Relationship of Iron Metabolism and Short-Term Cuprizone Treatment of C57BL/6 Mice
Abstract
:1. Introduction
2. Results
2.1. Effect of CZ on the Expression of Activation Markers
2.2. Effect of CZ Administration on the Expression Levels of Hepcidin
2.3. Effect of CZ Treatment on the Regulators of Hepcidin Expression
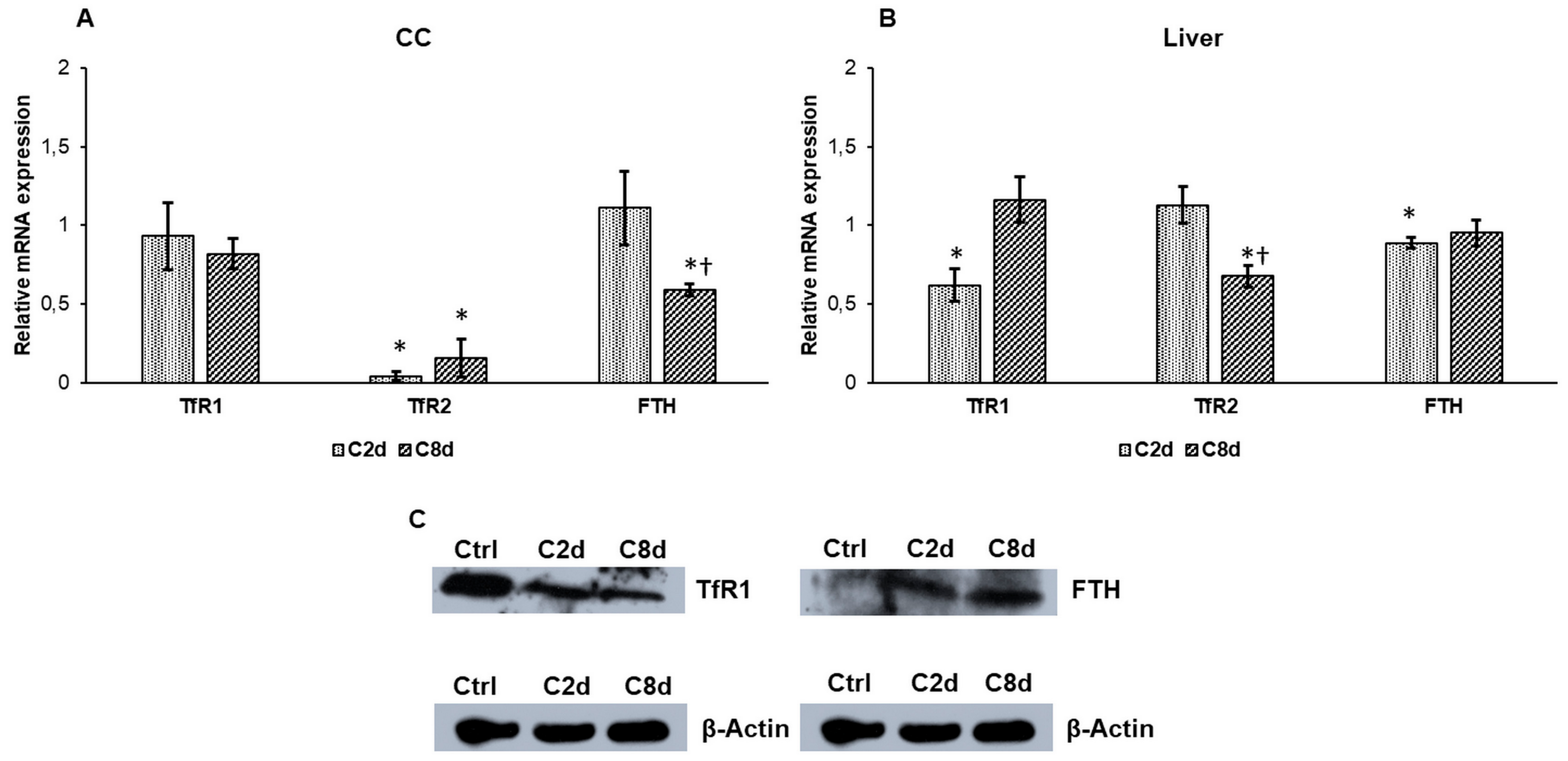
2.4. Effect of CZ Treatment on the Iron Storage and Transferrin Receptors Expressions
2.5. Effect of CZ Administration on the Expressions of Mitochondrial Iron Transport and Storage Genes
2.6. Expression Analysis of Mitochondrial Genes Involved in Heme and Iron-Sulfur Cluster Syntheses
2.7. Heme and Non-Heme Iron Contents of the Liver Following CZ Treatment
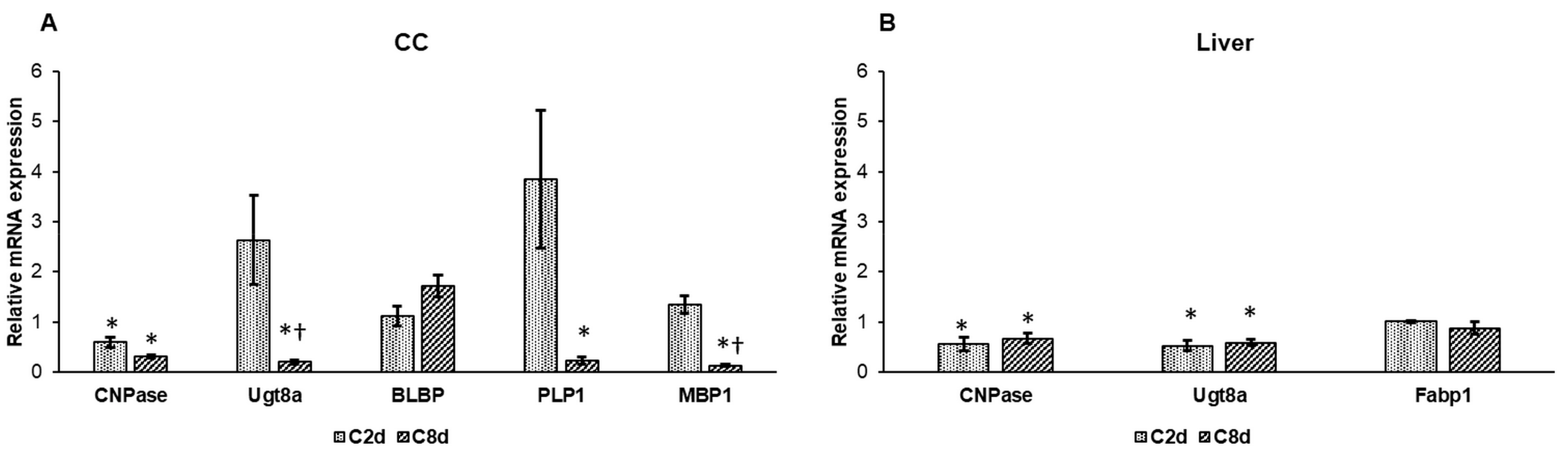
2.8. Expression Analysis of Lipid Metabolism Genes Following CZ Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RNA Isolation and Real Time PCR
4.3. Serum Hepcidin Measurements
4.4. Non-Heme Iron Measurements
4.5. Heme Measurements
4.6. Western Blot Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Mallucci, G.; Peruzzotti-Jametti, L.; Bernstock, J.D.; Pluchino, S. The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis. Prog. Neurobiol. 2015, 127, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Procaccini, C.; De Rosa, V.; Pucino, V.; Formisano, L.; Matarese, G. Animal models of Multiple Sclerosis. Eur. J. Pharmacol. 2015, 759, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Torkildsen, Ø.; Brunborg, L.A.; Myhr, K.-M.; Bø, L. The cuprizone model for demyelination. Acta Neurol. Scand. 2008, 117, 72–76. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Franklin, R.J.M. Remyelination in Experimental Models of Toxin-Induced Demyelination. Curr. Topics Microbiol. Immunol. 2008, 318, 193–212. [Google Scholar]
- Matsushima, G.K.; Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Johnson, A.J.; Bieber, A.J.; Warrington, A.E.; Rodriguez, M.; Pirko, I. The Relevance of Animal Models in Multiple Sclerosis Research. Pathophysiology 2011, 18, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Praet, J.; Guglielmetti, C.; Berneman, Z.; Van Der Linden, A.; Ponsaerts, P. Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci. Biobehav. Rev. 2014, 47, 485–505. [Google Scholar] [CrossRef] [Green Version]
