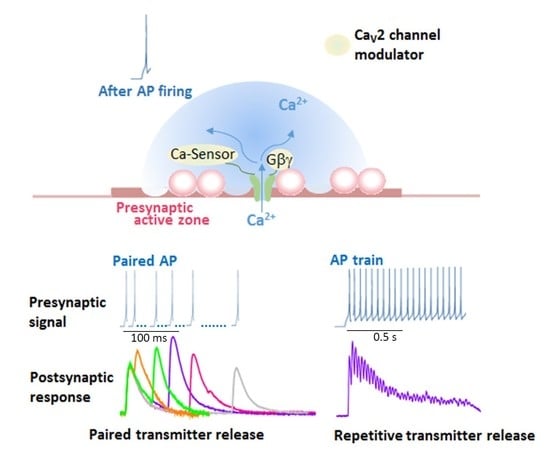
Presynaptic Calcium Channels
Abstract
:1. Introduction
2. Presynaptic Ca2+ Channels
3. Intracellular Molecules Modulate Presynaptic Ca2+ Channels Activity
3.1. G Proteins
3.2. Active Zone Proteins
3.3. t-SNAREs and Synaptotagmin-1
3.4. Ca2+-Sensor Proteins
4. Negative Regulation of Neurotransmitter Release by Gβγ protein/CaV2 Channel Complex
5. Synchronous Neurotransmitter Release Regulated by Ca2+ Channel/SNARE Proteins Complex
6. Presynaptic Plasticity Induced by Ca2+-Sensors-Mediated CaV2.1 Channel Modulation
6.1. Ca2+/CaM Mediates Synaptic Depression and Facilitation
6.2. Neuron-Specific Ca2+-Sensor Proteins Mediate Synaptic Depression and Facilitation
6.3. Temporal Regulation of Release Efficacy by Ca2+-Sensor Proteins
6.4. CaMKII Saves as Effector Checkpoint for Ca2+ Entry
7. Neuronal Firing and Presynaptic Short-Term Plasticity
7.1. Presynaptic Short-Term Facilitation
7.2. Presynaptic Short-Term Depression
7.3. CaMKII Regulates Short-Term Synaptic Plasticity
7.4. Ca2+-Binding Molecules Regulate Short-Term Synaptic Plasticity
8. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| APs | action potentials |
| AZ | active zone |
| CaV2 channels | voltage-gated Ca2+ channels |
| SVs | synaptic vesicles |
| SCG | superior cervical ganglion |
| EPSPs | excitatory postsynaptic potentials |
| RIM | Rab-interacting molecule |
| RIM-BPs | RIM-binding proteins |
| nCaS | neuron specific Ca2+ sensor proteins |
| CaM | calmodulin |
| CaBP1 | Ca2+-binding protein-1 |
| VILIP-2 | Visinin-like protein-2 |
| NCS-1 | neuronal calcium sensor-1 |
| CaMKII | Ca2+/CaM-dependent protein kinase II |
| PPF | paired-pulse facilitation |
| PPD | paired-pulse depression |
| ISI | inter-stimulus interval |
| PTP | post-tetanic potentiation |
References
- Dunlap, K.; Luebke, J.I.; Turner, T.J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995, 18, 89–98. [Google Scholar] [CrossRef]
- Snutch, T.P.; Reiner, P.B. Ca2+ channels: Diversity of form and function. Curr. Opin. Neurobiol. 1992, 2, 247–253. [Google Scholar] [CrossRef]
- Tedford, H.W.; Zamponi, G.W. Direct G protein modulation of Cav2 calcium channels. Pharm. Rev. 2006, 58, 837–862. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Few, A.P. Calcium channel regulation and presynaptic plasticity. Neuron 2008, 59, 882–901. [Google Scholar] [CrossRef]
- Tsien, R.W.; Lipscombe, D.; Madison, D.V.; Bley, K.R.; Fox, A.P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988, 11, 431–438. [Google Scholar] [CrossRef]
- Tsien, R.W.; Ellinor, P.T.; Horne, W.A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharm. Sci. 1991, 12, 349–354. [Google Scholar] [CrossRef]
- Miljanich, G.P.; Ramachandran, J. Antagonists of neuronal calcium channels: Structure, function, and therapeutic implications. Annu. Rev. Pharm. Toxicol. 1995, 35, 707–734. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef]
- Olivera, B.M.; Miljanich, G.P.; Ramachandran, J.; Adams, M. Calcium channel diversity and neurotransmitter release: The ω-conotoxins and ω-agatoxins. Annu. Rev. Biochem. 1994, 63, 823–867. [Google Scholar] [CrossRef]
- Takahashi, M.; Seagar, M.J.; Jones, J.F.; Reber, B.F.; Catterall, W.A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc. Natl. Acad. Sci. USA 1987, 84, 5478–5482. [Google Scholar] [CrossRef]
- Frank, H.Y.; Yarov-Yarovoy, V.; Gutman, G.A.; Catterall, W.A. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol. Rev. 2005, 57, 387–395. [Google Scholar]
- Wu, J.; Yan, Z.; Li, Z.; Yan, C.; Lu, S.; Dong, M.; Yan, N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science 2015, 350, aad2395. [Google Scholar] [CrossRef] [PubMed]
- Ertel, E.A.; Campbell, K.P.; Harpold, M.M.; Hofmann, F.; Mori, Y.; Perez-Reyes, E.; Schwartz, A.; Snutch, T.P.; Tanabe, T.; Birnbaumer, L.; et al. Nomenclature of voltage-gated calcium channels. Neuron 2000, 25, 533–535. [Google Scholar] [CrossRef]
