Increased Expression of Cell Surface SSEA-1 is Closely Associated with Naïve-Like Conversion from Human Deciduous Teeth Dental Pulp Cells-Derived iPS Cells
Abstract
:1. Introduction
2. Results
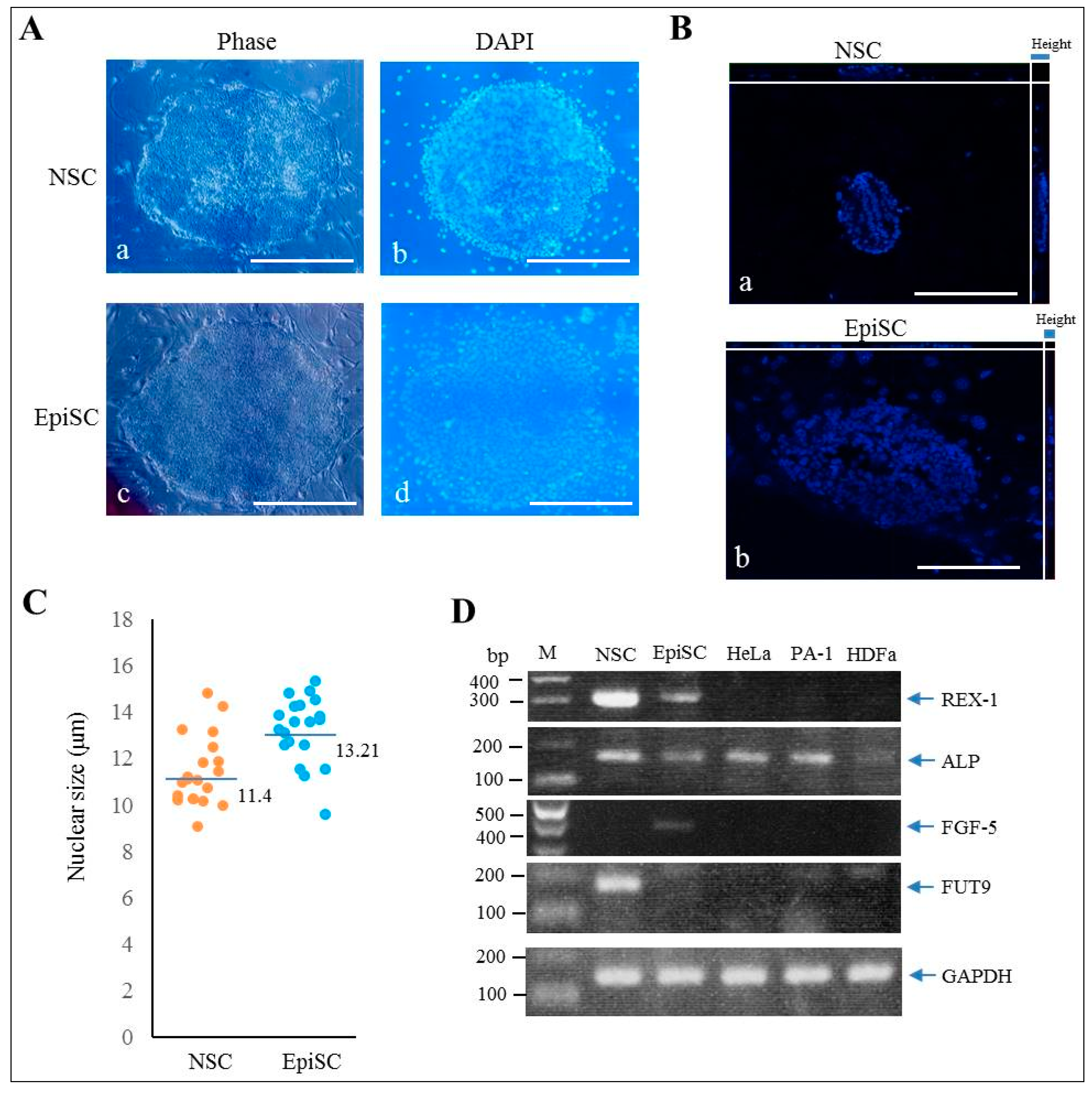
2.1. Generation of HDDPC-Derived Naïve iPSCs
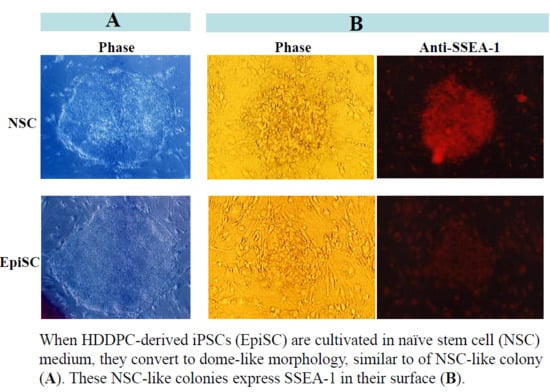
2.2. Characterization of NSC-Like Colonies
2.3. Immunocytochemical Staining of NSC-Like Colonies
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Generation and Maintenance of HDDPC-Derived iPSCs
4.3. Induction of NSC-Like iPSCs
4.4. In Vitro Differentiation
4.5. In Vivo Differentiation
4.6. Observation of Colony Morphology
4.7. RT-PCR Analysis
4.8. Immunocytochemical Staining Using Antibodies
4.9. Lectin Cytochemistry
4.10. Fluorescence Observation
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| iPSCs | induced pluripotent stem cells |
| ifhU-iPSCs | integration-free human urine induced pluripotent stem cells |
| EpiSCs | epiblast stem cells |
| ESCs | embryonic stem cells |
| NSCs | naïve stem cells |
| ROCK | Rho-associated coiled-coil forming kinase |
| FACS | fluorescence activated cell sorting |
| SSEA-1 | stage-specific embryonic antigen-1 |
| ECCs | embryonic carcinoma cells |
| FUT9 | alpha 1,3-fucosyltransferase IX gene |
| HDDPCs | human deciduous tooth dental pulp cells |
| MEF | mouse embryonic fibroblast |
| DAPI | 4′,6-diamidino-2-phenylindole |
| REX-1 | reduced expression protein-1 |
| ZEP42 | zinc finger protein 42 |
| EBs | embryoid bodies |
| ALP | alkaline phosphatase |
| FGF-5 | fibroblast growth factor-5 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| LIF | leukemia inhibitory factor |
| bFGF | basic fibroblast growth factor |
| TGFβ1 | transforming growth factor β1 |
| TECP2L1 | transcription factor CP2 like 1 |
| DPPA3 | developmental pluripotency associated 3 |
| ESRRB | estrogen related receptor β |
| TBX3 | T-box 3 |
| MMC | mitomycin C |
| PFA | paraformaldehyde |
| PBS | phosphate-buffered saline |
| H-E | hematoxylin and eosin |
| NGS | normal goat serum |
| AFP | α-fetoprotein |
| α-SMA | α-smooth muscle actin |
References
- Sunil, P.M. Induced pluripotent stem cells in dentistry. J. Pharm. Bioallied Sci. 2016, 8, S23–S27. [Google Scholar]
