Self-Assembled Supramolecular Ribbon-Like Structures Complexed to Single Walled Carbon Nanotubes as Possible Anticancer Drug Delivery Systems
Abstract
:1. Introduction
2. Results
2.1. Analysis of DOX Interaction with SWNT–CR
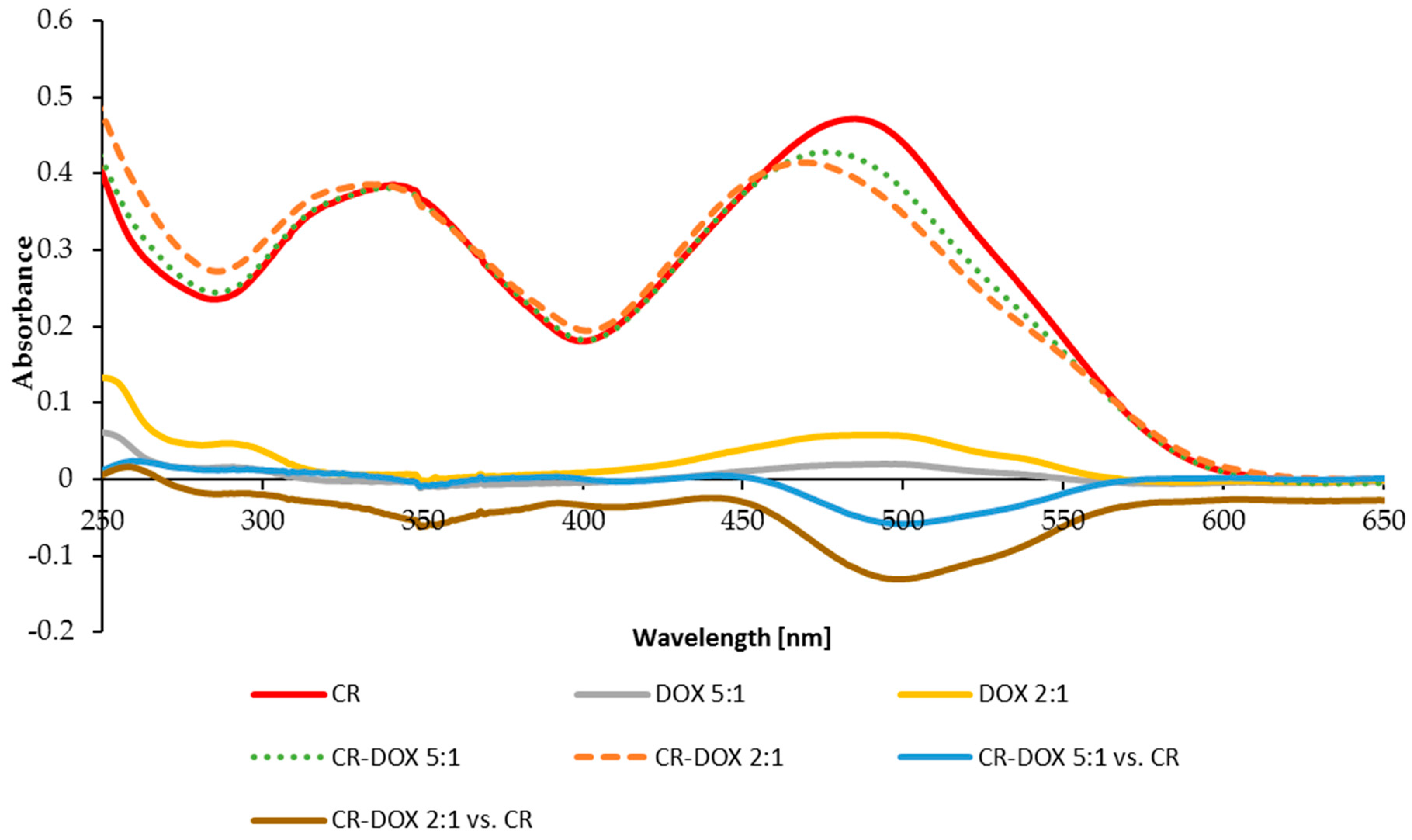
2.1.1. DOX Binding to CR
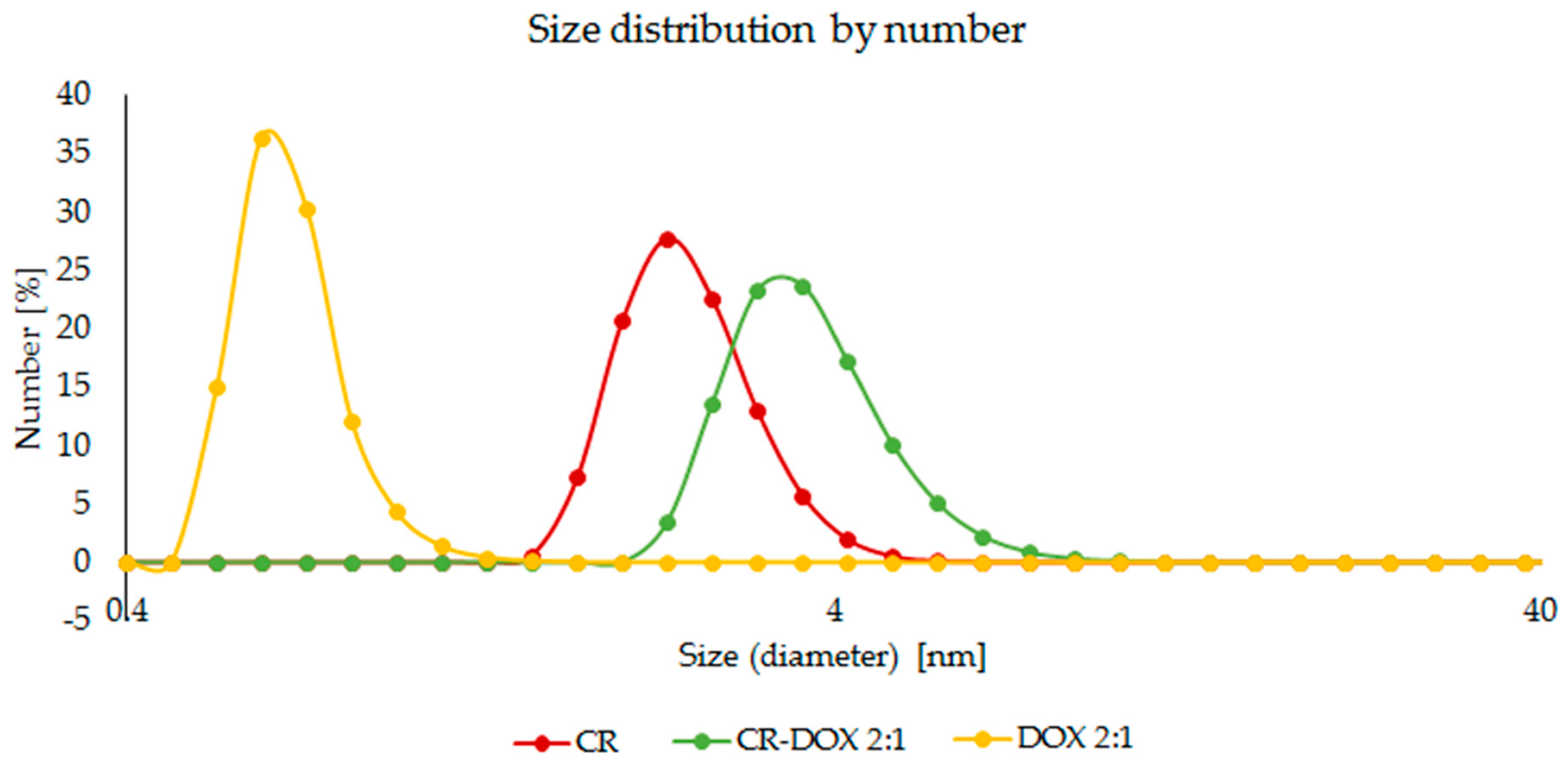
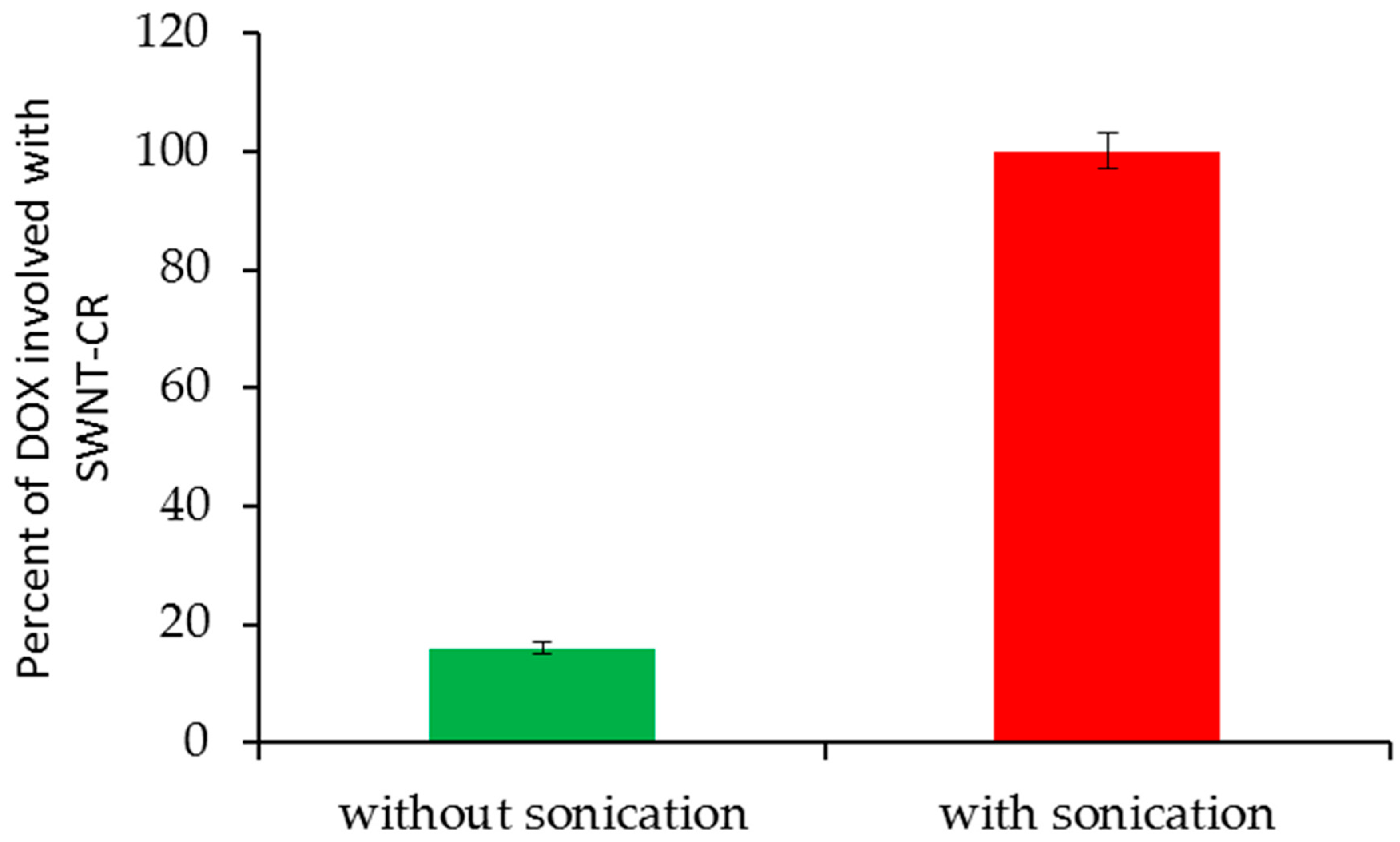
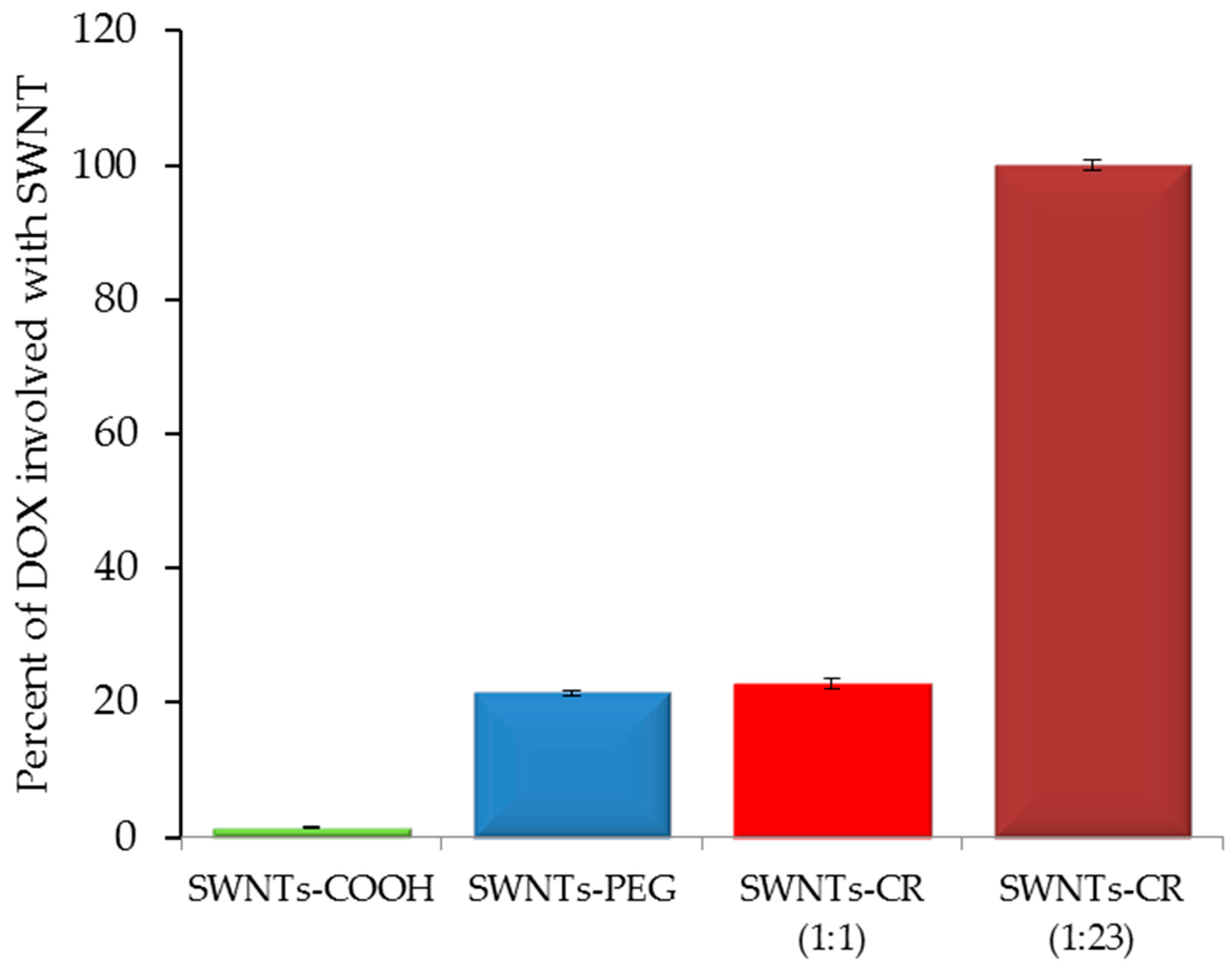
2.1.2. DOX Binding to SWNT–CR
2.2. The Effect of pH on Stability of SWNT–CR–DOX Complex: A Possibility of DOX Release
2.2.1. DOX Release Evaluated by Dialysis
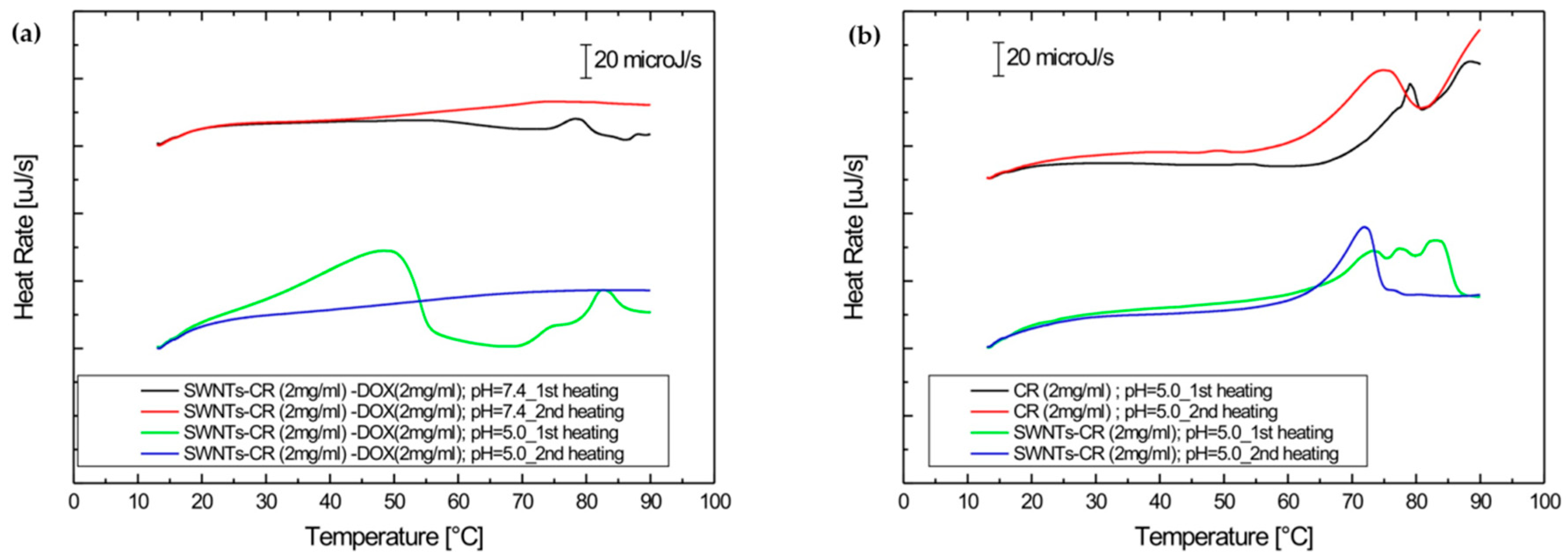
2.2.2. DOX Release Evaluated by Differential Scanning Calorimetry (DSC)
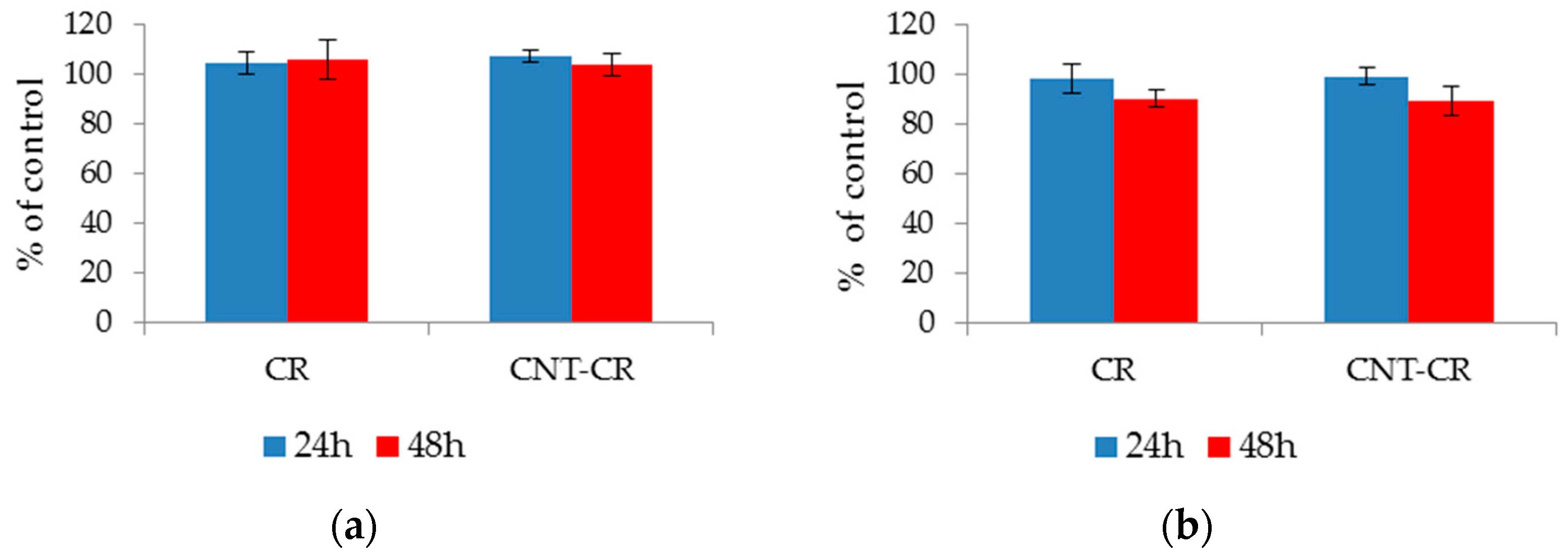
2.3. Evaluation of CR and SWNT-CR on Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of CR–DOX and SWNT–CR–DOX Complexes
4.3. Characterization of CR–DOX or SWNT–CR–DOX Complexes
4.3.1. Electrophoresis
4.3.2. Dynamic Light Scattering (DLS)
4.3.3. Scanning Electron Microscopy (SEM)
4.3.4. Effect of pH on DOX Release Dialysis
4.3.5. Differential Scanning Calorimetry (DSC)
4.4. Evaluation of Cell Proliferation after Addition of CR and SWNT–CR
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SRLS | Self-assembled ribbon-like structure |
| CR | Congo Red |
| CNT | Carbon nanotubes |
| SWNT | Single wall carbon nanotubes |
| DOX | Doxorubicin |
| MDR | Multidrug resistance |
| DLS | Dynamic light scattering |
| PTFE | Polytetrafluoroethylene |
| SEM | Scanning electron microscopy |
| DSC | Differential scanning calorimetry |
References
- Heister, E.; Neves, V.; Carmen, T. Triple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. Carbon 2009, 47, 2152–2160. [Google Scholar] [CrossRef] [Green Version]
- Lara, S.J.-M.; van Vlerken, L.E.; Yadav, S. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat. Rev. 2009, 34, 592–602. [Google Scholar] [CrossRef]