- Müller, C.S.; Haupt, A.; Bildl, W.; Schindler, J.; Knaus, H.G.; Meissner, M.; Meissner, B.; Striessnig, J.; Flockerzi, V.; Fakler, B.; et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc. Natl. Acad. Sci. USA 2010, 107, 14950–14957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolphin, A.C. Beta subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003, 35, 599–620. [Google Scholar] [CrossRef]
- Hofmann, F.; Lacinova, L.; Klugbauer, N. Voltage-dependent calcium channels: From structure to function. Rev. Physiol. Biochem. Pharm. 1999, 139, 33–87. [Google Scholar]
- Davies, A.; Hendrich, J.; Van Minh, A.T.; Wratten, J.; Douglas, L.; Dolphin, A.C. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharm. Sci. 2007, 28, 220–228. [Google Scholar] [CrossRef]
- Hoppa, M.B.; Lana, B.; Margas, W.; Dolphin, A.C.; Ryan, T.A. α2δ a expression sets presynaptic calcium channel abundance and release probability. Nature 2012, 486, 122–125. [Google Scholar] [CrossRef]
- Hille, B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994, 17, 531–536. [Google Scholar] [CrossRef]
- Ikeda, S.R.; Dunlap, K. Voltage-dependent modulation of N-type calcium channels: Role of G protein subunits. Adv. Second Messenger Phosphoprot. Res. 1999, 33, 131–151. [Google Scholar]
- Hertlitze, S.; Garcia, D.E.; Mackie, K.; Hille, B.; Scheuer, T.; Catterall, W.A. Modulation of Ca2+ channels by G-protein βγ subunits. Nature 1996, 380, 258–262. [Google Scholar] [CrossRef]
- Ikeda, S.R. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature 1996, 380, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Cantı, C.; Page, K.M.; Stephens, G.J.; Dolphin, A.C. Identification of residues in the N terminus of α1B critical for inhibition of the voltage-dependent calcium channel by Gβγ. J. Neurosci. 1999, 19, 6855–6864. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Bourinet, E.; Nelson, D.; Nargeot, J.; Snutch, T.P. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature 1997, 385, 442–446. [Google Scholar] [CrossRef]
- Li, B.; Zhong, H.; Scheuer, T.; Catterall, W.A. Functional role of a C-terminal G βγ-binding domain of Cav2.2 channels. Mol. Pharm. 2004, 66, 761–769. [Google Scholar]
- Bean, B.P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 1989, 340, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Carbone, E.; Lux, H.D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflug. Arch. 1986, 406, 104–111. [Google Scholar] [CrossRef]
- Tsunoo, A.; Yoshii, M.; Narahashi, T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc. Natl. Acad. Sci. USA 1986, 83, 9832–9836. [Google Scholar] [CrossRef]
- Canti, C.; Bogdanov, Y.; Dolphin, A.C. Interaction between G proteins and accessory subunits in the regulation of 1B calcium channels in Xenopus oocytes. J. Physiol. 2000, 527 Pt. 3, 419–432. [Google Scholar] [CrossRef]
- Feng, Z.P.; Arnot, M.I.; Doering, C.J.; Zamponi, G.W. Calcium channel beta subunits differentially regulate the inhibition of N-type channels by individual Gβ isoforms. J. Biol. Chem. 2001, 276, 45051–45058. [Google Scholar] [CrossRef]
- Dresviannikov, A.V.; Page, K.M.; Leroy, J.; Pratt, W.S.; Dolphin, A.C. Determinants of the voltage dependence of G protein modulation within calcium channel beta subunits. Pflug. Arch. 2009, 457, 743–756. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Currie, K.P. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta 2013, 1828, 1629–1643. [Google Scholar] [CrossRef]
- García, D.E.; Li, B.; García-Ferreiro, R.E.; Hernández-Ochoa, E.O.; Yan, K.; Gautam, N.; Catterall, W.A.; Mackie, K.; Hille, B. G-protein beta-subunit specificity in the fast membrane-delimited inhibition of Ca2+ channels. J. Neurosci. 1998, 18, 9163–9170. [Google Scholar] [CrossRef]
