; LIU, T.-S.; Tatsuhiko, S.; XU, Z.-J. Erect panicle super rice varieties enhance yield by harvest index advantages in high nitrogen and density conditions. J. Integr. Agric. 2017, 16, 1467–1473. [Google Scholar]
- Liu, L.; Chen, T.; Wang, Z.; Zhang, H.; Yang, J.; Zhang, J. Combination of site-specific nitrogen management and alternate wetting and drying irrigation increases grain yield and nitrogen and water use efficiency in super rice. Field Crops Res. 2013, 154, 226–235. [Google Scholar] [CrossRef]
- Lu, X.; Huang, X. Plant miRNAs and abiotic stress responses. Biochem. Biophys. Res. Commun. 2008, 368, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Li, X.; Yang, W.; Xia, K.; Ouyang, J.; Zhang, M. Rice Osa-miR171c mediates phase change from vegetative to reproductive development and shoot apical meristem maintenance by repressing four OsHAM transcription factors. PLoS ONE 2015, 10, e0125833. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Xue, D.; Wang, J.; Yan, M.; Liu, G.; Dong, G.; Zeng, D.; Lu, Z.; Zhu, X. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010, 42, 541–544. [Google Scholar] [CrossRef]
- Johnson, C.; Kasprzewska, A.; Tennessen, K.; Fernandes, J.; Nan, G.; Walbot, V.; Sundaresan, V.; Vance, V.; Bowman, L.H. Clusters and superclusters of phased small RNAs in the developing inflorescence of rice. Genome Res. 2009, 19, 1429–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
| Gene | Product | Function | References |
|---|---|---|---|
| APO1/APO2 | F-box protein | floral meristems | [42,45] |
| ASP1 | co-repressor | auxin signaling and spikelet development | [35] |
| DEP1/DEP2 | PEBP (PHOSPHATIDYLETHALAMINE binding) protein | panicle erectness | [76,77] |
| EP2 | Endoplasmic reticulum protein | panicle erectness | [75] |
| FZP | ERF transcription factor | floral meristem establishment | [37] |
| GN1 | CYTOKININ OXIDASE | panicle size | [99] |
| LAX1 | a bHLH transcription factor | inflorescence architecture | [28] |
| LAX2 | a novel nuclear protein | axillary meristems | [29] |
| LP | F-box protein | panicle size | [50] |
| MOC1 | GRAS family protein | axillary buds and meristems | [31] |
| OSH1 | HOMEOBOX PROTEIN KNOTTED-1-LIKE 6 | spikelet development | [33] |
| RCN1 and RCN2 | PEBP (PHOSPHATIDYL-ETHANOLAMINE-BINDING) protein | inflorescence meristem | [41] |
| SP1 | PTR transporter | panicle size | [65] |
| SPA | bHLH domain | axillary meristems | [30] |
| TAW1 | ALOG (Arabidopsis LSH2 and Oryza G1) protein | inflorescence architecture | [39] |
| LOG | CTK- activating enzyme | meristem development | [24] |
| SPL6 | IRE1-transducer | control PCD | [82] |
| OsCIPK31 | CIPK- protein | panicle development | [5] |
| OsALMT7 | ALMT protein | panicle development | [71] |
| OsSPL14 | SPL-protein | ideal plant architecture | [97] |
| Osc6 | LTP-protein | pollen development | [25] |
| TUTOU1 | cAMP/WAVE-like protein | actin organization | [8] |
| OsLAC | LACCASE-like protein | panicle branching | [56] |
| GNP1 | GA20ox1 | reproductive meristem | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Xu, P.; Riaz, A.; Wu, X. Current Advances in Molecular Mechanisms and Physiological Basis of Panicle Degeneration in Rice. Int. J. Mol. Sci. 2019, 20, 1613. https://doi.org/10.3390/ijms20071613
Ali A, Xu P, Riaz A, Wu X. Current Advances in Molecular Mechanisms and Physiological Basis of Panicle Degeneration in Rice. International Journal of Molecular Sciences. 2019; 20(7):1613. https://doi.org/10.3390/ijms20071613
Chicago/Turabian StyleAli, Asif, Peizhou Xu, Asad Riaz, and Xianjun Wu. 2019. "Current Advances in Molecular Mechanisms and Physiological Basis of Panicle Degeneration in Rice" International Journal of Molecular Sciences 20, no. 7: 1613. https://doi.org/10.3390/ijms20071613








