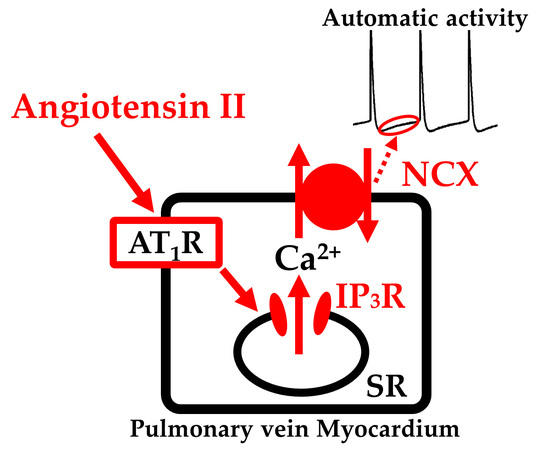
Angiotensin II Induces Automatic Activity of the Isolated Guinea Pig Pulmonary Vein Myocardium through Activation of the IP3 Receptor and the Na+-Ca2+ Exchanger
Abstract
:1. Introduction
2. Results
2.1. Induction of Automatic Contractile Activity by Angiotensin II
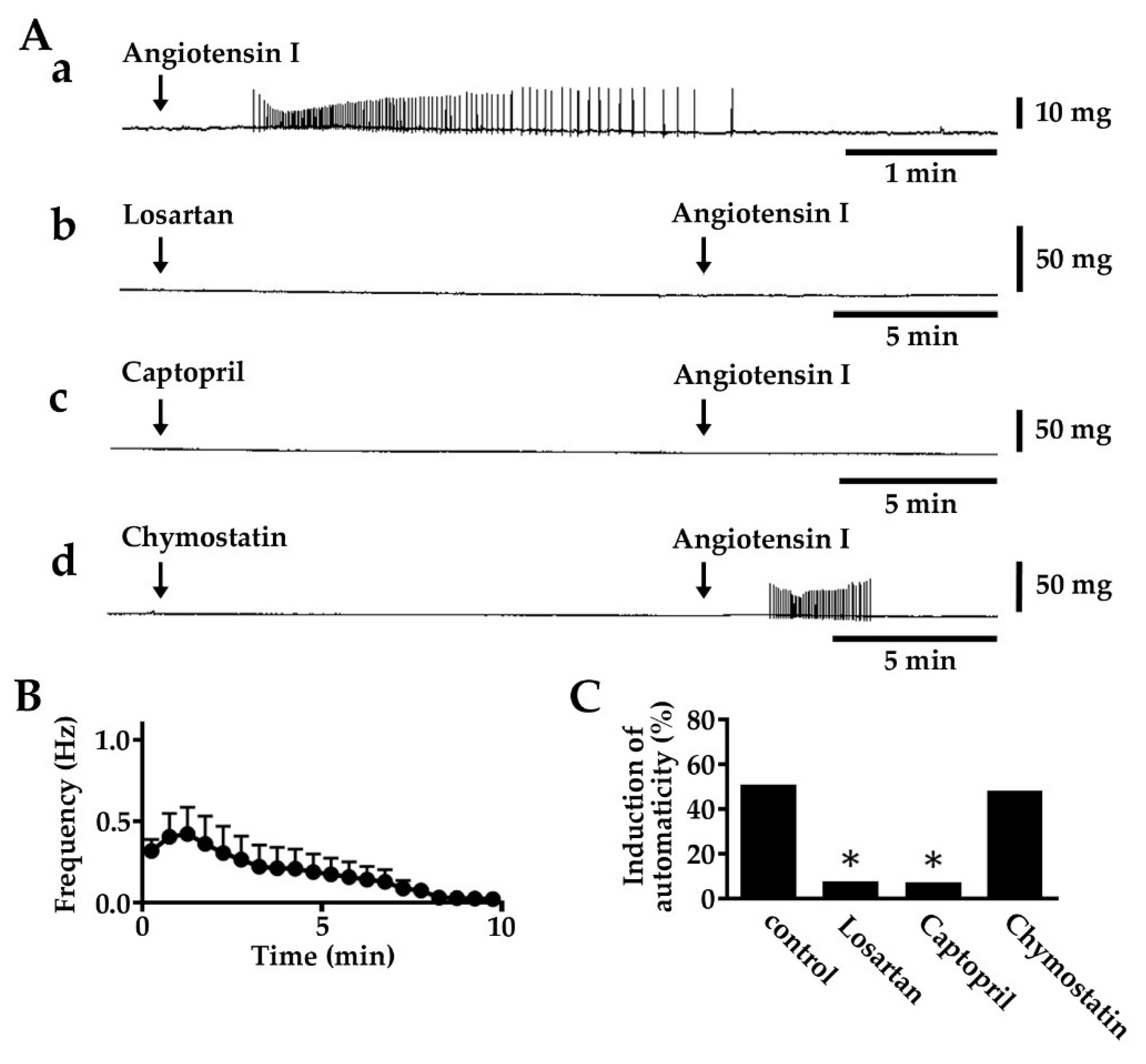
2.2. Induction of Automatic Contractile Activity by Angiotensin I
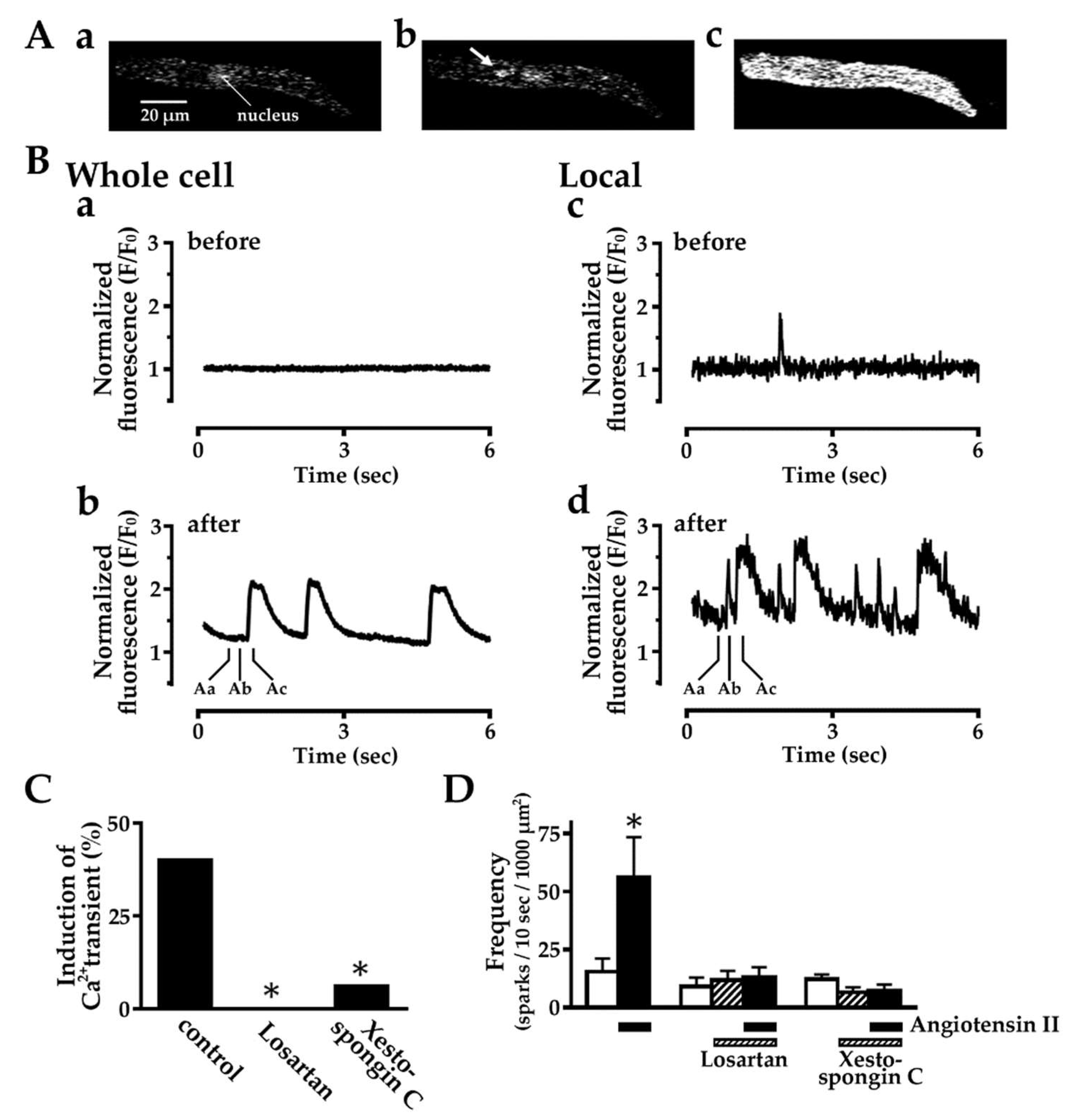
2.3. Effect of Angiotensin II on Intracellular Ca2+ Dynamics
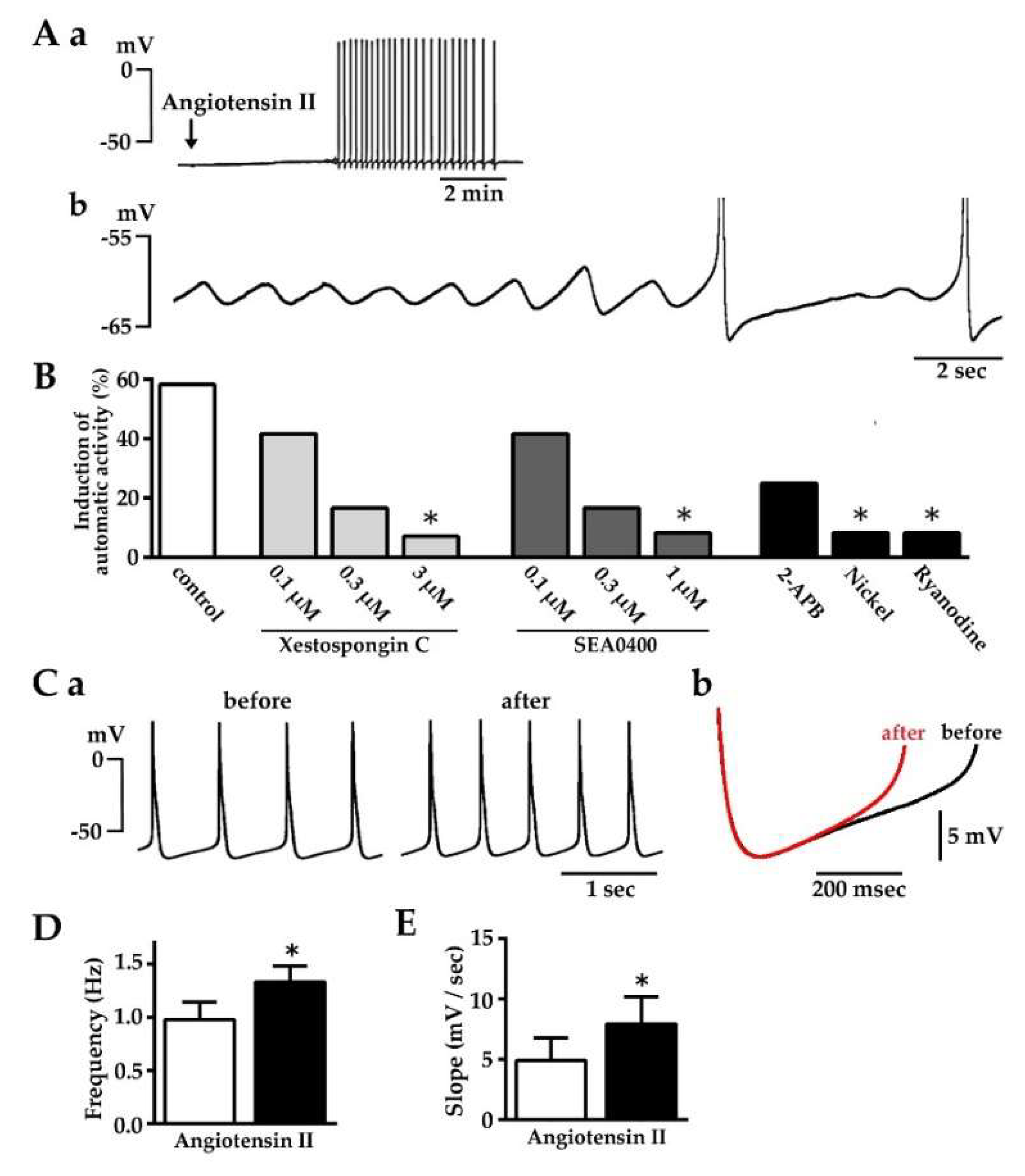
2.4. Induction of Automatic Action Potential Firing and Diastolic Depolarization by Angiotensin II
2.5. Enhancement of Diastolic Depolarization by Angiotensin II under Constant Firing Frequency
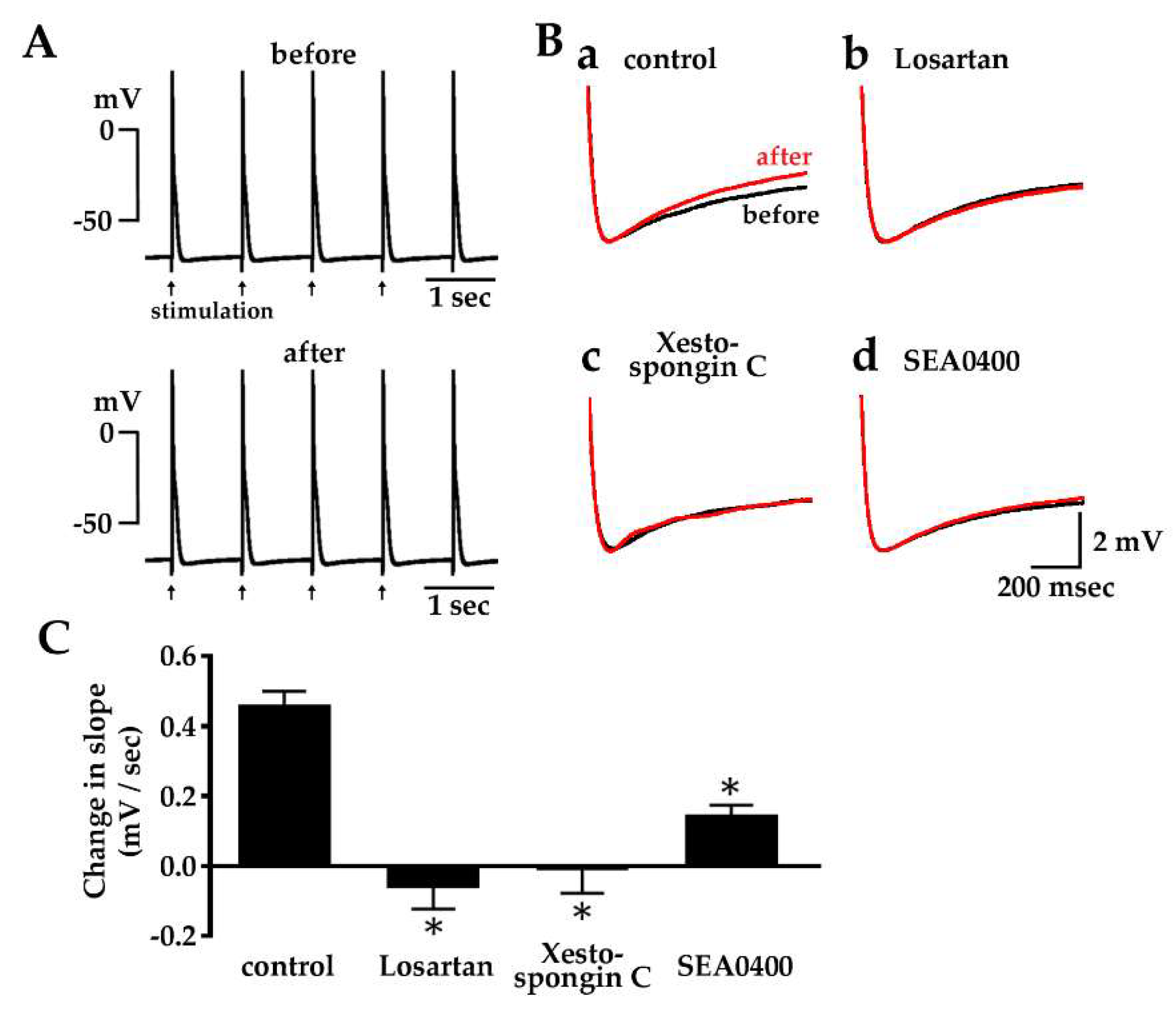
2.6. Effect of Losartan, Xestospongin C and SEA0400 on the Spontaneous Automatic Action Potentials
3. Discussion
4. Materials and Methods
4.1. General
4.2. Preparation of Guinea Pig Pulmonary Vein
4.3. Contractile Force Measurement
4.4. Action Potential Measurement
4.5. Isolation of Pulmonary Vein Cardiomyocytes and Confocal Microscopy
4.6. Chemicals
4.7. Data Analysis and Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Brunton, L.; Fayrer, J. Note on the independent pulsatio of the pulmonary veins and vena cava. Proc. R. Soc. Lond. 1976, 25, 174–176. [Google Scholar]
- Tasaki, H. Electrophysiological study of the striated muscle cells of the extrapulmonary vein of the guinea-pig. Jpn. Circ. J. 1969, 33, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.W. Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J. Physiol. 1981, 314, 445–456. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Chen, S.A.; Hsieh, M.H.; Tai, C.T.; Tsai, C.F.; Prakash, V.S.; Yu, W.C.; Hsu, T.L.; Ding, Y.A.; Chang, M.S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins. Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999, 100, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S. Basic electrophysiology of the pulmonary veins and their role in atrial fibrillation: Precipitators, perpetuaters, and perplexers. J. Cardiovas. Electrophysiol. 2003, 14, 1372–1375. [Google Scholar] [CrossRef]
- Namekata, I.; Tsuneoka, Y.; Tanaka, H. Electrophysiological and pharmacological properties of the pulmonary vein myocardium. Biol. Pharm. Bull. 2013, 36, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Takahara, A.; Hagiwara, M.; Namekata, I.; Tanaka, H. Pulmonary vein myocardium as a possible pharmacological target for the treatment of atrial fibrillation. J. Pharmacol. Sci. 2014, 126, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, S.A.; Chang, M.S.; Lin, C.I. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: Implication for the genesis of atrial fibrillation. Cardiovasc. Res. 2000, 48, 265–273. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, S.A.; Chen, Y.J.; Chang, M.S.; Chan, P.; Lin, C.I. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J. Am. Coll. Cardiol. 2002, 39, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, Y.; Fishbein, M.C.; Karagueuzian, H.S. Electrical current-induced atrial and pulmonary vein action potential duration shortening and repetitive activity. Am. J. Physiol. Heart. Circ. Physiol. 2004, 287, H178–H186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuneoka, Y.; Kobayashi, Y.; Honda, Y.; Namekata, I.; Tanaka, H. Electrical activity of the mouse pulmonary vein myocardium. J. Pharmacol. Sci. 2012, 119, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Tsuneoka, Y.; Irie, M.; Tanaka, Y.; Sugimoto, T.; Kobayashi, Y.; Kusakabe, T.; Kato, K.; Hamaguchi, S.; Namekata, I.; Tanaka, H. Permissive role of reduced inwardly-rectifying potassium current density in the automaticity of the guinea pig pulmonary vein myocardium. J. Pharmacol. Sci. 2017, 133, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Cha, T.J.; Zhang, L.; Chartier, D.; Melnyk, P.; Hohnloser, S.H.; Nattel, S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: Action potential and ionic current properties. J. Physiol. 2003, 551, 801–813. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, S.A.; Chen, Y.C.; Yeh, H.I.; Chang, M.S.; Lin, C.I. Electrophysiology of single cardiomyocytes isolated from rabbit pulmonary veins: Implication in initiation of focal atrial fibrillation. Basic. Res. Cardiol. 2002, 97, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Honjo, H.; Boyett, M.R.; Niwa, R.; Inada, S.; Yamamoto, M.; Mitsui, K.; Horiuchi, T.; Shibata, N.; Kamiya, K.; Kodama, I. Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation 2003, 107, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Namekata, I.; Tsuneoka, Y.; Takahara, A.; Shimada, H.; Sugimoto, T.; Takeda, K.; Nagaharu, M.; Shigenobu, K.; Kawanishi, T.; Tanaka, H. Involvement of the Na+/Ca2+ exchanger in the automaticity of guinea-pig pulmonary vein myocardium as revealed by SEA0400. J. Pharmacol. Sci. 2009, 110, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Takano, M.; Ohba, T.; Ono, K. Arrhythmogenic coupling between the Na+-Ca2+ exchanger and inositol 1,4,5-triphosphate receptor in rat pulmonary vein cardiomyocytes. J. Mol. Cell. Cardiol. 2012, 52, 988–997. [Google Scholar] [CrossRef]