- Reyes-Vaca, A.; de la Cruz, L.; Garduño, J.; Arenas, I.; Garcia, D.E. Fast Inactivation of CaV2.2 Channels Is Prevented by the Gβ1 Subunit in Rat Sympathetic Neurons. J. Mol. Neurosci 2017, 63, 377–384. [Google Scholar] [CrossRef]
- Hernández-Castellanos, J.M.; Vivas, O.; Garduño, J.; De la Cruz, L.; Arenas, I.; Elías-Viñas, D.; Mackie, K.; García, D.E. Gβ2 mimics activation kinetic slowing of CaV2.2 channels by noradrenaline in rat sympathetic neurons. Biochem. Biophys. Res. Commun. 2014, 445, 250–254. [Google Scholar] [CrossRef]
- Mahmoud, S.; Yun, J.K.; Ruiz-Velasco, V. Gβ2 and Gβ4 participate in the opioid and adrenergic receptor-mediated Ca2+ channel modulation in rat sympathetic neurons. J. Physiol. 2012, 590, 4673–4689. [Google Scholar] [CrossRef]
- Arnot, M.I.; Stotz, S.C.; Jarvis, S.E.; Zamponi, G.W. Differential modulation of N-type 1B and P/Q-type 1A calcium channels by different G protein subunit isoforms. J. Physiol. 2000, 527 Pt. 2, 203–212. [Google Scholar] [CrossRef]
- Stanley, E.F.; Mirotznik, R.R. Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels. Nature 1997, 385, 340–343. [Google Scholar] [CrossRef]
- Jarvis, S.E.; Magga, J.M.; Beedle, A.M.; Braun, J.E.; Zamponi, G.W. G protein modulation of N-type calcium channels is facilitated by physical interactions between syntaxin 1A and Gβγ. J. Biol. Chem. 2000, 275, 6388–6394. [Google Scholar] [CrossRef]
- Strock, J.; Diverse-Pierluissi, M.A. Ca2+ channels as integrators of G protein-mediated signaling in neurons. Mol. Pharm. 2004, 66, 1071–1076. [Google Scholar] [CrossRef]
- Delmas, P.; Coste, B.; Gamper, N.; Shapiro, M.S. Phosphoinositide lipid second messengers: New paradigms for calcium channel modulation. Neuron 2005, 47, 179–182. [Google Scholar] [CrossRef]
- Kubista, H.; Kosenburger, K.; Mahlknecht, P.; Drobny, H.; Boehm, S. Inhibition of transmitter release from rat sympathetic neurons via presynaptic M1 muscarinic acetylcholine receptors. Br. J. Pharm 2009, 156, 1342–1352. [Google Scholar] [CrossRef]
- Koushika, S.P.; Richmond, J.E.; Hadwiger, G.; Weimer, R.M.; Jorgensen, E.M.; Nonet, M.L. A post-docking role for active zone protein Rim. Nat. Neurosci. 2001, 4, 997–1005. [Google Scholar] [CrossRef]
- Schoch, S.; Mittelstaedt, T.; Kaeser, P.S.; Padgett, D.; Feldmann, N.; Chevaleyre, V.; Castillo, P.E.; Hammer, R.E.; Han, W.; Schmitz, F.; et al. Redundant functions of RIM1α and RIM2α in Ca2+-triggered neurotransmitter release. EMBO J. 2006, 25, 5852–5863. [Google Scholar] [CrossRef]
- Gracheva, E.O.; Hadwiger, G.; Nonet, M.L.; Richmond, J.E. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci. Lett. 2008, 444, 137–142. [Google Scholar] [CrossRef]
- Kaeser, P.S.; Deng, L.; Wang, Y.; Dulubova, I.; Liu, X.; Rizo, J.; Südhof, T.C. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 2011, 144, 282–295. [Google Scholar] [CrossRef]
- Deng, L.; Kaeser, P.S.; Xu, W.; Südhof, T.C. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron 2011, 69, 317–331. [Google Scholar] [CrossRef]
- Han, Y.; Kaeser, P.S.; Südhof, T.C.; Schneggenburger, R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron 2011, 69, 304–316. [Google Scholar] [CrossRef]
- Castillo, P.E.; Schoch, S.; Schmitz, F.; Südhof, T.C.; Malenka, R.C. RIM1α is required for presynaptic long-term potentiation. Nature 2002, 415, 327–330. [Google Scholar] [CrossRef]
- Coppola, T.; Magnin-Lüthi, S.; Perret-Menoud, V.; Gattesco, S.; Schiavo, G.; Regazzi, R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J. Biol. Chem. 2001, 276, 32756–32762. [Google Scholar] [CrossRef]
- Hibino, H.; Pironkova, R.; Onwumere, O.; Vologodskaia, M.; Hudspeth, A.J.; Lesage, F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca2+ channels. Neuron 2002, 34, 411–423. [Google Scholar] [CrossRef]
- Acuna, C.; Liu, X.; Gonzalez, A.; Südhof, T.C. RIM-BPs Mediate tight coupling of action potentials to Ca2+-triggered neurotransmitter release. Neuron 2015, 87, 1234–1247. [Google Scholar] [CrossRef]
