Electrospun Water-Borne Polyurethane Nanofibrous Membrane as a Barrier for Preventing Postoperative Peritendinous Adhesion
Abstract
:1. Introduction
2. Results
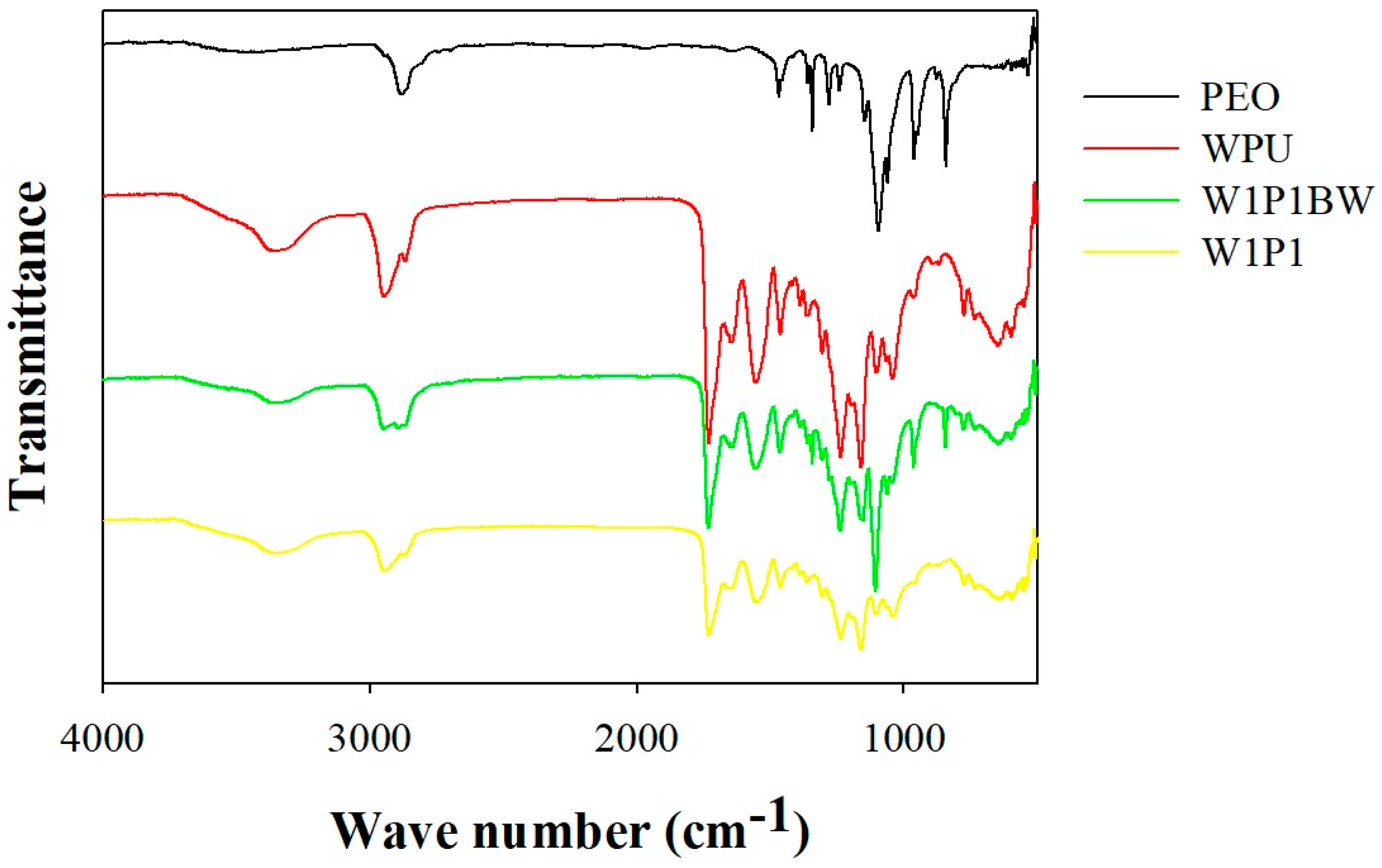
2.1. Preparation and Characterization of the Electrospun NFM
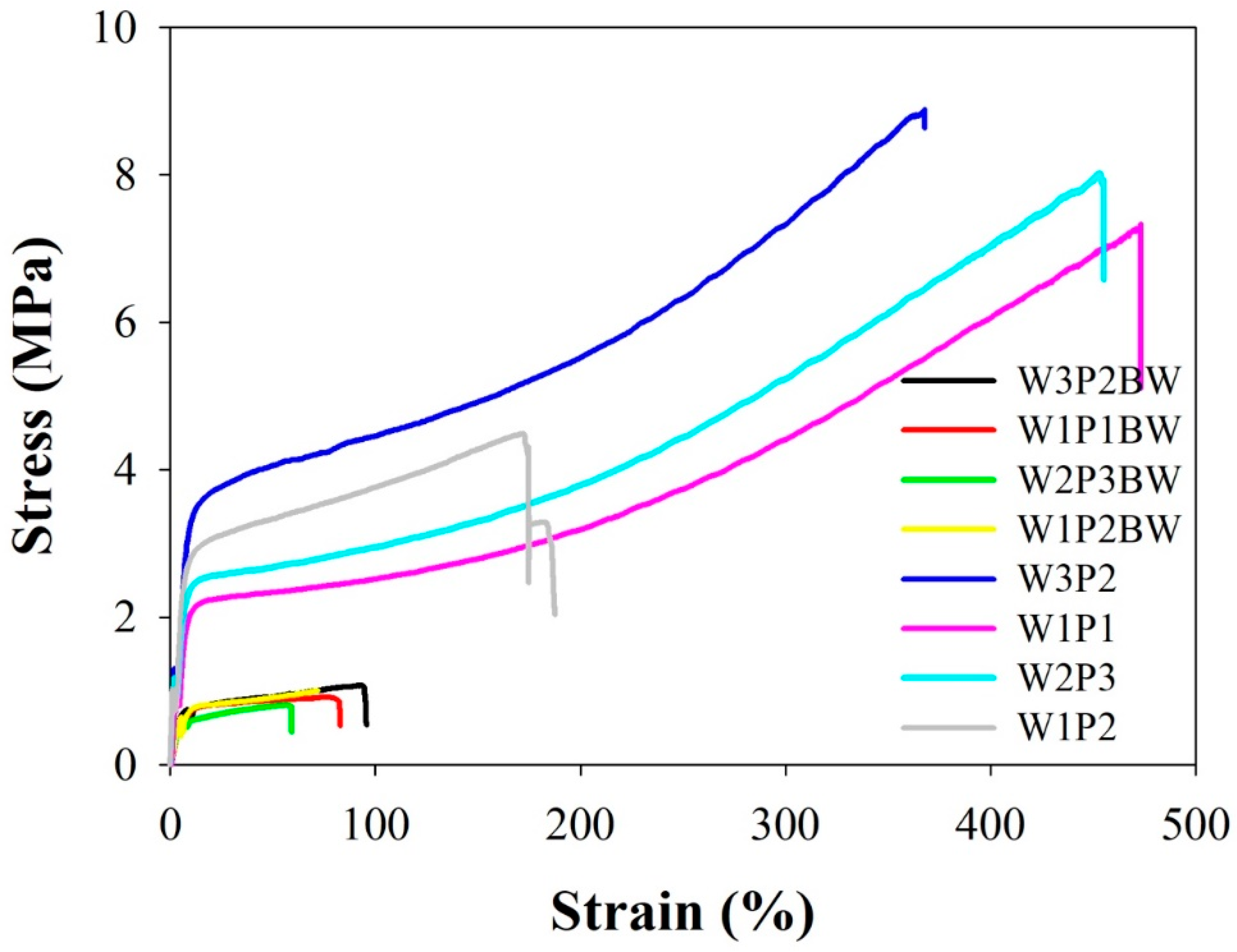
2.2. Mechanical Properties
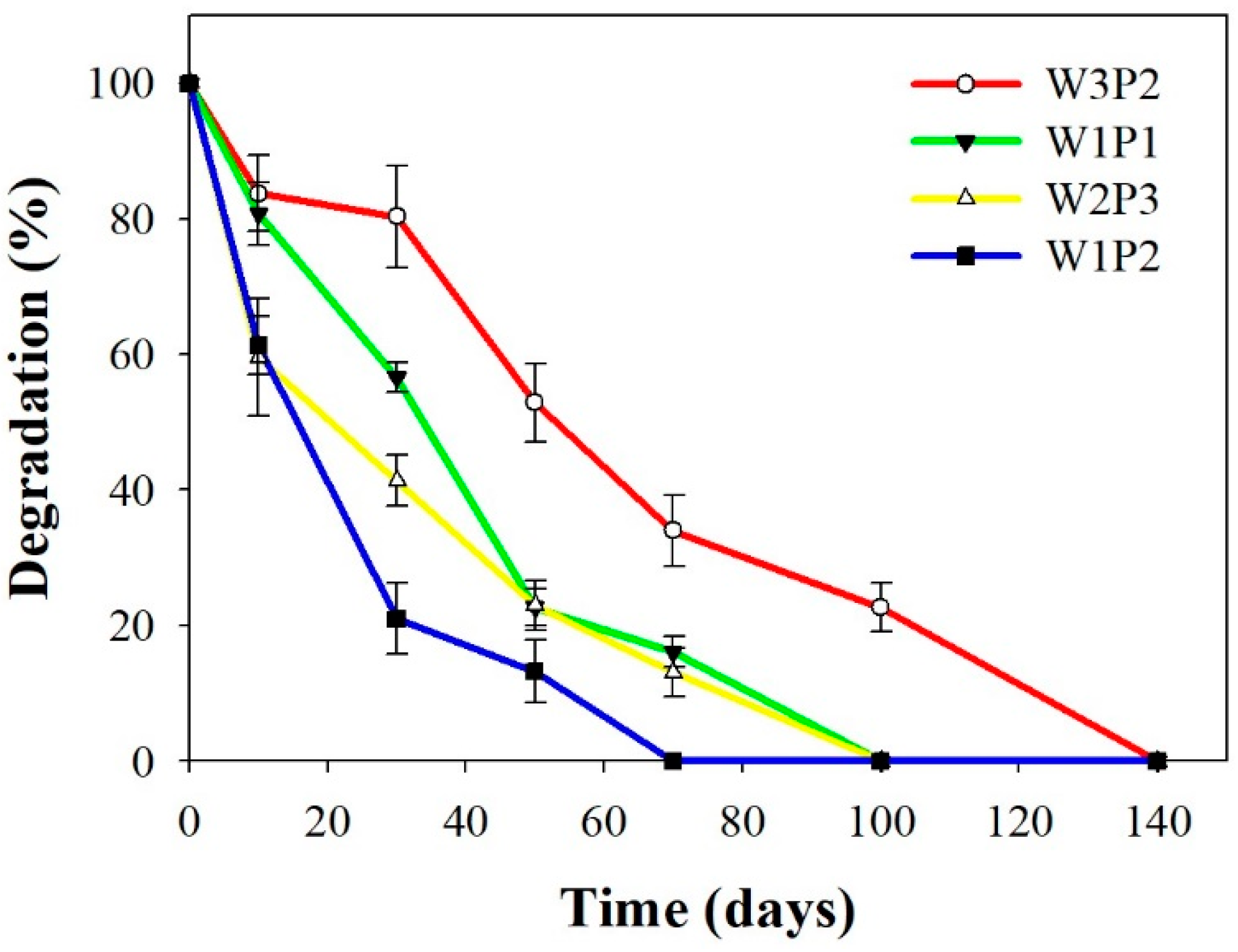
2.3. In Vitro Degradation
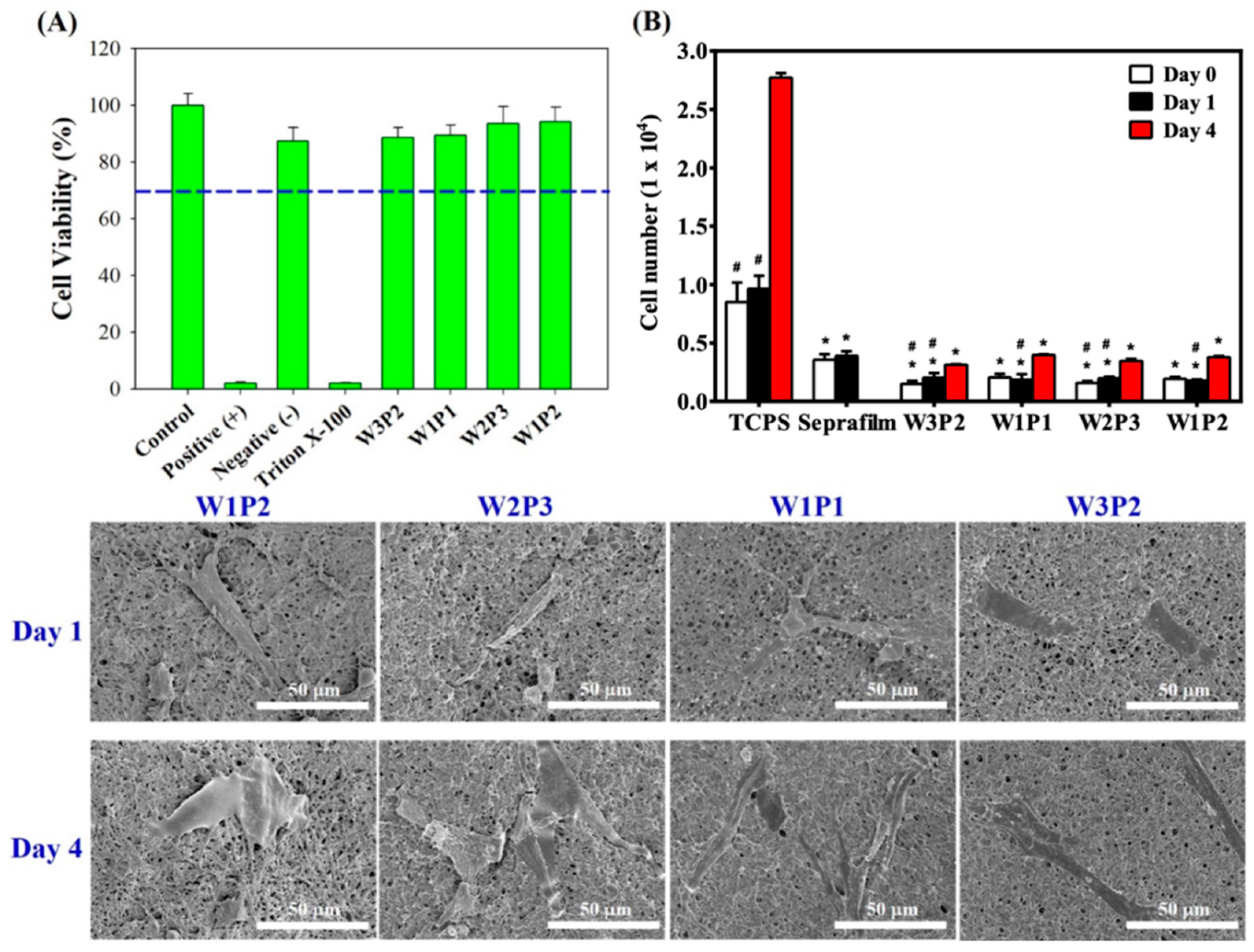
2.4. Cytotoxicity and Cell Attachment Test
2.5. Animal Study
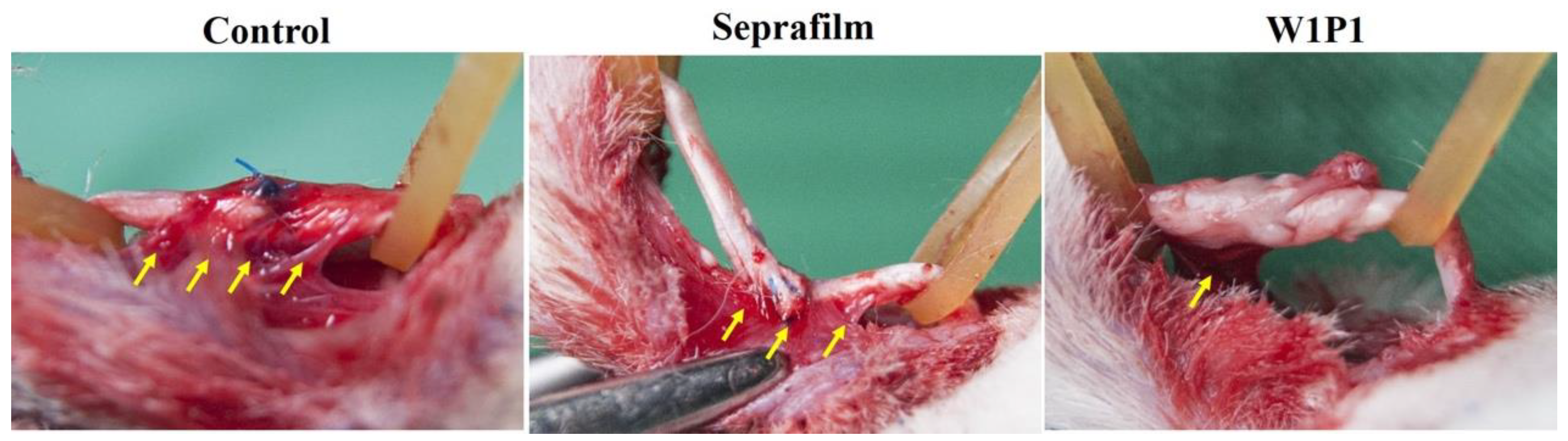
2.5.1. Gross Evaluation
2.5.2. Histology
2.5.3. Range of Motion
2.5.4. Pullout and Breaking Force
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Physicochemical Analyses of WPU
4.3. Preparation of Electrospun NFMs
4.4. Removal of PEO
4.5. Characterization of Electrospun NFMs
4.6. Confirmation of WPU NFM
4.7. Degradation Rate of WPU NFM
4.8. Cytotoxicity and Cell Attachment Test and In Vitro Cell Culture
4.9. Animal Study
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. 2008, 33, 102–112. [Google Scholar] [CrossRef]
- de Jong, J.P.; Nguyen, J.T.; Sonnema, A.J.M.; Nguyen, E.C.; Amadio, P.C.; Moran, S.L. The Incidence of Acute Traumatic Tendon Injuries in the Hand and Wrist: A 10-Year Population-based Study. Clin. Orthop. Surg. 2014, 6, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.W.; Peck, F. Rehabilitation of flexor and extensor tendon injuries in the hand: Current updates. Injury 2013, 44, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Dy, C.J.; Hernandez-Soria, A.; Ma, Y.; Roberts, T.R.; Daluiski, A. Complications After Flexor Tendon Repair: A Systematic Review and Meta-Analysis. J. Hand Surg. 2012, 37, 543–551.e1. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, B.; Trimbos-Kemper, T.; Trimbos, J.; Emeis, J.J.; Kooistra, T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil. Steril. 2000, 74, 203–212. [Google Scholar] [CrossRef]
- diZerega, G.S.; Campeau, J.D. Peritoneal repair and post-surgical adhesion formation. Hum. Reprod. Update 2001, 7, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taras, J.S.; Lamb, M.J. Treatment of flexor tendon injuries: Surgeons’ perspective. J. Hand Ther. 1999, 12, 141–148. [Google Scholar] [CrossRef]
- Lilly, S.I.; Messer, T.M. Complications after treatment of flexor tendon injuries. J. Am. Acad. Orthop. Surg. 2006, 14, 387–396. [Google Scholar] [CrossRef]
- Boyer, M.I. Flexor Tendon Biology. Hand Clin. 2005, 21, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, L.; Heinegard, D.; Ohlsson, K. The contents of macromolecule solutes in flexor tendon sheath fluid and their relation to synovial fluid. A quantitative analysis. J. Hand Surg. (Eur. Vol.) 1992, 17, 167–171. [Google Scholar] [CrossRef]
- Peterson, W.W.; Manske, P.R.; Dunlap, J.; Horwitz, D.S.; Kahn, B. Effect of various methods of restoring flexor sheath integrity on the formation of adhesions after tendon injury. J. Hand Surg. 1990, 15, 48–56. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Yang, D.-J.; Chen, F.; Xiong, Z.-C.; Xiong, C.-D.; Wang, Y.-Z. Tissue anti-adhesion potential of biodegradable PELA electrospun membranes. Acta Biomater. 