- Kam, N.W.; Liu, Z.; Dai, H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. Am. Chem. Soc. 2005, 127, 12492–12493. [Google Scholar] [CrossRef]
- Prato, M.; Kostarelos, K. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008, 41, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Więckowski, S.; Luangsivilay, J. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2007, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Chen, S.Y.; Zhao, X.R.; Kuznetsova, L.V.; Wong, S.S.; Ojima, I. Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor targeted delivery. J. Am. Chem. Soc. 2008, 130, 16778–16785. [Google Scholar] [CrossRef]
- Gao, L.; Nie, L.; Wang, T.; Qin, Y.; Guo, Z.; Yang, D.; Yan, X. Carbon nanotube delivery of the GFP gene into mammalian cells. ChemBioChem 2006, 7, 239–242. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Misra, S.K.; Bhattacharya, S. CNT loading into cationic cholesterol suspensions show improved DNA binding and serum stability and ability to internalize into cancer cells. Nanotechnology 2012, 23, 065101. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Graff, R.; Hoebeke, J.; Briand, J.-P.; Prato, M. Synthesis, structural characterization and immunological properties of carbon nanotubes functionalized with peptides. J. Am. Chem. Soc. 2003, 125, 6160–6164. [Google Scholar] [CrossRef]
- Kam, N.W.S.; Jessop, T.C.; Wender, P.A.; Dai, H.J. Nanotube molecular transporters: Internalization of carbon nanotube-protein conjugates into mammalian cells. J. Am. Chem. Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef]
- Pastorin, G. Carbon Nanotubes: From Bench Chemistry to Promising Biomedical Applications, 1st ed.; Pastorin, G., Ed.; Pan Stanford Publishing Pte., Ltd.: Singapore, 2011. [Google Scholar]
- Hampel, S.; Kunze, D.; Haase, D.; Krämer, K.; Rauschenbach, M. Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumor cell growth. Nanomedicine 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef]