- Kiyonaka, S.; Wakamori, M.; Miki, T.; Uriu, Y.; Nonaka, M.; Bito, H.; Beedle, A.M.; Mori, E.; Hara, Y.; De Waard, M.; et al. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat. Neurosci 2007, 10, 691–701. [Google Scholar] [CrossRef]
- Kiyonaka, S.; Nakajima, H.; Takada, Y.; Hida, Y.; Yoshioka, T.; Hagiwara, A.; Kitajima, I.; Mori, Y.; Ohtsuka, T. Physical and functional interaction of the active zone protein CAST/ERC2 and the β-subunit of the voltage-dependent Ca2+ channel. J. Biochem. 2012, 152, 149–159. [Google Scholar] [CrossRef]
- Calloway, N.; Gouzer, G.; Xue, M.; Ryan, T.A. The active-zone protein Munc13 controls the use-dependence of presynaptic voltage-gated calcium channels. Elife 2015, 4, 1–15. [Google Scholar] [CrossRef]
- Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci 2004, 27, 509–547. [Google Scholar] [CrossRef]
- Cohen, M.W.; Jones, O.T.; Angelides, K.J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J. Neurosci. 1991, 11, 1032–1039. [Google Scholar] [CrossRef]
- Westenbroek, R.E.; Hell, J.W.; Warner, C.; Dubel, S.J.; Snutch, T.P.; Catterall, W.A. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron 1992, 9, 1099–1115. [Google Scholar] [CrossRef]
- Westenbroek, R.E.; Sakurai, T.; Elliott, E.M.; Hell, J.W.; Starr, T.V.; Snutch, T.P.; Catterall, W.A. Immunochemical identification and subcellular distribution of the α1A subunits of brain calcium channels. J. Neurosci. 1995, 15, 6403–6418. [Google Scholar] [CrossRef]
- Bennett, M.K.; Calakos, N.; Scheller, R.H. Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 1992, 257, 255–259. [Google Scholar] [CrossRef]
- Leveque, C.; el Far, O.U.S.S.A.M.A.; Martin-Moutot, N.; Sato, K.; Kato, R.; Takahashi, M.; Seagar, M.J. Purification of the N-type calcium channel associated with syntaxin and synaptotagmin. A complex implicated in synaptic vesicle exocytosis. J. Biol. Chem. 1994, 269, 6306–6312. [Google Scholar]
- Yoshida, A.; Oho, C.; Omori, A.; Kuwahara, R.; Ito, T.; Takahashi, M. HPC-1 is associated with synaptotagmin and ω-conotoxin receptor. J. Biol. Chem. 1992, 267, 24925–24928. [Google Scholar]
- Sheng, Z.H.; Rettig, J.; Cook, T.; Catterall, W.A. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature 1996, 379, 451–454. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Rettig, J.; Takahashi, M.; Catterall, W.A. Identification of a syntaxin-binding site on N-type calcium channels. Neuron 1994, 13, 1303–1313. [Google Scholar] [CrossRef]
- Yokoyama, C.T.; Myers, S.J.; Fu, J.; Mockus, S.M.; Scheuer, T.; Catterall, W.A. Mechanism of SNARE protein binding and regulation of Cav2 channels by phosphorylation of the synaptic protein interaction site. Mol. Cell Neurosci. 2005, 28, 1–17. [Google Scholar] [CrossRef]
- Kim, D.K.; Catterall, W.A. Ca2+-dependent and -independent interactions of the isoforms of the alpha1A subunit of brain Ca2+ channels with presynaptic SNARE proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 14782–14786. [Google Scholar] [CrossRef]
- Rettig, J.; Sheng, Z.H.; Kim, D.K.; Hodson, C.D.; Snutch, T.P.; Catterall, W.A. Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc. Natl. Acad. Sci. USA 1996, 93, 7363–7368. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Scheller, R.H.; Tsien, R.W. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature 1995, 378, 623–626. [Google Scholar] [CrossRef]
- Wiser, O.; Bennett, M.K.; Atlas, D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. Embo J. 1996, 15, 4100–4110. [Google Scholar] [CrossRef]
- Zhong, H.; Yokoyama, C.T.; Scheuer, T.; Catterall, W.A. Reciprocal regulation of P/Q-type Ca2+ channels by SNAP-25, syntaxin and synaptotagmin. Nat. Neurosci. 1999, 2, 939–941. [Google Scholar] [CrossRef]
