A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration
Abstract
:1. Introduction
2. Results
2.1. Rabbits
2.1.1. Post-Implant and Macroscopic Evaluations
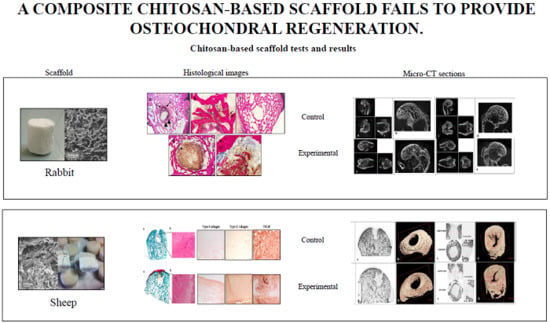
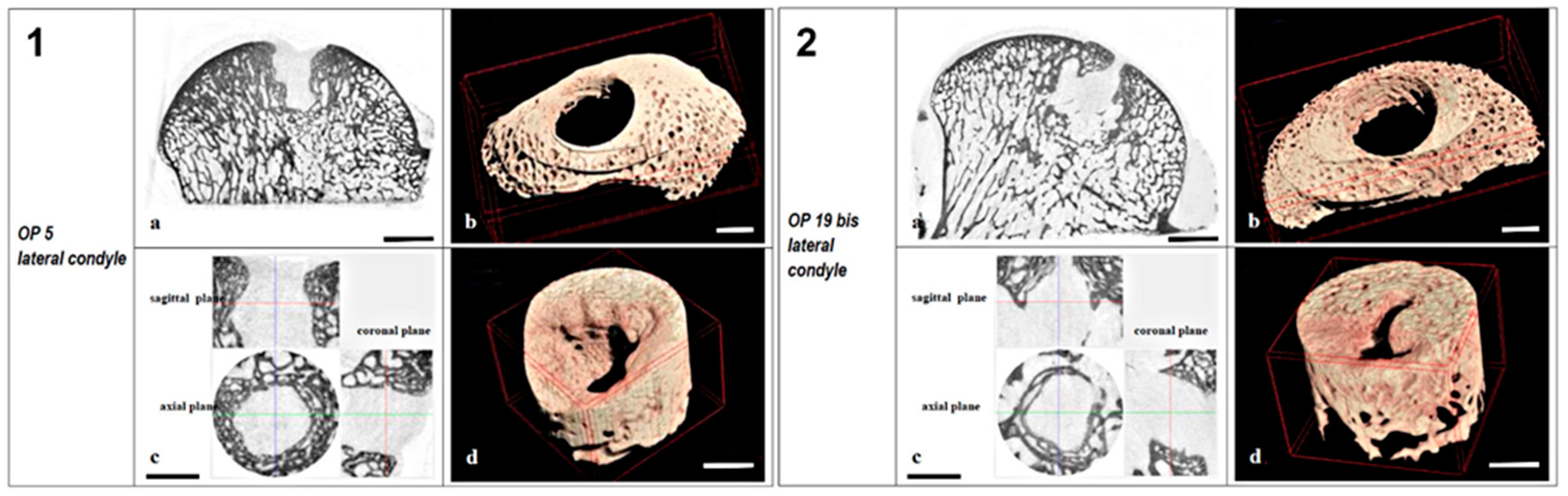
2.1.2. Microtomography
2.1.3. Histology and Histomorphometry
2.2. Sheep
2.2.1. Post-Implant and Macroscopic Evaluations
2.2.2. Microtomography
2.2.3. Histology
2.2.4. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Scaffold Preparation
4.1.1. Bone Scaffold Preparation
4.1.2. Cartilaginous Layer Scaffold Preparation
4.1.3. Osteochondral Scaffold Preparation
4.2. Surgical Procedures
4.2.1. Rabbit Model
4.2.2. Sheep Model
4.3. Explant Evaluation
4.3.1. Microtomography
4.3.2. Morphological Parameters
- Defect BV/TV1 (%), ratio between the volume of newly formed bone within the bone defect and the total volume of the bone defect;
- Defect Trabecular Thickness Tb.Th (mm), calculated in a model-independent way [47] over the total volume of the bone defect TV1;
- Defect Trabecular Number Tb.N (mm−1), the number of intersections through a trabecular structure per unit length of a random linear path through TV1;
- Defect Trabecular Separation Tb.Sp (mm), calculated as the Tb.Th;
- Peri-implant BV/TV2 (%), ratio between the volume of trabecular bone around the bone defect and the total volume of interest TV2;
- Peri-implant Tb.Th Trabecular Thickness, Tb.N Trabecular Number and Tb.Sp Trabecular Separation calculated as above (in TV2) (see Supplementary Materials).
