Metabolic Pathway Analysis of Nitrogen and Phosphorus Uptake by the Consortium between C. vulgaris and P. aeruginosa
Abstract
1. Introduction
2. Results and Discussion
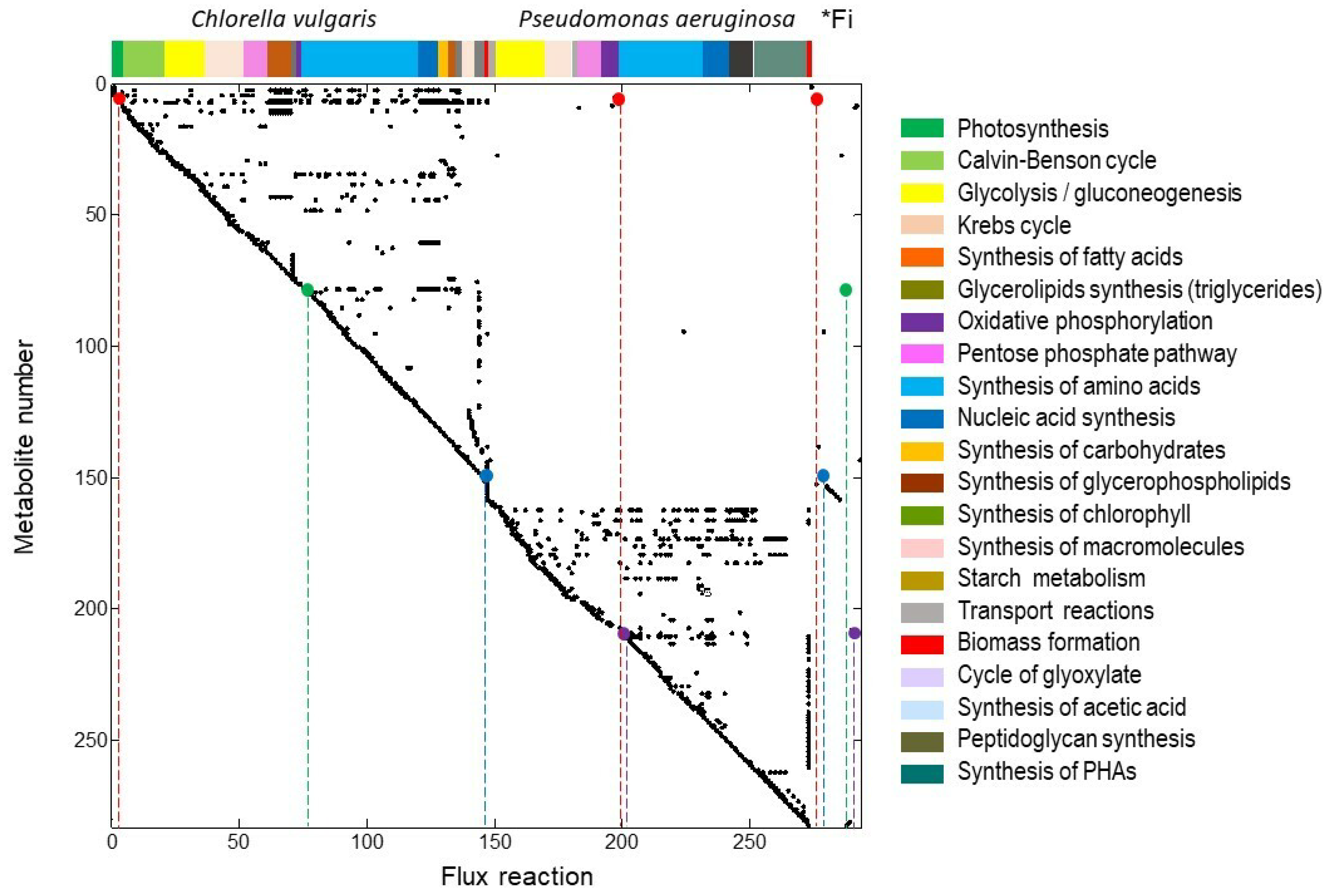
2.1. Stoichiometric Matrix S
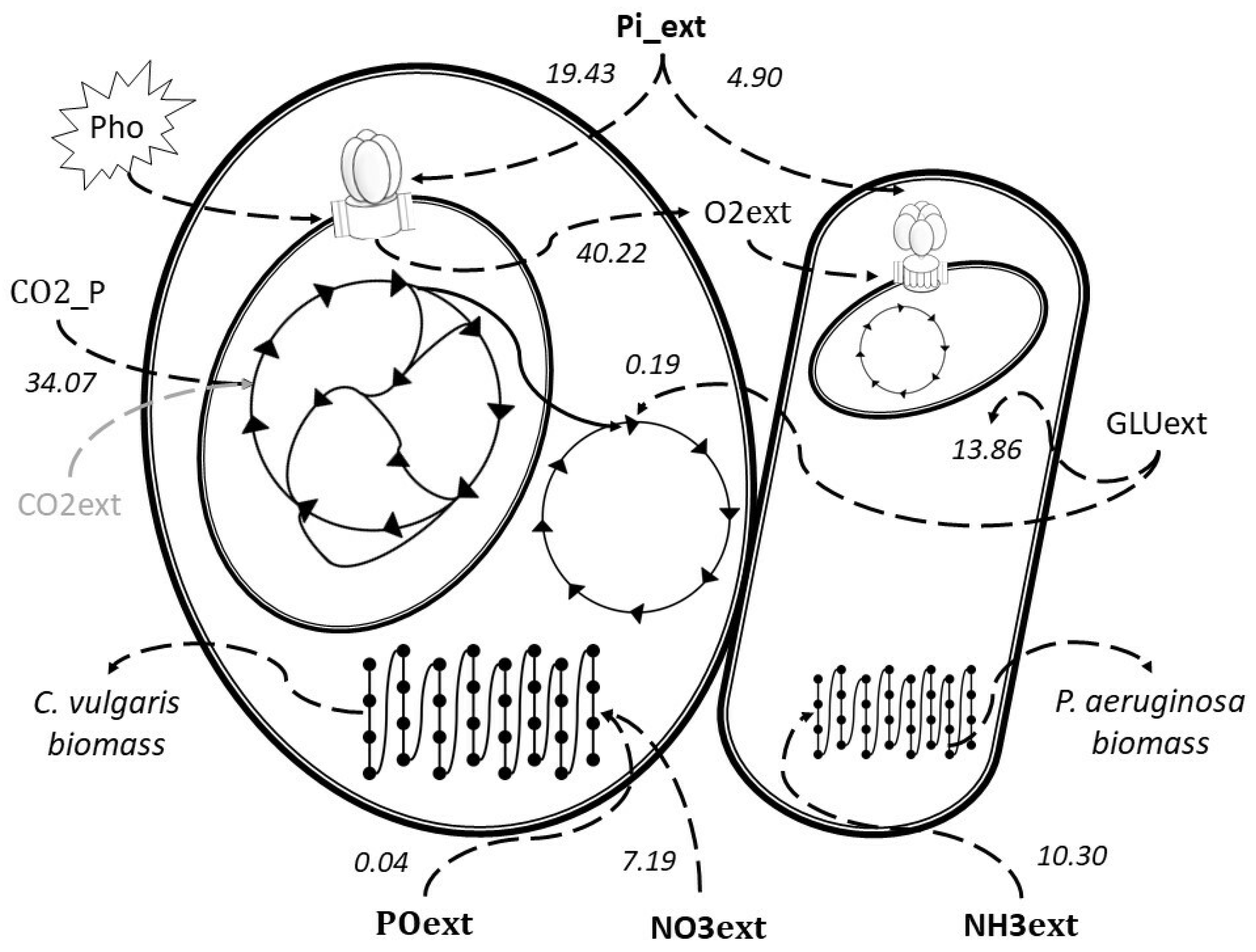
2.2. Extreme Pathways Analysis Featuring the Uptake of Phosphorous Species
2.3. Extreme Pathways Analysis Featuring the Uptake of Nitrogen Species
2.4. The Extreme Pathway with the Highest Yield of Nutrient Uptake
3. Methods
3.1. Reconstruction of the Genome-Scale Biochemical Reaction Network for the Co-Culture of C. vulgaris and P. aeruginosa
3.2. Extreme Pathway Analysis (ExPas)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2-Cv | Carbon dioxide in C. vulgaris |
| CO2-P | Carbon dioxide in P. aeruginosa |
| Glu-Cv | L-Glutamate in C. vulgaris |
| Glu-P | L-Glutamate in P. aeruginosa |
| NH3ext | External ammonia |
| NO3ext | External nitrate |
| O2-Cv | Oxygen in C. vulgaris |
| O2-P | Oxygen in P. aeruginosa |
| Piext | External phosphorus |
| Pho | Photon |
| PO4ext | External phosphate |
| ExPas | Extreme Pathways |
References
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2014, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Alsan, S.; Kapdan, I.K. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol. Eng. 2006, 28, 64–70. [Google Scholar]
- Mohan, S.V.; Rohit, M.; Chiranjeevi, P.; Chandra, R.; Navaneeth, B. Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: Progress and perspectives. Bioresour. Technol. 2015, 184, 169–178. [Google Scholar] [CrossRef]
- Oberhardt, M.A.; Puchatka, J.; Fryer, K.E.; dos Santos, V.A.P.M.; Papin, J.A. Genome-Scale Metabolic Network Analysis of the Opportunistic Pathogen Pseudomonas aeruginosa PAO1. J. Bacteriol. 2008, 190, 2790–2803. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A.S. Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutierrez, R.; Ferrer, I.; Garcia, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef]