- Barajas-Martinez, H.; Goodrow, R.J.; Hu, D.; Patel, P.; Desai, M.; Panama, B.K.; Treat, J.A.; Aistrup, G.L.; Cordeiro, J.M. Biophysical and molecular comparison of sodium current in cells isolated from canine atria and pulmonary vein. Pflugers Arch. 2017, 469, 703–712. [Google Scholar] [CrossRef]
- Chang, S.L.; Chen, Y.C.; Yeh, Y.H.; Lin, Y.K.; Wu, T.J.; Lin, C.I.; Chen, S.A.; Chen, Y.J. Heart failure enhanced pulmonary vein arrhythmogenesis and dysregulated sodium and calcium homeostasis with increased calcium sparks. J. Cardiovasc. Electrophysiol. 2011, 22, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kawamura, K.; Kawamura, K.; Ono, K. Pathological impact of hyperpolarization-activated chloride current peculiar to rat pulmonary vein cardiomyocytes. J. Mol. Cell. Cardiol. 2014, 66, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, S.; Hikita, K.; Tanaka, Y.; Tsuneoka, Y.; Namekata, I.; Tanaka, H. Enhancement of automaticity by mechanical stretch of the isolated guinea pig pulmonary vein myocardium. Biol. Pharm. Bull. 2016, 39, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belenger, A.J.; Wolf, P.A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhan, Y.; Izumi, Y.; Yasumoto, H.; Yano, M.; Iwao, H. In vivo activation of rat aortic platelet-derived growth factor and epidermal growth factor receptors by angiotensin II and hypertension. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Hunyady, L.; Catt, K.J. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006, 20, 953–970. [Google Scholar] [CrossRef]
- Nakashima, H.; Kumagai, K.; Urata, H.; Gondo, N.; Ideishi, M.; Arakawa, K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation 2000, 101, 2612–2617. [Google Scholar] [CrossRef]
- Kumagai, K.; Nakashima, H.; Urata, H.; Gondo, N.; Arakawa, K.; Saku, K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J. Am. Coll. Cardiol. 2003, 41, 2197–2204. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, H.; Kumagai, K. Reverse-remodeling effects of angiotensin II type 1 receptor blocker in a canine atrial fibrillation model. Circ. J. 2007, 71, 1977–1982. [Google Scholar] [CrossRef]
- Novo, G.; Guttilla, D.; Fazio, G.; Cooper, D.; Novo, S. The role of the renin-angiotensin system in atrial fibrillation and the therapeutic effects of ACE-Is and ARBS. Br. J Clin. Pharmacol. 2008, 66, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.J.; Zang, Y.L.; Chen, Y.L.; Geng, N.; Ma, S.M.; Li, X.D. Renin-angiotensin system inhibitors for prevention of recurrent atrial fibrillation: A meta-analysis. Int. J. Clin. Pract. 2013, 67, 536–543. [Google Scholar] [CrossRef]