- Jarvis, S.E.; Zamponi, G.W. Distinct molecular determinants govern syntaxin 1A-mediated inactivation and G-protein inhibition of N-type calcium channels. J. Neurosci. 2001, 21, 2939–2948. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Zhong, P.; Scheller, R.H.; Tsien, R.W. Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating. Proc. Natl. Acad. Sci. USA 2000, 97, 13943–13948. [Google Scholar] [CrossRef]
- Geppert, M.; Goda, Y.; Hammer, R.E.; Li, C.; Rosahl, T.W.; Stevens, C.F.; Südhof, T.C. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell 1994, 79, 717–727. [Google Scholar] [CrossRef]
- Xu, J.; Mashimo, T.; Sudhof, T.C. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 2007, 54, 567–581. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Yokoyama, C.T.; Catterall, W.A. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA 1997, 94, 5405–5410. [Google Scholar] [CrossRef]
- Wiser, O.; Tobi, D.; Trus, M.; Atlas, D. Synaptotagmin restores kinetic properties of a syntaxin-associated N-type voltage sensitive calcium channel. FEBS Lett. 1997, 404, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, C.T.; Sheng, Z.H.; Catterall, W.A. Phosphorylation of the synaptic protein interaction site on N-type calcium channels inhibits interactions with SNARE proteins. J. Neurosci. 1997, 17, 6929–6938. [Google Scholar] [CrossRef]
- DeMaria, C.D.; Soong, T.W.; Alseikhan, B.A.; Alvania, R.S.; Yue, D.T. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 2001, 411, 484–489. [Google Scholar] [CrossRef]
- Lee, A.; Scheuer, T.; Catterall, W.A. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J. Neurosci. 2000, 20, 6830–6838. [Google Scholar] [CrossRef]
- Lee, A.; Wong, S.T.; Gallagher, D.; Li, B.; Storm, D.R.; Scheuer, T.; Catterall, W.A. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature 1999, 399, 155–159. [Google Scholar] [CrossRef]
- Lee, A.; Zhou, H.; Scheuer, T.; Catterall, W.A. Molecular determinants of Ca2+/calmodulin-dependent regulation of CaV2.1 channels. Proc. Natl. Acad. Sci. USA 2003, 100, 16059–16064. [Google Scholar] [CrossRef]
- Few, A.P.; Lautermilch, N.J.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Differential regulation of CaV2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation. J. Neurosci. 2005, 25, 7071–7080. [Google Scholar] [CrossRef]
- Lautermilch, N.J.; Few, A.P.; Scheuer, T.; Catterall, W.A. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J. Neurosci. 2005, 25, 7062–7070. [Google Scholar] [CrossRef]
- Lee, A.; Westenbroek, R.E.; Haeseleer, F.; Palczewski, K.; Scheuer, T.; Catterall, W.A. Differential modulation of CaV2.1 channels by calmodulin and Ca2+-binding protein 1. Nat. Neurosci. 2002, 5, 210–217. [Google Scholar] [CrossRef]
- Yan, J.; Leal, K.; Magupalli, V.G.; Nanou, E.; Martinez, G.Q.; Scheuer, T.; Catterall, W.A. Modulation of CaV2.1 channels by neuronal calcium sensor-1 induces short-term synaptic facilitation. Mol. Cell Neurosci. 2014, 63, 124–131. [Google Scholar] [CrossRef]
- Liu, H.; De Waard, M.; Scott, V.E.; Gurnett, C.A.; Lennon, V.A.; Campbell, K.P. Identification of three subunits of the high affinity omega-conotoxin MVIIC-sensitive Ca2+ channel. J. Biol. Chem. 1996, 271, 13804–13810. [Google Scholar] [CrossRef]
- Forsythe, I.D.; Tsujimoto, T.; Barnes-Davies, M.; Cuttle, M.F.; Takahashi, T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron 1998, 20, 797–807. [Google Scholar] [CrossRef]
- Xu, J.; Wu, L.G. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron 2005, 46, 633–645. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Alseikhan, B.A.; Chang, S.Y.; Soong, T.W.; Yue, D.T. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J. Neurosci. 2005, 25, 8282–8294. [Google Scholar] [CrossRef]
- Mochida, S.; Few, A.P.; Scheuer, T.; Catterall, W.A. Regulation of presynaptic CaV2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron 2008, 57, 210–216. [Google Scholar]