2009, 5, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-J.; Lee, Y.-H.; Wu, M.-H.; Yang, M.-C.; Chien, C.-T. Electrospun anti-adhesion barrier made of chitosan alginate for reducing peritoneal adhesions. Carbohydr. Polym. 2012, 88, 1304–1312. [Google Scholar] [CrossRef]
- Dinarvand, P.; Hashemi, S.M.; Seyedjafari, E.; Shabani, I.; Mohammadi-Sangcheshmeh, A.; Farhadian, S.; Soleimani, M. Function of Poly (lactic-co-glycolic acid) Nanofiber in Reduction of Adhesion Bands. J. Surg. Res. 2012, 172, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, J.; Ruan, H.; Tang, T.; Liu, G.; Yu, D.; Cui, W.; Fan, C. Biomimetic Sheath Membrane via Electrospinning for Antiadhesion of Repaired Tendon. Biomacromolecules 2012, 13, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chen, S.-H.; Shalumon, K.T.; Chen, J.-P. Dual functional core-sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater. 2015, 26, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, C.; Li, F.; Li, X.-J.; Cui, W.; Fan, C. Prevention of Peritendinous Adhesions with Electrospun Ibuprofen-Loaded Poly( l-Lactic Acid)-Polyethylene Glycol Fibrous Membranes. Tissue Eng. Part A 2013, 19, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Wu, Y.F.; Tang, J.B. Tendon healing, edema, and resistance to flexor tendon gliding: Clinical implications. Hand Clin. 2013, 29, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Myer, C.; Fowler, J.R. Flexor Tendon Repair: Healing, Biomechanics, and Suture Configurations. Orthop. Clin. N. Am. 2016, 47, 219–226. [Google Scholar] [CrossRef]