- Schneider, M.P.; Hua, T.A.; Böhm, M.; Wachtell, K.; Kjeldsen, S.E.; Schmieder, R.E. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Chaugai, S.; Meng, W.Y.; Sepehry, A.A. Effects of RAAS blockers on atrial fibrillation prophylaxis: An updated systematic review and meta-analysis of randomized controlled trials. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Hypertension and atrial fibillation. Doubts and certainties from basic and clinical studies. Circ. Res. 2018, 122, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Holubarsch, C.; Hasenfuss, G.; Schmidt-Schweda, S.; Knorr, A.; Pieske, B.; Ruf, T.; Fasol, R.; Just, H. Angiotensin I and II exert inotropic effects in atrial but not in ventricular human myocardium. An in vitro study under physiological experimental conditions. Circulation 1993, 88, 1228–1237. [Google Scholar] [CrossRef]
- Sekine, T.; Kusano, H.; Nishimaru, K.; Tanaka, Y.; Tanaka, H.; Shigenobu, K. Developmental conversion of inotropism by endothelin I from positive to negative in mice. Eur. J. Pharmacol. 1999, 374, 411–415. [Google Scholar] [CrossRef]
- Endoh, M. Signal transduction and Ca2+ signalling in intact myocardium. J. Pharmacol. Sci. 2006, 100, 525–537. [Google Scholar] [CrossRef]
- Kashihara, T.; Nakada, T.; Kojima, K.; Takeshita, T.; Yamada, M. Angiotensin II activates Cav1.2 Ca2+ channels through β-arrestin2 and casein kinase 2 in mouse immature cardiomyocytes. J. Physiol. 2017, 595, 4207–4225. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Hohnloser, S.H.; Nattel, S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: Clinical and experimental evidence. Eur. Heart J. 2006, 27, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.S.; Cohen, N.M.; Dhallan, R.S.; Gaa, S.T.; Lederer, W.J.; Rogers, T.B. Angiotensin II increases spontaneous contractile frequency and stimulates calcium current in cultured neonatal rat heart myocytes: Insights into the underlying biochemical mechanisms. Circ. Res. 1988, 62, 524–534. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.C.; Tai, C.T.; Yeh, H.I.; Lin, C.I.; Chen, S.A. Angiotensin II and angiotensin II receptor blocker modulate the arrhythmogenic activity of pulmonary veins. Br. J. Pharmacol. 2006, 147, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Felode, E.; Vigne, P.; Frelin, C. Angiotensin AT1 receptors mediate a positive inotropic effect of angiotensin II in guinea pig atria. Eur. J. Pharmacol. 1993, 245, 63–66. [Google Scholar] [CrossRef]