- Xie, H.; Dubey, N.; Shim, W.; Ramachandra, C.J.A.; Min, K.S.; Cao, T.; Rosa, V. Functional odontoblastic-like cells derived from human iPSCs. J. Dent. Res. 2018, 97, 77–83. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Y.; Liu, P.; Chen, S.; Wu, X.; Sun, Y.; Li, A.; Huang, K.; Luo, R.; Wang, L.; et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen. (Lond) 2013, 2, 6. [Google Scholar] [CrossRef]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Cheng, A.W.; Saha, K.; Kim, J.; Lengner, C.J.; Soldner, F.; Cassady, J.P.; Muffat, J.; Carey, B.W.; Jaenisch, R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA 2010, 107, 9222–9227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murayama, H.; Masaki, H.; Sato, H.; Hayama, T.; Yamaguchi, T.; Nakauchi, H. Successful reprogramming of epiblast stem cells by blocking nuclear localization of beta-catenin. Stem Cell Rep. 2015, 4, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Solter, D.; Knowles, B.B. Immunosurgery of mouse blastocyst. Proc. Natl. Acad. Sci. USA 1975, 72, 5099–5102. [Google Scholar] [CrossRef] [PubMed]
- Eguizabal, C.; Shovlin, T.C.; Durcova-Hills, G.; Surani, A.; McLaren, A. Generation of primordial germ cells from pluripotent stem cells. Differentiation 2009, 78, 116–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Johkura, K.; Yue, F.; Ogiwara, N.; Okouchi, Y.; Asanuma, K.; Sasaki, K. Spatial distribution and initial changes of SSEA-1 and other cell adhesion-related molecules on mouse embryonic stem cells before and during differentiation. J. Histochem. Cytochem. 2004, 52, 1447–1457. [Google Scholar] [CrossRef]
- Draper, J.S.; Pigott, C.; Thomson, J.A.; Andrews, P.W. Surface antigens of human embryonic stem cells: Changes upon differentiation in culture. J. Anat. 2002, 200, 249–258. [Google Scholar] [CrossRef]
- Pruszak, J.; Ludwig, W.; Blak, A.; Alavian, K.; Isacson, O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells 2009, 27, 2928–2940. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Lopez, D.; Castella, E.M.; Munoz, C.; Zujar, M.J.; Mate, J. Expression of CD15 in normal and metaplastic Paneth cells of the digestive tract. J. Clin. Pathol. 1996, 49, 474–477. [Google Scholar] [CrossRef]
- Trubiani, O.; Zalzal, S.F.; Paganelli, R.; Marchisio, M.; Giancola, R.; Pizzicannella, J.; Buhring, H.J.; Piattelli, M.; Caputi, S.; Nanci, A. Expression profile of the embryonic markers nanog, OCT-4, SSEA-1, SSEA-4, and frizzled-9 receptor in human periodontal ligament mesenchymal stem cells. J. Cell. Physiol. 2010, 225, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kaneko, M.; Iwasaki, H.; Togayachi, A.; Nishihara, S.; Abe, K.; Narimatsu, H. Normal embryonic and germ cell development in mice lacking alpha 1,3-fucosyltransferase IX (Fut9) which show disappearance of stage-specific embryonic antigen 1. Mol. Cell. Biol. 2004, 24, 4221–4228. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Ojima, T.; Shibata, E.; Saito, S.; Toyoda, M.; Nakajima, H.; Yamazaki-Inoue, M.; Miyagawa, Y.; Kiyokawa, N.; Fujimoto, J.; Sato, T.; et al. Glycolipid dynamics in generation and differentiation of induced pluripotent stem cells. Sci. Rep. 2015, 5, 14988. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Allegrucci, C.; Alberio, R. Modulation of pluripotency in the porcine embryo and iPS cells. PLoS ONE 2012, 7, e49079. [Google Scholar] [CrossRef]