4.3.3. Histology and Histomorphometry
4.3.4. Immunohistochemical Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomoll, A.H.; Filardo, G.; Almqvist, F.K.; Bugbee, W.D.; Jelic, M.; Monllau, J.C.; Puddu, G.; Rodkey, W.G.; Verdonk, P.; Verdonk, R.; et al. Surgical treatment for early osteoarthritis. Part II: Allografts and concurrent procedures. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 468–486. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Filardo, G.; de Girolamo, L.; Espregueira-Mendes, J.; Marcacci, M.; Rodkey, W.G.; Steadman, J.R.; Zaffagnini, S.; Kon, E. Surgical treatment for early osteoarthritis. Part I: Cartilage repair procedures. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 767–775. [Google Scholar] [CrossRef]
- Abarrategi, A.; Lopiz-Morales, Y.; Ramos, V.; Civantos, A.; Lopez-Duran, L.; Marco, F.; Lopez-Lacomba, J.L. Chitosan scaffolds for osteochondral tissue regeneration. J. Biomed. Mater. Res. Part A 2010, 95, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Torricelli, P.; Panzavolta, S.; Parrilli, A.; Fini, M.; Bigi, A. Multi-Layered Scaffolds for Osteochondral Tissue Engineering: In Vitro Response of Co-Cultured Human Mesenchymal Stem Cells. Macromol. Biosci. 2015, 15, 1535–1545. [Google Scholar] [CrossRef]
- Melton, J.T.K.; Wilson, A.J.; Chapman-Sheath, P.; Cossey, A.J. TruFit CB (R) bone plug: Chondral repair, scaffold design, surgical technique and early experiences. Expert Rev. Med. Devic. 2010, 7, 333–341. [Google Scholar] [CrossRef]
- Williams, R.J.; Gamradt, S.C. Articular cartilage repair using a resorbable matrix scaffold. Instr. Course Lect. 2008, 57, 563–571. [Google Scholar]
- Tampieri, A.; Sandri, M.; Landi, E.; Pressato, D.; Francioli, S.; Quarto, R.; Martin, I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 2008, 29, 3539–3546. [Google Scholar] [CrossRef] [Green Version]
- Filardo, G.; Kon, E.; Perdisa, F.; Di Matteo, B.; Di Martino, A.; Iacono, F.; Zaffagnini, S.; Balboni, F.; Vaccari, V.; Marcacci, M. Osteochondral scaffold reconstruction for complex knee lesions: A comparative evaluation. Knee 2013, 20, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Zaffagnini, S.; Kon, E.; Muccioli, G.M.M.; Di Martino, A.; Di Matteo, B.; Bonanzinga, T.; Iacono, F.; Filardo, G. Unicompartmental osteoarthritis: An integrated biomechanical and biological approach as alternative to metal resurfacing. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2509–2517. [Google Scholar] [CrossRef]
- Perdisa, F.; Kon, E.; Sessa, A.; Andriolo, L.; Busacca, M.; Marcacci, M.; Filardo, G. Treatment of Knee Osteochondritis Dissecans With a Cell-Free Biomimetic Osteochondral Scaffold: Clinical and Imaging Findings at Midterm Follow-up. Am. J. Sports Med. 2018, 46, 314–321. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am. J. Sports Med. 2014, 42, 158–165. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2704–2715. [Google Scholar] [CrossRef]
- Kon, E.; Ronga, M.; Filardo, G.; Farr, J.; Madry, H.; Milano, G.; Andriolo, L.; Shabshin, N. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Feng, Q.L.; Jiao, Y.F.; Cui, F.H. Collagen-based scaffolds reinforced by chitosan fibres for bone tissue engineering. Polym. Int. 2005, 54, 1034–1040. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Kennedy, C.J.; Wess, T.J. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Tan, Y.; Zhang, B.; Gu, Z.; Li, X. Synthesis and evaluation of collagen-chitosan-hydroxyapatite nanocomposites for bone grafting. J. Biomed. Mater. Res. Part A 2009, 89, 1079–1087. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Hurtig, M.; Rossomacha, E.; Sun, J.; Chevrier, A.; Shive, M.S.; Buschmann, M.D. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J. Bone Jt. Surg. Am. Vol. 2005, 87, 2671–2686. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef]