- Monopoli, A.; Forlani, A.; Bevilacqua, M.; Vago, T.; Norbiato, G.; Bertora, P.; Biglioli, P.; Alamanni, F.; Ongini, E. Interaction of selected vasodilating β-blockers with adrenergic receptors in human cardiovascular tissues. J. Cardiovas. Res. 1989, 14, 114–120. [Google Scholar] [CrossRef]
- Herman, A.G. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am. J. Cardiol. 1992, 70, 102C–108C. [Google Scholar] [CrossRef]
- Prosser, H.C.G.; Forster, M.E.; Richards, A.M.; Pemberton, C.J. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: Effects of PA12 upon cardiac hemodynamics. Cardiovas. Res. 2009, 82, 40–50. [Google Scholar] [CrossRef]
- Miyamoto, S.; Izumi, M.; Hori, M.; Kobayashi, M.; Ozaki, H.; Karaki, H. Xestospongin C, a selective and mambrane-permeable inhibitor of IP3 receptor, attenuates the positive inotropic effect of α-adrenergic stimulation in guinea-pig papillary muscle. Brit. J. Pharmacol. 2000, 130, 650–654. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishimaru, K.; Aikawa, T.; Hirayama, W.; Tanaka, Y.; Shigenobu, K. Effect of SEA0400, a novel inhibitor of sodium-calcium exchanger, on myocardial currents. Br. J. Pharmacol. 2002, 135, 1096–1100. [Google Scholar] [CrossRef]
- Mackenzie, L.; Bootman, M.D.; Laine, M.; Berridge, M.J.; Thuring, J.; Holmes, A.; Li, W.H.; Lipp, P. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J. Physiol. 2002, 541, 395–409. [Google Scholar] [CrossRef]
- Kimura, J.; Miyamae, S.; Noma, A. Indentification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J. Physiol. 1987, 384, 199–222. [Google Scholar] [CrossRef]
- Zucchi, R.; Ronca-Testoni, S. The sarcoplasmic Ca2+ channel/ryanodine receptor: Modulation by endogenous effectors, drugs and disease states. Pharmacol. Rev. 1997, 49, 1–51. [Google Scholar]
- Ferguson, S.S. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev. 2001, 53, 1–24. [Google Scholar]
- Guo, D.F.; Sun, Y.L.; Hamet, P.; Inagami, T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001, 11, 165–180. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.C.; Zhu, Y.Z.; Lu, N.; Wang, M.J.; Wang, Y.X.; Yao, T. Role of angiotensin AT1 and AT2 receptors in cardiac hypertrophy and cardiac remodelling. Clin. Exp. Pharmacol. Physiol. 2003, 30, 911–918. [Google Scholar] [CrossRef]
- Brasch, H.; Sieroslawski, L.; Dominiak, P. Angiotensin II increases norepinephrine release from atria by acting on angiotensin subtype I receptors. Hypertension 1993, 22, 699–704. [Google Scholar] [CrossRef]