- Saitoh, I.; Inada, E.; Iwase, Y.; Noguchi, H.; Murakami, T.; Soda, M.; Kubota, N.; Hasegawa, H.; Akasaka, E.; Matsumoto, Y.; et al. Choice of feeders is important when first establishing iPSCs derived from primarily cultured human deciduous tooth dental pulp cells. Cell Med. 2015, 8, 9–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, C.; Zhang, P.; Li, X.; Liu, T.; Pu, Y.; Li, Y.; Cao, Z.; Cao, H.; Liu, Y.; et al. Efficient reprogramming of naive-like induced pluripotent stem cells from porcine adipose-derived stem cells with a feeder-independent and serum-free system. PLoS ONE 2014, 9, e85089. [Google Scholar] [CrossRef]
- Ginsburg, M.; Snow, M.H.; McLaren, A. Primordial germ cells in the mouse embryo during gastrulation. Development 1990, 110, 521–528. [Google Scholar]
- Brons, I.G.; Smithers, L.E.; Trotter, M.W.; Rugg-Gunn, P.; Sun, B.; Chuva de Sousa Lopes, S.M.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007, 448, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Yang, J.; Nichols, J.; Hall, J.S.; Eyres, I.; Mansfield, W.; Smith, A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 2009, 136, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Kumari, D. States of pluripotency: Naïve and primed pluripotent stem cells. In Pluripotent Stem Cells; InTechOpen: Rijeka, Croatia, 2016; pp. 31–45. [Google Scholar]
- Sato, M.; Saitoh, I.; Murakami, T.; Kubota, N.; Nakamura, S.; Watanabe, S.; Inada, E. Intrapancreatic parenchymal injection of cells as a useful tool for allowing a small number of proliferative cells to grow in vivo. Int. J. Mol. Sci. 2017, 18, 1678. [Google Scholar] [CrossRef] [PubMed]
- Solter, D.; Knowles, B.B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 1978, 75, 5565–5569. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.B.; Aden, D.P.; Solter, D. Monoclonal antibody detecting a stage-specific embryonic antigen (SSEA-1) on preimplantation mouse embryos and teratocarcinoma cells. Curr. Top. Microbiol. Immunol. 1978, 81, 51–53. [Google Scholar]