- Kajikawa, Y.; Saitoh, N.; Takahashi, T. GTP-binding protein βγ subunits mediate presynaptic calcium current inhibition by GABAB receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 8054–8058. [Google Scholar] [CrossRef]
- Takahashi, T.; Forsythe, I.D.; Tsujimoto, T.; Barnes-Davies, M.; Onodera, K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science 1996, 274, 594–597. [Google Scholar] [CrossRef]
- Brown, S.P.; Safo, P.K.; Regehr, W.G. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J. Neurosci. 2004, 24, 5623–5631. [Google Scholar] [CrossRef]
- Brody, D.L.; Yue, D.T. Relief of G-protein inhibition of calcium channels and short-term synaptic facilitation in cultured hippocampal neurons. J. Neurosci. 2000, 20, 889–898. [Google Scholar] [CrossRef]
- Han, J.; Mark, M.D.; Li, X.; Xie, M.; Waka, S.; Rettig, J.; Herlitze, S. RGS2 determines short-term synaptic plasticity in hippocampal neurons by regulating Gi/o-mediated inhibition of presynaptic Ca2+ channels. Neuron 2006, 51, 575–586. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Regehr, W.G. Modulation of transmission during trains at a cerebellar synapse. J. Neurosci. 2000, 20, 1348–1357. [Google Scholar] [CrossRef]
- Stephens, G.J.; Mochida, S. G protein βγ subunits mediate presynaptic inhibition of transmitter release from rat superior cervical ganglion neurones in culture. J. Physiol. 2005, 563, 765–776. [Google Scholar] [CrossRef] [Green Version]
- Bucci, G.; Mochida, S.; Stephens, G.J. Inhibition of synaptic transmission and G protein modulation by synthetic CaV2.2 Ca2+ channel peptides. J. Physiol. 2011, 589, 3085–3101. [Google Scholar] [CrossRef]
- Mochida, S.; Sheng, Z.H.; Baker, C.; Kobayashi, H.; Catterall, W.A. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron 1996, 17, 781–788. [Google Scholar] [CrossRef]
- Rettig, J.; Heinemann, C.; Ashery, U.; Sheng, Z.H.; Yokoyama, C.T.; Catterall, W.A.; Neher, E. Alteration of Ca2+ dependence of neurotransmitter release by disruption of Ca2+ channel/syntaxin interaction. J. Neurosci. 1997, 17, 6647–6656. [Google Scholar] [CrossRef]
- Inchauspe, C.G.; Forsythe, I.D.; Uchitel, O.D. Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels. J. Physiol. 2007, 584, 835–851. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, S.; Momiyama, A.; Uchitel, O.D.; Takahashi, T. Developmental changes in calcium channel types mediating central synaptic transmission. J. Neurosci. 2000, 20, 59–65. [Google Scholar] [CrossRef]
- Wu, L.G.; Borst, J.G. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron 1999, 23, 821–832. [Google Scholar] [CrossRef]
- Wadel, K.; Neher, E.; Sakaba, T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron 2007, 53, 563–575. [Google Scholar] [CrossRef]
- Wu, L.G.; Westenbroek, R.E.; Borst, J.G.G.; Catterall, W.A.; Sakmann, B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J. Neurosci. 1999, 19, 726–736. [Google Scholar] [CrossRef]
- Keith, R.K.; Poage, R.E.; Yokoyama, C.T.; Catterall, W.A.; Meriney, S.D. Bidirectional modulation of transmitter release by calcium channel/syntaxin interactions in vivo. J. Neurosci. 2007, 27, 265–269. [Google Scholar] [CrossRef]
- Mochida, S.; Nonomura, Y.; Kobayashi, H. Analysis of the mechanism for acetylcholine release at the synapse formed between rat sympathetic neurons in culture. Microsc. Res. Tech. 1994, 29, 94–102. [Google Scholar] [CrossRef]
- Ma, H.; Mochida, S. A cholinergic model synapse to elucidate protein function at presynaptic terminals. Neurosci. Res. 2007, 57, 491–498. [Google Scholar] [CrossRef]
- Mochida, S.; Saisu, H.; Kobayashi, H.; Abe, T. Impairment of syntaxin by botulinum neurotoxin C1 or antibodies inhibits acetylcholine release but not Ca2+ channel activity. Neuroscience 1995, 65, 905–915. [Google Scholar] [CrossRef]