- Suzuki, H.; Maehara, K.; Yaoita, H.; Maruyama, Y. Altered effects of angiotensin II type 1 and type 2 receptor blockers on cardiac norepinephrine release and inotropic responses during cardiac sympathetic nerve stimulation in aorto-caval shunt rats. Circ. J. 2004, 68, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, A.L.; Ackerly, J.A.; Peach, M.J. Differentiation of neurogenic and myocardial angiotensin II receptors in isolated rabbit atria. Circ. Res. 1975, 36, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.; Schemann, M.; Sann, H.; Kummer, W. Innervation pattern of guinea pig pulmonary vasculature depends on vascular diameter. J. Appl. Physiol. 1997, 82, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.Y.; Chen, P.S.; Chen, L.S.; Fishbein, M.C. Autonomic nerves in pulmonary veins. Heart Rhythm 2007, 4, S57–S60. [Google Scholar] [CrossRef] [PubMed]
- Maupoil, V.; Bronquard, C.; Freslon, J.L.; Cosnay, P.; Findlay, I. Ectopic activity in the rat pulmonary vein can arise from simultaneous activation of alpha1- and beta1-adrenoceptors. Br. J. Pharmacol. 2007, 150, 899–905. [Google Scholar] [CrossRef]
- Irie, M.; Tsuneoka, Y.; Shimobayashi, M.; Hasegawa, N.; Tanaka, Y.; Mochizuki, S.; Ichige, S.; Hamaguchi, S.; Namekata, I.; Tanaka, H. Involvement of alpha- and beta-adrenoceptors in the automaticity of the isolated guinea pig pulmonary vein myocardium. J. Pharmacol. Sci. 2017, 133, 247–253. [Google Scholar] [CrossRef]
- Erdös, E.G. Conversion of angiotensin I to angiotensin II. Am. J. Med. 1976, 60, 749–759. [Google Scholar] [CrossRef]
- Goutsouliak, V.; Rabkin, S.W. Comparison of angiotensin II type-1 and type-2 receptor antagonists on angiotensin II-induced IP3 generation in cardiomyocytes. Gen. Pharmacol. 1998, 30, 367–372. [Google Scholar] [CrossRef]
- Sedan, O.; Dolnikov, K.; Zeevi-Levin, N.; Fleishmann, N.; Spiegel, I.; Berdichevski, S.; Amit, M.; Itskovitz-Eldor, J.; Binah, O. Human embryonic stem cell-derived cardiomyocytes can mobilize 1,4,5-inositol trisphosphate-operated [Ca2+]i stores: The functionality of angiotensin-II/endothelin-1 signaling pathways. Ann. N. Y. Acad. Sci. 2010, 1188, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Bodi, I.; Maillet, M.; DeSantiago, J.; Domeier, T.L.; Mikoshiba, K.; Lorenz, J.N.; Blatter, L.A.; Bers, D.M.; Molkentin, J.D. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ. Res. 2010, 107, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.K.; Woodcock, E.A.; Allen, D.G.; Cannell, M.B. Inositol 1,4,5-trisphosphate receptors and pacemaker rhythms. J. Mol. Cell. Cardiol. 2012, 53, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Logantha, S.J.; Cruickshank, S.F.; Rowan, E.G.; Drummond, R.M. Spontaneous and electrically evoked Ca2+ transients in cardiomyocytes of the rat pulmonary vein. Cell Calcium. 2010, 48, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J.; Xu, Y.; Slayter, H.S.; Izumo, S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 1993, 75, 977–984. [Google Scholar] [CrossRef]