- Yuan, B.; He, C.; Dong, X.; Wang, J.; Gao, Z.; Wang, Q.; Tian, H.; Chen, X. 5-Fluorouracil loaded thermosensitive PLGA–PEG–PLGA hydrogels for the prevention of postoperative tendon adhesion. RSC Adv. 2015, 5, 25295–25303. [Google Scholar] [CrossRef]
- Na, S.Y.; Oh, S.H.; Song, K.S.; Lee, J.H. Hyaluronic acid/mildly crosslinked alginate hydrogel as an injectable tissue adhesion barrier. J. Mater. Sci. Mater. Med. 2012, 23, 2303–2313. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, N.; He, T.; Shang, J.; Li, L.; Song, L.; Yang, X.; Li, X.; Luo, N.; Zhang, W.; et al. Thermosensitive hydrogel containing dexamethasone micelles for preventing postsurgical adhesion in a repeated-injury model. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Wang, Y.-L.; Qu, Y.; Liao, J.-F.; Chu, B.-Y.; Zhang, H.-P.; Luo, F.; Qian, Z.Y. Synthesis, characterization, and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci. Rep. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Yeo, Y.; Kohane, D.S. Polymers in the prevention of peritoneal adhesions. Eur. J. Pharm. Biopharm. 2008, 68, 57–66. [Google Scholar] [CrossRef]
- Choung, H.-K.; Hwang, J.-M. The Use of Surgi Wrap in Delayed Adjustable Strabismus Surgery. Am. J. Ophthalmol. 2005, 140, 433–436. [Google Scholar] [CrossRef]
- Park, H.B.; Lee, Y.M. Separation of toluene/nitrogen through segmented polyurethane and polyurethane urea membranes with different soft segments. J. Membr. Sci. 2002, 197, 283–296. [Google Scholar] [CrossRef]
- Isfahani, A.P.; Ghalei, B.; Bagheri, R.; Kinoshita, Y.; Kitagawa, H.; Sivaniah, E.; Sadeghi, M. Polyurethane gas separation membranes with ethereal bonds in the hard segments. J. Membr. Sci. 2016, 513, 58–66. [Google Scholar] [CrossRef]