- Van Norden, R. The trials of new carbon. Nature 2011, 14, 469. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005, 3, 571–577. [Google Scholar] [CrossRef]
- Foldvari, M.; Bagonluri, M. Carbon nanotubes as functional excipients for nanomedicines: I. Pharmaceutical properties. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 173–182. [Google Scholar] [CrossRef]
- Lacerda, L.; Bianco, A.; Prato, M.; Kostarelos, K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv. Drug Deliv. Rev. 2006, 58, 1460–1470. [Google Scholar] [CrossRef]
- Kim, S.N.; Rusling, J.F.; Papadimitrakopoulos, F. Carbon nanotubes for electronic and electrochemical detection of biomolecules. Adv. Mater. 2007, 19, 3214–3228. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon nanotubes: An emerging drug carrier for targeting cancer cells. J. Drug Deliv. 2014, 6, 670815. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H.J. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ASC Nano 2007, 1, 50–56. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Lorents, D.C. Mechanical and thermal properties of carbon nanotubes. Carbon 1995, 33, 925–930. [Google Scholar] [CrossRef]
- Król, M.; Roterman, I.; Piekarska, B.; Konieczny, L.; Rybarska, J.; Stopa, B.; Spólnik, P.; Szneler, E. An approach to understand the complexation of supramolecular dye Congo Red with immunoglobulin L chain lambda. Biopolymers 2005, 77, 155–162. [Google Scholar] [CrossRef]
- Skowronek, M.; Stopa, B.; Konieczny, L.; Rybarska, J.; Piekarska, B.; Szneler, E. Self-assembly of Congo Red-A theoretical and experimental approach to identify its supramolecular organization in water and salt solutions. Biopolymers 1998, 46, 267–281. [Google Scholar] [CrossRef]
- Rybarska, J.; Piekarska, B.; Stopa, B.; Zemanek, G.; Konieczny, L. Chapter 1: Supramolecular Systems as Protein Ligands. In Self-Assembled Molecules—New Kind of Protein Ligands. Supramolecular Ligands, 1st ed.; Roterman, I., Konieczny, L., Eds.; Springer Nature: Cham, Switzerlandnd, 2017; pp. 1–20. [Google Scholar] [CrossRef]