- Disertori, M.; Latini, R.; Barlera, S.; Franzosi, M.G.; Staszewsky, L.; Maggioni, A.P.; Lucci, D.; Di Pasquale, G.; Tognoni, G. Valsartan for prevention of recurrent atrial fibrillation. GISSI-AF Investigators. N. Engl. J. Med. 2009, 360, 1606–1617. [Google Scholar] [PubMed]
- Hamaguchi, S.; Tsuneoka, Y.; Tanaka, A.; Irie, M.; Tsuruta, M.; Nakayama, T.; Namekata, I.; Nada, M.; Aimoto, M.; Takahara, A.; et al. Manifestation of automaticity in the pulmonary-vein myocardium of rats with abdominal aorto-venocaval shunt. J. Pharmacol. Sci. 2015, 128, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.M.; Luk, H.N.; Sheu, J.R.; Wu, H.P.; Chiang, C.E. Inducibility of abnormal automaticity and triggered activity in myocardial sleeves of canine pulmonary veins. Int. J. Cardiol. 2005, 104, 59–66. [Google Scholar] [CrossRef]
- Udyavar, A.R.; Chen, Y.C.; Chen, Y.J.; Cheng, C.C.; Lin, C.I.; Chen, S.A. Endothelin-1 modulates the arrhythmogenic activity of pulmonary veins. J. Cardiovasc. Electrophysiol. 2008, 19, 285–292. [Google Scholar] [CrossRef]
- Lin, Y.K.; Lu, Y.Y.; Chen, Y.C.; Chen, Y.J.; Chen, S.A. Nitroprusside modulates pulmonary vein arrhythmogenic activity. J. Biomed. Sci. 2010, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, H.; Yasuda, N.; Komuro, I. Mechanisms and functions of agonist-independent activation in the angiotensin II type 1 receptor. Mol. Cell. Endocrinol. 2009, 302, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Drexler, H. The renin-angiotensin system and experimental heart failure. Cardiovasc. Res. 1999, 43, 838–849. [Google Scholar] [CrossRef]
- Goette, A.; Arndt, M.; Röcken, C.; Spiess, A.; Staack, T.; Geller, J.C.; Huth, C.; Ansorge, S.; Klein, H.U.; Lendeckel, U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 2000, 13, 2678–2681. [Google Scholar] [CrossRef]
- De la Cruz, G.G.; Groschner, K.; Kappe, C.O.; Glasnov, T.N. High-speed microwave assisted synthesis of SEA0400, a selective inhibitor of the Na+/Ca2+ exchanger. Tetrahedron Lett. 2012, 53, 3731–3734. [Google Scholar] [CrossRef]


| Induction of Automatic Activity | Parameters of Induced Automatic Activity | ||||
|---|---|---|---|---|---|
| Latency (sec) | Duration (min) | Maximum Frequency (Hz) | n | ||
| Angiotensin II (100 nM) | 4/15 (26.7%) | 66.4 ± 23.1 | 4.0 ± 1.6 | 0.4 ± 0.3 | 4 |