- Choix, F.; de Bashan, L.; Bashan, Y. Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzym. Microb. Technol. 2012, 51, 294–299. [Google Scholar] [CrossRef]
- Qu, L.; Wang, R.; Zhao, P.; Chen, R.; Zhou, W.; Tang, L.; Tang, X. Interaction between Chlorella vulgaris and bacteria: interference and resource competition. Acta Oceanol. Sin. 2014, 33, 135–140. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Simoes, M. Wastewater treatment to enhance the economic viability of microalgae culture. Environ. Sci. Pollut. Res. 2013, 20, 5096–5105. [Google Scholar]
- Mujtaba, G.; Rizwan, M.; Lee, K. Simultaneous removal of inorganic nutrients and organic carbon by symbiotic co-culture of Chlorella vulgaris and Pseudomonas putida. Biotechnol. Bioprocess Eng. 2015, 20, 114–122. [Google Scholar] [CrossRef]
- Gómez-Guzmán, A.; Jiménez-Magaña, S.; Guerra-Rentería, A.S.; Gómez-Hermosillo, C.; Parra-Rodríguez, F.J.; Velázquez, S.; Aguilar-Uscanga, B.R.; Solis-Pacheco, J.; González-Reynoso, O. Evaluation of nutrients removal (NO3-N, NH3-N and PO4-P) with Chlorella vulgaris, Pseudomonas putida, Bacillus cereus and a consortium of these microorganisms in the treatment of wastewater effluents. Water Sci. Technol. 2017, 76, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lananan, F.; Hamid, S.H.A.; Din, W.N.S.; Ali, N.; Khatoon, H.; Jusoh, A.; Endut, A. Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae Chlorella sp. Int. Biodeterior. Biodegrad. 2014, 95, 127–134. [Google Scholar] [CrossRef]
- Posadas, E.; Bochon, S.; Coca, M.; Garcia-González, M.; Muñoz, P.G.E.R. Microalgae-based agro-industrial wastewater treatment: a preliminary screening of biodegradability. J. Appl. Phycol. 2014, 26, 2335–2345. [Google Scholar] [CrossRef]