- Spólnik, P.; Król, M.; Stopa, B.; Konieczny, L.; Piekarska, B.; Rybarska, J.; Zemanek, G.; Jagusiak, A.; Piwowar, P.; Szoniec, G. Influence of the electric field on supramolecular structure and properties of amyloid-specific reagent Congo Red. Eur. Biophys. J. 2011, 40, 1187–1196. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Shen, A.; Shen, X.; Li, J.; Hu, S. Water-soluble single-walled carbon nanotubes via noncovalent functionalization by a rigid, planar and conjugated diazo dye. Carbon 2006, 44, 428–434. [Google Scholar] [CrossRef]
- Szlachta, M.; Wójtowicz, P. Adsorption of methylene blue and Congo Red from aqueous solution by activated carbon and carbon nanotubes. Water Sci. Technol. 2013, 68, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chatterjee, T.; Lim, S.-R.; Woo, S.H. Effect of the addition mode of carbon nanotubes for the production of chitosan. Bioresour. Technol. 2011, 102, 4402–4409. [Google Scholar] [CrossRef] [PubMed]
- Jagusiak, A.; Piekarska, B.; Pańczyk, T.; Jemioła-Rzemińska, M. Dispersion of single walled carbon nanotubes with supramolecular Congo Red—Properties of the complexes and mechanism of the interaction. Beilstein J. Nanotechnol. 2017, 8, 636–648. [Google Scholar] [CrossRef]
- Jagusiak, A.; Piekarska, B.; Chłopaś, K.; Bielańska, E.; Pańczyk, T. Shortening and dispersion of single-walled carbon nanotubes upon interaction with mixed supramolecular compounds. Bio-Algorithms Med-Syst. 2016, 12, 123–132. [Google Scholar] [CrossRef]
- Chłopaś, K.; Jagusiak, A.; Konieczny, L. The use of titan yellow dye as a metal ion binding marker for studies on the formation of specific complexes by supramolecular Congo Red. Bio-Algorithms Med-Syst. 2015, 11, 9–17. [Google Scholar] [CrossRef]
- Rybarska, J.; Konieczny, L.; Jagusiak, A. Silver ions as EM marker of Congo Red ligation sites in amyloids and amyloid-like aggregates. Acta Biochim. Pol. 2017, 1, 161–169. [Google Scholar] [CrossRef]