- Tateno, H.; Matsushima, A.; Hiemori, K.; Onuma, Y.; Ito, Y.; Hasehira, K.; Nishimura, K.; Ohtaka, M.; Takayasu, S.; Nakanishi, M.; et al. Podocalyxin is a glycoprotein ligand of the human pluripotent stem cell-specific probe rBC2LCN. Stem Cells Transl. Med. 2013, 2, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hasehira, K.; Tateno, H.; Onuma, Y.; Ito, Y.; Asashima, M.; Hirabayashi, J. Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol. Cell. Proteom. 2012, 11, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, N.; Furukawa, J.; Araki, K.; Fujioka, T.; Takegawa, Y.; Piao, J.; Nishioka, T.; Tamura, T.; Nikaido, T.; Ito, M.; et al. Total cellular glycomics allows characterizing cells and streamlining the discovery process for cellular biomarkers. Proc. Natl. Acad. Sci. USA 2013, 110, 2105–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwazaki, H.; Kakizaki, M.; Ikehara, Y.; Togayachi, A.; Narimatsu, H.; Watanabe, R. Mice lacking alpha1,3-fucosyltransferase 9 exhibit modulation of in vivo immune responses against pathogens. Pathol. Int. 2014, 64, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kilens, S.; Meistermann, D.; Moreno, D.; Chariau, C.; Gaignerie, A.; Reignier, A.; Lelievre, Y.; Casanova, M.; Vallot, C.; Nedellec, S.; et al. Parallel derivation of isogenic human primed and naive induced pluripotent stem cells. Nat. Commun. 2018, 9, 360. [Google Scholar] [CrossRef] [Green Version]
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Genz, K.; Shi, L.; et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 2014, 15, 471–487. [Google Scholar] [CrossRef]
- Takashima, Y.; Guo, G.; Loos, R.; Nichols, J.; Ficz, G.; Kueger, F.; Oxley, D.; Santos, F.; Clarke, J.; Mansfield, W.; et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 2014, 158, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Kubota, N.; Soda, M.; Matsueda, K.; Murakami, T.; Sawami, T.; Kagoshima, A.; Yamasaki, Y.; Sato, M. Alkaline phosphatase and OCT-3/4 as useful markers for predicting susceptibility of human deciduous teeth-derived dental pulp cells to reprogramming factor-induced iPS cells. J. Investig. Clin. Dent. 2017, 8, e12236. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Tsukiyama, T.; Kimura, K.; Matsuyama, S.; Minami, N.; Yamada, M.; Imai, H. Generation of naive bovine induced pluripotent stem cells using piggyBac transposition of doxycycline-inducible transcription factors. PLoS ONE 2015, 10, e0135403. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castro, I.L.; Garcia-Lopez, G.; Avila-Gonzalez, D.; Flores-Herrera, H.; Molina-Hernandez, A.; Portillo, W.; Ramon-Gallegos, E.; Diaz, N.F. Markers of pluripotency in human amniotic epithelial cells and their differentiation to progenitor of cortical neurons. PLoS ONE 2015, 10, e0146082. [Google Scholar] [CrossRef]