- Bergero, M.F.; Lucchesi, G.I. Immobilization of Pseudomonas putida (ATCC 12633) cells: A promising tool for effective degradation of quaternary ammonium compounds in industrial effluents. Int. Biodeterior. Biodegrad. 2015, 100, 38–43. [Google Scholar] [CrossRef]
- Cooper, M.B.; Smith, A.G. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Price, N.D.; Reed, J.L.; Palsson, B.O. Genome-scale models of microbial cells: evaluating the consequences of constraints. Nat. Rev. Microbiol. 2004, 2, 886–897. [Google Scholar] [CrossRef]
- Varma, A.; Bosech, B.W.; Palsson, B.O. Stoichiometric Interpretation of Escherichia coli Glucose Catabolism under Various Oxygenation Rates. Appl. Environ. Microbiol. 1993, 59, 2465–2473. [Google Scholar]
- Papin, J.A.; Price, N.D.; Palsson, B.O. Extreme Pathway Lengths and Reaction Participation in Genome-Scale Metabolic Networks. Genome Res. 2002, 12, 1889–1900. [Google Scholar] [CrossRef]
- Schilling, C.H.; Letscher, D.; Palsson, B.O. Theory for the Systemic Definition of Metabolic Pathways and their use in Interpreting Metabolic Function from a Pathway-Oriented Perspective. J. Theor. Biol. 2000, 203, 229–248. [Google Scholar] [CrossRef]
- Guo, Z.; Tong, Y.W. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J. Appl. Phycol. 2013, 26, 1483–1492. [Google Scholar] [CrossRef]
- Allen, J.F. Photosynthesis of ATP: Electrons, Proton Pumps, Rotors, and Poise. Cell 2002, 110, 273–276. [Google Scholar] [CrossRef]
- Orth, J.D.; Thiele, I.; Palsson, B.O. KEGG: Kyoto Encyclopedia of Genes and Genomes. 2016. Available online: https://www.genome.jp/kegg/ (accessed on 16 November 2017).
- Rojas Rosas O, González Reynoso O, N.A.J. Producción de Polihidroxialcanoatos y análisis de la capacidad metabólica de Pseudomonas aeruginosa ATCC 9027, mediante el estudio de la distribución de flujos de carbono. Ph.D. Thesis, Universidad de Guadalajara, Guadalajara, Mexico, 2006.