- Konieczny, L.; Piekarska, B.; Rybarska, J. The use of Congo Red as a lyotropic liquid crystal to carry stain in a model immunotargeting system—Microscopic studies. Folia Histochem. Cytobiol. 1997, 35, 203–210. [Google Scholar]
- Jagusiak, A.; Pańczyk, T. Interaction of Congo Red, Evans Blue and Titan Yellow with doxorubicin in aqueous solutions. A molecular dynamics study. J. Mol. Liq. 2019, 279, 640–648. [Google Scholar] [CrossRef]
- Konieczny, L.; Piekarska, B.; Rybarska, J.; Stopa, B.; Krzykwa, B.; Noworolski, J.; Pawlicki, R.; Roterman, I. Bis azo dye liquid crystalline micelles as possible drug carriers in immunotergeting technique. J. Physiol. Pharm. 1994, 45, 441–454. [Google Scholar]
- Stopa, B.; Piekarska, B.; Jagusiak, A.; Kusior, D.; Zemanek, G.; Konieczny, L.; Rybarska, J. Supramolecular Congo Red as a potential drug carrier. Properties of Congo Red-doxorubicin complexes. In Proceedings of the 2nd Congress of Biochemistry and Cell Biology, 46th Meeting of Polish Biochemical society and 11th Conference of the Polish Cell Biology Society, Krakow, Poland, 5–9 September 2011. [Google Scholar]
- Stopa, B.; Piekarska, B.; Konieczny, L.; Rybarska, J.; Spólnik, P.; Zemanek, G.; Roterman, I.; Król, M. The structure and protein binding of amyloid-specific dye reagents. Acta Biochim. Pol. 2003, 50, 1213–1227. [Google Scholar]
- Rybarska, J.; Piekarska, B.; Stopa, B.; Spólnik, P.; Zemanek, G.; Konieczny, L.; Roterman, I. In vivo accumulation of self-assembling dye Congo Red in an area marked by specific immune complexes: Possible relevance to chemotherapy. Folia Histochem. Cytobiol. 2004, 42, 101–110. [Google Scholar]
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? Semin. Oncol. 1992, 19, 670–686. [Google Scholar]
- Singal, P.K.; Iliskovic, N. Doxorubicin-Induced cardiomyopathy. New Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for drug delivery of small molecule drug. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef]