| Angiotensin II (1 μM) | 11/17 (64.7%) | 50.4 ± 7.7 | 4.4 ± 1.1 | 0.8 ± 0.2 | 11 |
| Losartan + Angiotensin II (1 μM) | 0/10 (0%) | - | - | - | 0 |
| PD123,319 + Angiotensin II (1 μM) | 6/13 (46.2%) | 59.6 ± 17.8 | 3.5 ± 0.4 | 0.6 ± 0.1 | 6 |
| Carvedilol + Angiotensin II (1 μM) | 6/10 (60.0%) | 33.1 ± 7.9 | 6.3 ± 1.0 | 0.6 ± 0.1 | 6 |
| Induction of Automatic Activity | Parameters of Induced Automatic Activity | ||||
|---|---|---|---|---|---|
| Latency (sec) | Duration (min) | Maximum Frequency (Hz) | n | ||
| Angiotensin I | 10/20 (50.0%) | 47.8 ± 9.2 | 9.2 ± 3.1 | 0.4 ± 0.2 | 10 |
| Losartan + Angiotensin I | 1/15 (6.7%) | 7.6 | 6.1 | 1.3 | 1 |
| Captopril + Angiotensin I | 1/16 (6.3%) | 79.6 | 2.7 | 0.3 | 1 |
| Chymostatin + Angiotensin I | 9/19 (47.4%) | 62.2 ± 16.1 | 2.6 ± 0.2 | 0.4 ± 0.1 | 9 |
| Angiotensin II-Induced Activity (n = 7) | Spontaneous Activity (n = 6) | Stimulation-Induced Activity (n = 6) | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Frequency (Hz) | 0.51 ± 0.14 | 0.98 ± 0.02 | 1.33 ± 0.11 * | - | - |
| MDP (mV) | −71.0 ± 1.0 | −70.4 ± 2.4 | −71.5 ± 2.0 | −75.6 ± 1.5 | −74.7 ± 1.2 |
| Slope (mV/sec) | 2.70 ± 0.40 | 4.90 ± 1.79 | 7.94 ± 2.12 * | 2.66 ± 0.37 | 3.12 ± 0.38 * |
| (dV/dt)max (V/sec) | 99.3 ± 24.5 | 116.2 ± 18.2 | 122.6 ± 16.5 | 150.7 ± 7.8 | 148.3 ± 7.8 |
| Amplitude (mV) | 92.5 ± 2.5 | 97.3 ± 3.3 | 100.7 ± 3.1 * | 106.7 ± 1.1 | 107.5 ± 1.4 |
| APD90 (msec) | 84.2 ± 2.6 | 82.3 ± 3.8 | 85.2 ± 3.3 | 84.6 ± 2.7 | 90.7 ± 2.3 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Obata, K.; Ohmori, T.; Ishiwata, K.; Abe, M.; Hamaguchi, S.; Namekata, I.; Tanaka, H. Angiotensin II Induces Automatic Activity of the Isolated Guinea Pig Pulmonary Vein Myocardium through Activation of the IP3 Receptor and the Na+-Ca2+ Exchanger. Int. J. Mol. Sci. 2019, 20, 1768. https://doi.org/10.3390/ijms20071768
Tanaka Y, Obata K, Ohmori T, Ishiwata K, Abe M, Hamaguchi S, Namekata I, Tanaka H. Angiotensin II Induces Automatic Activity of the Isolated Guinea Pig Pulmonary Vein Myocardium through Activation of the IP3 Receptor and the Na+-Ca2+ Exchanger. International Journal of Molecular Sciences. 2019; 20(7):1768. https://doi.org/10.3390/ijms20071768
Chicago/Turabian StyleTanaka, Yusuke, Kae Obata, Tamano Ohmori, Kohei Ishiwata, Manato Abe, Shogo Hamaguchi, Iyuki Namekata, and Hikaru Tanaka. 2019. "Angiotensin II Induces Automatic Activity of the Isolated Guinea Pig Pulmonary Vein Myocardium through Activation of the IP3 Receptor and the Na+-Ca2+ Exchanger" International Journal of Molecular Sciences 20, no. 7: 1768. https://doi.org/10.3390/ijms20071768