- Abraham, G.A.; de Queiroz, A.A.A.; Román, J.S. Hydrophilic hybrid IPNs of segmented polyurethanes and copolymers of vinylpyrrolidone for applications in medicine. Biomaterials 2001, 22, 1971–1985. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Li, X.; Chen, W. Synthesis of vegetable oil-based waterborne polyurethane/silver-halloysite antibacterial nanocomposites. Compos. Sci. Technol. 2016, 126, 86–93. [Google Scholar] [CrossRef]
- Sun, W.; Jia, L.; Wang, Z.; Jia, Z.; Technology, O.S.A. Optical fiber sensor encapsulated by polyurethane. Opt. Int. J. Light Electron Opt. 2018, 165, 124–131. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Ugarte, L.; Gonzalez, K.; Martin, L.; Irusta, L.; Gonzalez, A.; Corcuera, M.A.; Eceiza, A. The role of cellulose nanocrystals incorporation route in waterborne polyurethane for preparation of electrospun nanocomposites mats. Carbohydr. Polym. 2017, 166, 146–155. [Google Scholar] [CrossRef]
- Lin, H.-H.; Hsieh, F.-Y.; Tseng, C.-S.; Hsu, S.-H. Preparation and characterization of a biodegradable polyurethane hydrogel and the hybrid gel with soy protein for 3D cell-laden bioprinting. J. Mater. Chem. B 2016, 4, 6694–6705. [Google Scholar] [CrossRef]
- Valério, A.; Conti, D.S.; Araújo, P.H.H.; Sayer, C.; da Rocha, S.R.P. Synthesis of PEG-PCL-based polyurethane nanoparticles by miniemulsion polymerization. Colloids Surf. B Biointerfaces 2015, 135, 35–41. [Google Scholar] [CrossRef]