- Schellman, J.A.; Reese, H.R. Extensions to the theory of intercalation. Biopolymers 1995, 39, 161–171. [Google Scholar] [CrossRef]
- Tuite, E.; Kelly, J.M. The interaction of methylene blue, azure B, and thionine with DNA: Formation of complexes with polynucleotides as model systems. Biopolymers 1994, 35, 419–433. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Lu, Q.; Fei, Z.; Dyson, P.J. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef] [PubMed]
- Ali-Boucetta, H.; Khuloud, T.; Al-Jamal, K.T.; Mc Carthy, D.; Prato, M.; Bianco, A.; Kostarelos, K. Multiwalled carbon nanotubes-doxorubicin supramolecular complexes for cancer therapeutics. Chem. Commun. (Camb.) 2008, 3, 459–461. [Google Scholar] [CrossRef]
- Niu, L.; Meng, L.; Lu, Q. Folate-conjugated PEG on single walled carbon nanotubes for targeting delivery of doxorubicin to cancer cells. Macromol. Biosci. 2013, 13, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yuan, Q.; Shah, J.S.; Misra, R.D.K. A new family of folate-decorated and carbon nanotube-mediated drug delivery system: Synthesis and drug delivery response. Adv. Drug Deliv. Rev. 2011, 63, 1332–1339. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffman, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hover, S. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic Extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Nehoff, H.; Parayath, N.N.; Domanovitch, L.; Taurin, S.; Greish, K. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 9, 2539–2555. [Google Scholar] [CrossRef]
- Oda, T.; Sato, F.; Maeda, H. Facilitated internalization of neocarcinostatin and its lipophilic polymer conjugate, SMANCS, into cytosol in acidic pH. J. Natl. Cancer Inst. 1987, 79, 1205–1211. [Google Scholar] [PubMed]