- Stephanopoulos, G.; Aristidou, A.A.; Nielsen, J. Metabolic Engineering: Principles and Methodologies; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Bell, S.L.; Palsson, B.O. Expa: A program for calculating extreme pathways in biochemical reaction networks. Bioinformatics 2005, 21, 1739–1740. [Google Scholar] [CrossRef]
- Orth, J.D.; Thiele, I.; Palsson, B.O. What is flux balance analysis? Nat. Biotechnol. 2013, 28, 245–248. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Renteria, A.S.; García-Ramírez, M.A.; Gómez-Hermosillo, C.; Gómez-Guzmán, A.; González-García, Y.; González-Reynoso, O. Metabolic Pathway Analysis of Nitrogen and Phosphorus Uptake by the Consortium between C. vulgaris and P. aeruginosa. Int. J. Mol. Sci. 2019, 20, 1978. https://doi.org/10.3390/ijms20081978
Guerra-Renteria AS, García-Ramírez MA, Gómez-Hermosillo C, Gómez-Guzmán A, González-García Y, González-Reynoso O. Metabolic Pathway Analysis of Nitrogen and Phosphorus Uptake by the Consortium between C. vulgaris and P. aeruginosa. International Journal of Molecular Sciences. 2019; 20(8):1978. https://doi.org/10.3390/ijms20081978
Chicago/Turabian StyleGuerra-Renteria, A. Suggey, M. Alberto García-Ramírez, César Gómez-Hermosillo, Abril Gómez-Guzmán, Yolanda González-García, and Orfil González-Reynoso. 2019. "Metabolic Pathway Analysis of Nitrogen and Phosphorus Uptake by the Consortium between C. vulgaris and P. aeruginosa" International Journal of Molecular Sciences 20, no. 8: 1978. https://doi.org/10.3390/ijms20081978
APA StyleGuerra-Renteria, A. S., García-Ramírez, M. A., Gómez-Hermosillo, C., Gómez-Guzmán, A., González-García, Y., & González-Reynoso, O. (2019). Metabolic Pathway Analysis of Nitrogen and Phosphorus Uptake by the Consortium between C. vulgaris and P. aeruginosa. International Journal of Molecular Sciences, 20(8), 1978. https://doi.org/10.3390/ijms20081978