- Shah, P.N.; Manthe, R.L.; Lopina, S.T.; Yun, Y.H. Electrospinning of l-tyrosine polyurethanes for potential biomedical applications. Polymer 2009, 50, 2281–2289. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Akins, R.E., Jr.; Parrag, I.C.; Woodhouse, K.A.; Rabolt, J.F. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials 2008, 29, 4783–4791. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Hung, S.-C.; Hsu, S.-H. The effect of elastic biodegradable polyurethane electrospun nanofibers on the differentiation of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2014, 122, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. 2003, 67B, 675–679. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Singh, B.N.; Panda, N.N.; Pramanik, K. A novel electrospinning approach to fabricate high strength aqueous silk fibroin nanofibers. Int. J. Biol. Macromol. 2016, 87, 201–207. [Google Scholar] [CrossRef]
- Coutinho, F.; Delpech, M.C.; Alves, L.S. Anionic waterborne polyurethane dispersions based on hydroxyl-terminated polybutadiene and poly(propylene glycol): Synthesis and characterization. J. Appl. Polym. Sci. 2001, 80, 566–572. [Google Scholar] [CrossRef]
- Yang, C.H.; Yang, H.J.; Wen, T.C.; Wu, M.S.; Chang, J.S. Mixture design approaches to IPDI-H6XDI-XDI ternary diisocyanate-based waterborne polyurethanes. Polymer 1999, 40, 871–885. [Google Scholar] [CrossRef]
- Buruaga, L.; Sardon, H.; Irusta, L.; Gonzalez, A.; Fernández-Berridi, M.J.; Iruin, J.J. Electrospinning of waterborne polyurethanes. J. Appl. Polym. Sci. 2010, 115, 1176–1179. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, X.; Yu, Q.; Liu, S.; Guo, D.; Yu, R.; Hu, J. Synthesis and characterization of low crystalline waterborne polyurethane for potential application in water-based ink binder. Prog. Org. Coat. 2014, 77, 61–71. [Google Scholar] [CrossRef]