- Ye, S.; Li, P.; Tong, C.; Ying, Q.L. Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. EMBO J. 2013, 32, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, T.; Cai, Y.; Wang, H. Preserving self-renewal of porcine pluripotent stem cells in serum-free 3i culture condition and independent of LIF and b-FGF cytokines. Cell Death Discov. 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Inada, E.; Saitoh, I.; Watanabe, S.; Aoki, R.; Miura, H.; Ohtsuka, M.; Murakami, T.; Sawami, T.; Yamasaki, Y.; Sato, M.; et al. PiggyBac transposon-mediated gene delivery efficiently generates stable transfectants derived from cultured primary human deciduous tooth dental pulp cells (HDDPCs) and HDDPC-derived iPS cells. Int. J. Oral Sci. 2015, 7, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Toriumi, T.; Takayama, N.; Murakami, M.; Sato, M.; Yuguchi, M.; Yamazaki, Y.; Eto, K.; Otsu, M.; Nakauchi, H.; Shirasawa, T.; et al. Characterization of mesenchymal progenitor cells in the crown and root pulp of primary teeth. Biomed. Res. 2015, 36, 31–45. [Google Scholar] [CrossRef] [Green Version]


| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Size (bp) | Reference |
|---|---|---|---|---|
| REX-1 | CAGATCCTAAACAGCTCGCAGAAT | GCGTACGCAAATTAAAGTCCAGA | 306 | [41] |
| ALP | TGGCCCCCATGCTGAGTGACAC | TGGCGCAGGGGCACAGCAGAC | 160 | [35] |
| FGF-5 | ATCCCACGAAGCCAATATGT | GCAGAAAGGGGAATCTTTGAC | 420 | GenBank M37825.1 |
| FUT9 | TCTACGTGCTTTCCATGATAT | CAGAGCTGGCTGATTCCATTG | 180 | NCBI NM_006581 |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA | 138 | [41] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inada, E.; Saitoh, I.; Kubota, N.; Iwase, Y.; Murakami, T.; Sawami, T.; Yamasaki, Y.; Sato, M. Increased Expression of Cell Surface SSEA-1 is Closely Associated with Naïve-Like Conversion from Human Deciduous Teeth Dental Pulp Cells-Derived iPS Cells. Int. J. Mol. Sci. 2019, 20, 1651. https://doi.org/10.3390/ijms20071651
Inada E, Saitoh I, Kubota N, Iwase Y, Murakami T, Sawami T, Yamasaki Y, Sato M. Increased Expression of Cell Surface SSEA-1 is Closely Associated with Naïve-Like Conversion from Human Deciduous Teeth Dental Pulp Cells-Derived iPS Cells. International Journal of Molecular Sciences. 2019; 20(7):1651. https://doi.org/10.3390/ijms20071651
Chicago/Turabian StyleInada, Emi, Issei Saitoh, Naoko Kubota, Yoko Iwase, Tomoya Murakami, Tadashi Sawami, Youichi Yamasaki, and Masahiro Sato. 2019. "Increased Expression of Cell Surface SSEA-1 is Closely Associated with Naïve-Like Conversion from Human Deciduous Teeth Dental Pulp Cells-Derived iPS Cells" International Journal of Molecular Sciences 20, no. 7: 1651. https://doi.org/10.3390/ijms20071651