- Byrne, D.J.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, L.; Bian, X.; Kong, J.; Yang, P.; Liu, B. pH-controlled delivery of doxorubicin to cancer cells, based on small mesoporous carbon nanospheres. Small 2012, 8, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Stopa, B.; Jagusiak, A.; Konieczny, L.; Piekarska, B.; Rybarska, J.; Zemanek, G.; Król, M.; Piwowar, P.; Roterman, I. The use of supramolecular structures as protein ligands. J. Mol. Model. 2013, 19, 4731–4740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźnicka, O.; Rybarska, J.; Jagusiak, A.; Konieczny, L.; Stopa, B.; Roterman, I. Chapter 4: Metal ions introduced to proteins by supramolecular ligands. In Self-Assembled Molecules—New Kind of Protein Ligands. Supramolecular Ligands, 1st ed.; Roterman, I., Konieczny, L., Eds.; Springer Nature: Cham, Switzerlandnd, 2017; pp. 61–76. [Google Scholar] [CrossRef]
- Jagusiak, A.; Piekarska, B.; Stopa, B.; Zemanek, G.; Chłopaś, K. Supramolecular compounds interaction with chemotherapeutic drugs, antibodies and carbon nanotubes—Hybrid nanocomplexes with potential drug delivery systems applications. In Proceedings of the 43th FEBS Congress, Prague, Czech Republic, 7–12 July 2018. [Google Scholar] [CrossRef]
- Jagusiak, A.; Rybarska, J.; Piekarska, B.; Stopa, B.; Konieczny, L. Chapter 2: Supramolecular Congo Red as specific ligand of antibodies engaged in immune complex. In Self-Assembled Molecules—New Kind of Protein Ligands. Supramolecular Ligands, 1st ed.; Roterman, I., Konieczny, L., Eds.; Springer Nature: Cham, Switzerlandnd, 2017; pp. 21–42. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, A.C.; Rakhra, K.; Sherlock, S.; Goodwin, A.; Chen, X.; Yang, Q.; Felsher, D.W.; Dai, H. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew. Chem. Int. Ed. 2009, 48, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, A.; Drozd, A.; Konieczny, L.; Król, M. The indirect generation of long-distance structural changes in antibodies upon their binding to antigen. Chem. Biol. Drug. Des. 2006, 68, 276–283. [Google Scholar] [CrossRef] [PubMed]