- Dai, L.; Long, Z.; Ren, X.-H.; Deng, H.-B.; He, H.; Liu, W. Electrospun polyvinyl alcohol/waterborne polyurethane composite nanofibers involving cellulose nanofibers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Lih, E.; Oh, S.H.; Joung, Y.K.; Lee, J.H.; Han, D.K. Polymers for cell/tissue anti-adhesion. Prog. Polym. Sci. 2015, 44, 28–61. [Google Scholar] [CrossRef]
- Fairhurst, D.; Rowell, R.L.; Monahan, I.M.; Key, S.; Stieh, D.; McNeil-Watson, F.; Morfesis, A.; Mitchnick, M.; Shattock, R.A. Microbicides for HIV/AIDS. 2. Electrophoretic Fingerprinting of CD4+ T-Cell Model Systems. Langmuir 2007, 23, 2680–2687. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Hung, K.-C.; Hung, H.-S.; Hsu, S.-H. Modulation of Macrophage Phenotype by Biodegradable Polyurethane Nanoparticles: Possible Relation between Macrophage Polarization and Immune Response of Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19436–19448. [Google Scholar] [CrossRef] [PubMed]
- Yen, K.-C.; Chen, C.-Y.; Huang, J.-Y.; Kuo, W.-T.; Lin, F.-H. Fabrication of keratin/fibroin membranes by electrospinning for vascular tissue engineering. J. Mater. Chem. B 2016, 4, 237–244. [Google Scholar] [CrossRef]



| Groups | Fiber Diameter (nm) | Fiber Diameter after Immersed in PBS 24 h (nm) | Pore Size (µm) |
|---|---|---|---|
| W1P2 | 362.3 ± 81.4 | 339.3 ± 83.4 | 0.78 ± 0.31 * |