- Shive, M.S.; Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Methot, S.; Vehik, K.; Restrepo, A. BST-CarGel(R) Treatment Maintains Cartilage Repair Superiority over Microfracture at 5 Years in a Multicenter Randomized Controlled Trial. Cartilage 2015, 6, 62–72. [Google Scholar] [CrossRef]
- Fortier, L.A.; Mohammed, H.O.; Lust, G.; Nixon, A.J. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J. Bone Jt. Surg. Br. Vol. 2002, 84, 276–288. [Google Scholar] [CrossRef] [Green Version]
- Niederauer, G.G.; Slivka, M.A.; Leatherbury, N.C.; Korvick, D.L.; Harroff, H.H.; Ehler, W.C.; Dunn, C.J.; Kieswetter, K. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials 2000, 21, 2561–2574. [Google Scholar] [CrossRef]
- Paul, K.; Lee, B.Y.; Abueva, C.; Kim, B.; Choi, H.J.; Bae, S.H.; Lee, B.T. In vivo evaluation of injectable calcium phosphate cement composed of Zn− and Si-incorporated beta-tricalcium phosphate and monocalcium phosphate monohydrate for a critical sized defect of the rabbit femoral condyle. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 260–271. [Google Scholar] [CrossRef]
- Brehm, W.; Aklin, B.; Yamashita, T.; Rieser, F.; Trub, T.; Jakob, R.P.; Mainil-Varlet, P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: Implantation method and short-term results. Osteoarthr. Cartil. 2006, 14, 1214–1226. [Google Scholar] [CrossRef]
- Buma, P.; Schreurs, W.; Verdonschot, N. Skeletal tissue engineering-from in vitro studies to large animal models. Biomaterials 2004, 25, 1487–1495. [Google Scholar] [CrossRef]
- Shive, M.S.; Hoemann, C.D.; Restrepo, A.; Hurtig, M.B.; Duval, N.; Ranger, P.; Stanish, W.D.; Buschman, M.D. BST-CarGel: In situ Chondro Induction for cartilage repair. Oper. Tech. Orthop. 2006, 16, 271–278. [Google Scholar] [CrossRef]
- Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Restrepo, A.; Shive, M.S. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J. Bone Jt. Surg. Am. Vol. 2013, 95, 1640–1650. [Google Scholar] [CrossRef]
- Methot, S.; Changoor, A.; Tran-Khanh, N.; Hoemann, C.D.; Stanish, W.D.; Restrepo, A.; Shive, M.S.; Buschmann, M.D. Osteochondral Biopsy Analysis Demonstrates That BST-CarGel Treatment Improves Structural and Cellular Characteristics of Cartilage Repair Tissue Compared with Microfracture. Cartilage 2016, 7, 16–28. [Google Scholar] [CrossRef]
- Steinwachs, M.R.; Waibl, B.; Mumme, M. Arthroscopic Treatment of Cartilage Lesions with Microfracture and BST-CarGel. Arthrosc. Tech. 2014, 3, e399–e402. [Google Scholar] [CrossRef]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of three-dimensional chitosan-agarose-gelatin cryogel scaffold for the repair of subchondral cartilage defects: An in vivo study in a rabbit model. Tissue Eng. Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef]
- Deng, J.; She, R.; Huang, W.; Dong, Z.; Mo, G.; Liu, B. A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J. Mater. Sci. Mater. Med. 2013, 24, 2037–2046. [Google Scholar] [CrossRef]