- Benetti, F.; Ventura, M.; Salmini, B.; Ceola, S.; Carbonera, D.; Mammi, S.; Zitolo, A.; D’Angelo, P.; Urso, E.; Maffia, M.; et al. Cuprizone neurotoxicity, copper deficiency and neurodegeneration. NeuroToxicology 2010, 31, 509–517. [Google Scholar] [CrossRef]
- Bénardais, K.; Kotsiari, A.; Skuljec, J.; Koutsoudaki, P.N.; Gudi, V.; Singh, V.; Vulinović, F.; Skripuletz, T.; Stangel, M. Cuprizone [Bis(Cyclohexylidenehydrazide)] is Selectively Toxic for Mature Oligodendrocytes. Neurotox. Res. 2013, 24, 244–250. [Google Scholar] [CrossRef]
- Bai, C.B.; Sun, S.; Roholt, A.; Benson, E.; Edberg, D.; Medicetty, S.; Dutta, R.; Kidd, G.; Macklin, W.B.; Trapp, B. A mouse model for testing remyelinating therapies. Exp. Neurol. 2016, 283, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheld, M.; Fragoulis, A.; Nyamoya, S.; Zendedel, A.; Denecke, B.; Krauspe, B.; Teske, n.; Kipp, M.; Beyer, C.; Clarner, T. Mitochondrial Impairment in Oligodendroglial Cells Induces Cytokine Expression and Signaling. J. Mol. Neurosci. 2019, 67, 265–275. [Google Scholar] [CrossRef]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Clemente, D.; Ortega, M.C.; Melero-Jerez, C.; De Castro, F. The effect of glia-glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases. Front. Cell. Neurosci. 2013, 7, 268. [Google Scholar] [CrossRef]
- Dutta, R.; McDonough, J.; Yin, X.; Peterson, J.; Chang, A.; Torres, T.; Gudz, T.; Macklin, W.B.; Lewis, D.A.; Fox, R.J.; et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006, 59, 478–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Mena, N.P.; Urrutia, P.J.; Lourido, F.; Carrasco, C.M.; Núñez, M.T. Mitochondrial iron homeostasis and its dysfunctions in neurodegenerative disorders. Mitochondrion 2015, 21, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Finazzi, D. Neurodegeneration with brain iron accumulation: Update on pathogenic mechanisms. Front. Pharmacol. 2014, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Righy, C.; Bozza, M.T.; Oliveira, M.F.; Bozza, F.A. Molecular, Cellular and Clinical Aspects of Intracerebral Hemorrhage: Are the Enemies Within? Curr. Neuropharmacol. 2016, 14, 392–402. [Google Scholar] [CrossRef]
- Morelli, A.; Ravera, S.; Calzia, D.; Panfoli, I. Impairment of heme synthesis in myelin as potential trigger of multiple sclerosis. Med. Hypotheses 2012, 78, 707–710. [Google Scholar] [CrossRef]