- Mochida, S.; Westenbroek, R.E.; Yokoyama, C.T.; Itoh, K.; Catterall, W.A. Subtype-selective reconstitution of synaptic transmission in sympathetic ganglion neurons by expression of exogenous calcium channels. Proc. Natl. Acad. Sci. USA 2003, 100, 2813–2818. [Google Scholar] [CrossRef] [Green Version]
- Voleti, R.; Tomchick, D.R.; Südhof, T.C.; Rizo, J. Exceptionally tight membrane-binding may explain the key role of the synaptotagmin-7 C2A domain in asynchronous neurotransmitter release. Proc. Natl. Acad. Sci. USA 2017, 114, E8518–E8527. [Google Scholar] [CrossRef]
- Voleti, R.; Tomchick, D.R.; Südhof, T.C.; Rizo, J. Calmodulin as a direct detector of Ca2+ signals. Nat. Neurosci. 2011, 14, 301–304. [Google Scholar]
- Leal, K.; Mochida, S.; Scheuer, T.; Catterall, W.A. Fine-tuning synaptic plasticity by modulation of CaV2.1 channels with Ca2+ sensor proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 17069–17074. [Google Scholar] [CrossRef]
- Haeseleer, F.; Sokal, I.; Verlinde, C.L.; Erdjument-Bromage, H.; Tempst, P.; Pronin, A.N.; Benovic, J.L.; Fariss, R.N.; Palczewski, K. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J. Biol. Chem. 2000, 275, 1247–1260. [Google Scholar] [CrossRef]
- Burgoyne, R.D.; Weiss, J.L. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem. J. 2001, 353, 1–12. [Google Scholar] [CrossRef]
- Mikhaylova, M.; Hradsky, J.; Kreutz, M.R. Between promiscuity and specificity: Novel roles of EF-hand calcium sensors in neuronal Ca2+ signaling. J. Neurochem. 2011, 118, 695–713. [Google Scholar] [CrossRef]
- Abbott, L.F.; Regehr, W.G. Synaptic computation. Nature 2004, 431, 796–803. [Google Scholar] [CrossRef]
- Kennedy, M.B.; Bennett, M.K.; Bulleit, R.F.; Erondu, N.E.; Jennings, V.R.; Miller, S.G.; Molloy, S.S.; Patton, B.L.; Schenker, L.J. Structure and regulation of type II calcium/calmodulin-dependent protein kinase in central nervous system neurons. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 101–110. [Google Scholar] [CrossRef]
- Lüscher, C.; Nicoll, R.A.; Malenka, R.C.; Muller, D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 2000, 3, 545–550. [Google Scholar] [CrossRef]
- Schulman, H.; Greengard, P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by “calcium-dependent regulator”. Proc. Natl. Acad. Sci. USA 1978, 75, 5432–5436. [Google Scholar] [CrossRef]
- Shepherd, J.D.; Huganir, R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007, 23, 613–643. [Google Scholar] [CrossRef]
- Llinas, R.; McGuinness, T.L.; Leonard, C.S.; Sugimori, M.; Greengard, P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc. Natl. Acad. Sci. USA 1985, 82, 3035–3039. [Google Scholar] [CrossRef]
- Llinas, R.; Gruner, J.A.; Sugimori, M.; McGuinness, T.L.; Greengard, P. Regulation by synapsin I and Ca2+-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J. Physiol. 1991, 436, 257–282. [Google Scholar] [CrossRef]
- Chapman, P.F.; Frenguelli, B.G.; Smith, A.; Chen, C.M.; Silva, A.J. The α-Ca2+/calmodulin kinase II: A bidirectional modulator of presynaptic plasticity. Neuron 1995, 14, 591–597. [Google Scholar] [CrossRef]
- Lu, F.M.; Hawkins, R.D. Presynaptic and postsynaptic Ca2+ and CamKII contribute to long-term potentiation at synapses between individual CA3 neurons. Proc. Natl. Acad. Sci. USA 2006, 103, 4264–4269. [Google Scholar] [CrossRef]
- Jiang, X.; Lautermilch, N.J.; Watari, H.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc. Natl. Acad. Sc. USA 2008, 105, 341–346. [Google Scholar] [CrossRef]
- Magupalli, V.G.; Mochida, S.; Yan, J.; Jiang, X.; Westenbroek, R.E.; Nairn, A.C.; Scheuer, T.; Catterall, W.A. Ca2+-independent activation of Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain of CaV2.1 calcium channels. J. Biol. Chem. 2013, 288, 4637–4648. [Google Scholar] [CrossRef]