- Suh, J.K.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Chang, C.P.; Lin, S.M. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2007, 57, 250–255. [Google Scholar] [CrossRef]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.K. In vivo study of chitosan-natural nano hydroxyapatite scaffolds for bone tissue regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Galed, G.; Miralles, B.; Panos, I.; Santiago, A.; Heras, A. N-Deacetylation and depolymerization reactions of chitin/chitosan: Influence of the source of chitin. Carbohydr. Polym. 2005, 62, 316–320. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical sciences. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar]
- Rodriguez-Vazquez, M.; Vega-Ruiz, B.; Ramos-Zuniga, R.; Saldana-Koppel, D.A.; Quinones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- Sprio, S.; Sandri, M.; Iafisco, M.; Panseri, S.; Filardo, G.; Kon, E.; Marcacci, M.; Tampieri, A. Composite Biomedical Foams for Engineering Bone Tissue. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 249–280. [Google Scholar]
- Sprio, S.; Sandri, M.; Panseri, S.; Iafisco, M.; Ruffini, A.; Minardi, S.; Tampieri, A. Bone Substitutes Based on Biomineralization; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Tampieri, A.; Celotti, G.; Landi, E.; Sandri, M.; Roveri, N.; Falini, G. Biologically inspired synthesis of bone-like composite: Self-assembled collagen fibers/hydroxyapatite nanocrystals. J. Biomed. Mater. Res. Part A 2003, 67, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J. Orthop. Res. 2010, 28, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Qin, C.; Wang, W.; Chi, W.; Li, W. Absorption and distribution of chitosan in mice after oral administration. Carbohydr. Polym. 2008, 71, 435–440. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput. Methods Biomech. Biomed. Eng. 1997, 1, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Pineda, S.; Pollack, A.; Stevenson, S.; Goldberg, V.; Caplan, A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat. 1992, 143, 335–340. [Google Scholar] [CrossRef]



| Group | Gross Appearance Score Modified by Fortier et al. | Gross Morphology Score Modified by Niederaurer et al. | ||
|---|---|---|---|---|
| CFM 15 min < 0 max | CFL 15 min < 0 max | CFM 0 min < 8 max | CFL 0 min < 8 max | |
| Untreated group | 5.5 | 8 | 3 | 3 |
| Experimental group | 9.5 | 9.25 | 3.25 | 3 |
| Parameters | Group | N | Mean | Std. Deviation |
|---|---|---|---|---|
| Defect Filling | Untreated | 4 | 3.00 | 0.82 |
| Chitosan | 8 | 1.75 | 0.89 | |
| Osteochondral Junction Reconstruction | Untreated | 4 | 1.00 | 0.82 |
| Chitosan | 8 | 0.63 | 0.52 | |
| Matrix Staining | Untreated | 4 | 2.25 | 1.50 |
| Chitosan | 8 | 1.25 | 1.39 | |
| Cell Morphology | Untreated | 4 | 2.50 | 1.29 |
| Chitosan | 8 | 2.38 | 0.74 | |
| Total | Untreated | 4 | 8.75 | 3.59 |
| Chitosan | 8 | 6.00 | 2.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roffi, A.; Kon, E.; Perdisa, F.; Fini, M.; Di Martino, A.; Parrilli, A.; Salamanna, F.; Sandri, M.; Sartori, M.; Sprio, S.; et al. A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration. Int. J. Mol. Sci. 2019, 20, 2227. https://doi.org/10.3390/ijms20092227
Roffi A, Kon E, Perdisa F, Fini M, Di Martino A, Parrilli A, Salamanna F, Sandri M, Sartori M, Sprio S, et al. A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration. International Journal of Molecular Sciences. 2019; 20(9):2227. https://doi.org/10.3390/ijms20092227
Chicago/Turabian StyleRoffi, Alice, Elizaveta Kon, Francesco Perdisa, Milena Fini, Alessandro Di Martino, Annapaola Parrilli, Francesca Salamanna, Monica Sandri, Maria Sartori, Simone Sprio, and et al. 2019. "A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration" International Journal of Molecular Sciences 20, no. 9: 2227. https://doi.org/10.3390/ijms20092227