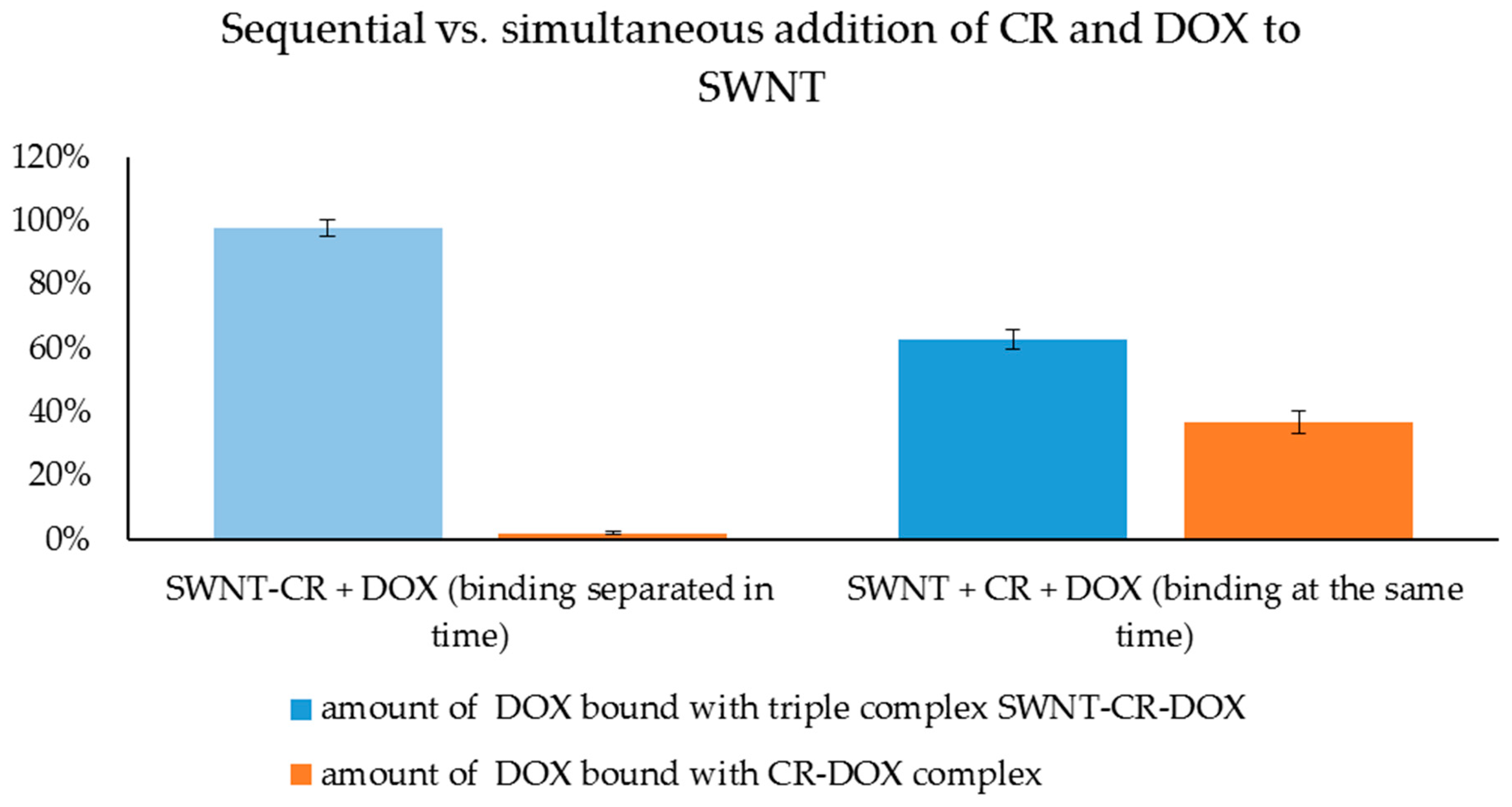


| Binding of SWNT, CR and DOX Separated in time | Simultaneous Binding of SWNT, CR and DOX | ||
|---|---|---|---|
| SWNT + CR Sonication 1 + DOX Sonication 2 | SWNT + CR + DOX Sonication | ||
| Filtration (AmiconUltra);present in filtrate: | |||
| CR | - | CR | - |
| DOX | - | DOX | - |
RESULT (1A):
| RESULT (1B):
| ||
| Filtration (PTFE);present in filtrate: | |||
| CR | - | CR | + |
| DOX | - | DOX | + |
RESULT (2A):
| RESULT (2B):
| ||
| Chromatography (after filtration through PTFE) | |||
| Chromatographic analysis of filtrate: | |||
| CR | - | CR | + |
| DOX | - | DOX | + |
| Chromatographic analysis of complexes bound to SWNT, retained on the membrane: | |||
| CR | + | CR | + |
| DOX | + | DOX | + |
RESULT (3A):
| RESULT (3B):
| ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagusiak, A.; Chłopaś, K.; Zemanek, G.; Jemioła-Rzemińska, M.; Piekarska, B.; Stopa, B.; Pańczyk, T. Self-Assembled Supramolecular Ribbon-Like Structures Complexed to Single Walled Carbon Nanotubes as Possible Anticancer Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 2064. https://doi.org/10.3390/ijms20092064
Jagusiak A, Chłopaś K, Zemanek G, Jemioła-Rzemińska M, Piekarska B, Stopa B, Pańczyk T. Self-Assembled Supramolecular Ribbon-Like Structures Complexed to Single Walled Carbon Nanotubes as Possible Anticancer Drug Delivery Systems. International Journal of Molecular Sciences. 2019; 20(9):2064. https://doi.org/10.3390/ijms20092064
Chicago/Turabian StyleJagusiak, Anna, Katarzyna Chłopaś, Grzegorz Zemanek, Małgorzata Jemioła-Rzemińska, Barbara Piekarska, Barbara Stopa, and Tomasz Pańczyk. 2019. "Self-Assembled Supramolecular Ribbon-Like Structures Complexed to Single Walled Carbon Nanotubes as Possible Anticancer Drug Delivery Systems" International Journal of Molecular Sciences 20, no. 9: 2064. https://doi.org/10.3390/ijms20092064