- Chang, B.H.; Mukherji, S.; Soderling, T.R. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc. Natl. Acad. Sci. USA 1998, 95, 10890–10895. [Google Scholar] [CrossRef] [Green Version]
- Benfenati, F.; Valtorta, F.; Chieregatti, E.; Greengard, P. Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron 1992, 8, 377–386. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef]
- Inchauspe, C.G.; Martini, F.J.; Forsythe, I.D.; Uchitel, O.D. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of Held presynaptic terminal. J. Neurosci. 2004, 24, 10379–10383. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kaneko, M.; Shin, H.S.; Takahashi, T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J. Physiol. 2005, 568, 199–209. [Google Scholar] [CrossRef]
- Habets, R.L.; Borst, J.G. Post-tetanic potentiation in the rat calyx of Held synapse. J. Physiol 2005, 564, 173–187. [Google Scholar] [CrossRef]
- Xie, M.; Li, X.; Han, J.; Vogt, D.L.; Wittemann, S.; Mark, M.D.; Herlitze, S. Facilitation versus depression in cultured hippocampal neurons determined by targeting of Ca2+ channel Cavbeta4 versus Cavβ2 subunits to synaptic terminals. J. Cell Biol. 2007, 178, 489–502. [Google Scholar] [CrossRef]
- Nanou, E.; Sullivan, J.M.; Scheuer, T.; Catterall, W.A. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to short-term synaptic plasticity in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2016, 113, 1062–1067. [Google Scholar] [CrossRef]
- Nanou, E.; Scheuer, T.; Catterall, W.A. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to long-term potentiation and spatial learning. Proc. Natl. Acad. Sci. USA 2016, 113, 13209–13214. [Google Scholar] [CrossRef]
- Maximov, A.; Lao, Y.; Li, H.; Chen, X.; Rizo, J.; Sørensen, J.B.; Südhof, T.C. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc. Natl. Acad. Sci. USA 2008, 105, 3986–3991. [Google Scholar] [CrossRef] [Green Version]
- Jackman, S.L.; Turecek, J.; Belinsky, J.E.; Regehr, W.G. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 2016, 529, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Schwaller, B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010, 2, a004051. [Google Scholar] [CrossRef]
- Gainey, M.A.; Feldman, D.E. Multiple shared mechanisms for homeostatic plasticity in rodent somatosensory and visual cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2017, 372, 1–7. [Google Scholar] [CrossRef]
- Chevaleyre, V.; Piskorowski, R. Modulating excitation through plasticity at inhibitory synapses. Front. Cell Neurosci. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Cheron, G.; Servais, L.; Dan, B. Cerebellar network plasticity: From genes to fast oscillation. Neuroscience 2008, 153, 1–19. [Google Scholar] [CrossRef]
- Lee, S.H.; Schwaller, B.; Neher, E. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: Implications for Ca2+ transients of neuronal dendrites. J. Physiol. 2000, 525, 419–432. [Google Scholar] [CrossRef]
- Müller, M.; Felmy, F.; Schwaller, B.; Schneggenburger, R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of Held that accelerates the decay of Ca2+ and short-term facilitation. J. Neurosci. 2007, 27, 2261–2271. [Google Scholar] [CrossRef]
- Nägerl, U.V.; Novo, D.; Mody, I.; Vergara, J.L. Binding kinetics of calbindin-D(28k) determined by flash photolysis of caged Ca2+. Biophys. J. 2000, 79, 3009–3018. [Google Scholar] [CrossRef]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef]




© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochida, S. Presynaptic Calcium Channels. Int. J. Mol. Sci. 2019, 20, 2217. https://doi.org/10.3390/ijms20092217
Mochida S. Presynaptic Calcium Channels. International Journal of Molecular Sciences. 2019; 20(9):2217. https://doi.org/10.3390/ijms20092217
Chicago/Turabian StyleMochida, Sumiko. 2019. "Presynaptic Calcium Channels" International Journal of Molecular Sciences 20, no. 9: 2217. https://doi.org/10.3390/ijms20092217