| W2P3 | 349.5 ± 63.3 | 324.5 ± 78.1 | 0.84 + 0.31 * |
| W1P1 | 369.3 ± 70.2 | 368.7 ± 56.3 | 0.95 ± 0.36 |
| W3P2 | 352.4 ± 131.5 | 372.1 ± 159.5 | 1.05 ± 0.44 |
| Membrane | Ultimate Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| W3P2BW | 1.00 ± 0.14 | 84.8 ± 22.7 | 0.14 ± 0.03 |
| W1P1BW | 0.82 ± 0.09 | 63.6 ± 16.2 | 0.13 ± 0.01 |
| W2P3BW | 0.82 ± 0.02 | 41.4 ± 14.8 | 0.11 ± 0.03 |
| W1P2BW | 0.79 ± 0.19 | 89.8 ± 25.2 | 0.13 ± 0.04 |
| W3P2 | 8.00 ± 1.25 *,#,‡ | 411.69 ± 85.0 *,# | 0.68 ± 0.17 * |
| W1P1 | 7.25 ± 0.91 *,§ | 432.4 ± 66.6 *,# | 0.64 ± 0.13 * |
| W2P3 | 8.58 ± 0.79 *#,‡ | 445.0 ± 68.8 *,# | 0.85 ± 0.30 *,‡ |
| W1P2 | 4.80 ± 1.58 *,§ | 236.5 ± 109.1 * | 0.92 ± 0.37 *,‡ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-H.; Chou, P.-Y.; Chen, Z.-Y.; Lin, F.-H. Electrospun Water-Borne Polyurethane Nanofibrous Membrane as a Barrier for Preventing Postoperative Peritendinous Adhesion. Int. J. Mol. Sci. 2019, 20, 1625. https://doi.org/10.3390/ijms20071625
Chen S-H, Chou P-Y, Chen Z-Y, Lin F-H. Electrospun Water-Borne Polyurethane Nanofibrous Membrane as a Barrier for Preventing Postoperative Peritendinous Adhesion. International Journal of Molecular Sciences. 2019; 20(7):1625. https://doi.org/10.3390/ijms20071625
Chicago/Turabian StyleChen, Shih-Heng, Pang-Yun Chou, Zhi-Yu Chen, and Feng-Huei Lin. 2019. "Electrospun Water-Borne Polyurethane Nanofibrous Membrane as a Barrier for Preventing Postoperative Peritendinous Adhesion" International Journal of Molecular Sciences 20, no. 7: 1625. https://doi.org/10.3390/ijms20071625



_周(Chou).jpg)