- Chiabrando, D.; Fiorito, V.; Petrillo, S.; Tolosano, E. Unraveling the Role of Heme in Neurodegeneration. Front. Neurosci. 2018, 12, 712. [Google Scholar] [CrossRef]
- Hagemeier, J.; Ramanathan, M.; Schweser, F.; Dwyer, M.G.; Lin, F.; Bergsland, N.; Weinstock-Guttman, B.; Zivadinov, R. Iron-related gene variants and brain iron in multiple sclerosis and healthy individuals. NeuroImage Clin. 2017, 17, 530–540. [Google Scholar] [CrossRef]
- Braymer, J.J.; Lill, R. Iron–sulfur cluster biogenesis and trafficking in mitochondria. J. Boil. Chem. 2017, 292, 12754–12763. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Teunissen, C.; Langkammer, C. Iron and Neurodegeneration in Multiple Sclerosis. Mult. Scler. Int. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hametner, S.; Wimmer, I.; Haider, L.; Pfeifenbring, S.; Brück, W.; Lassmann, H. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 2013, 74, 848–861. [Google Scholar] [CrossRef] [Green Version]
- Stankiewicz, J.M.; Neema, M.; Ceccarelli, A. Iron and multiple sclerosis. Neurobiol. Aging 2014, 35, S51–S58. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Reichmann, H. Role of iron in neurodegenerative diseases. J. Neurovisc. Relations 2016, 123, 389–399. [Google Scholar] [CrossRef]
- Monteros, A.E.D.L.; Kumar, S.; Zhao, P.; Huang, C.J.; Nazarian, R.; Pan, T.; Scully, S.; Chang, R.; De Vellis, J. Transferrin is an essential factor for myelination. Neurochem. Res. 1999, 24, 235–248. [Google Scholar]
- Marta, C.B.; Cabrera, O.E.E.; Garcia, C.I.; Villar, M.J.; Pasquini, J.M.; Soto, E.F. Oligodendroglial cell differentiation in rat brain is accelerated by the intracranial injection of apotransferrin. Cell. Mol. Boil. 2000, 46, 529–539. [Google Scholar]
- Marta, C.B.; Paez, P.; Lopez, M.; De Iraldi, A.P.; Soto, E.F.; Pasquini, J.M. Morphological Changes of Myelin Sheaths in Rats Intracranially Injected with Apotransferrin. Neurochem. Res. 2003, 28, 101–110. [Google Scholar] [CrossRef]
- Paez, P.M.; Soto, E.F.; García, C.I.; Pasquini, J.M. Differential Gene Expression during Development in Two Oligodendroglial Cell Lines Overexpressing Transferrin: A cDNA Array Analysis. Dev. Neurosci. 2007, 29, 413–426. [Google Scholar]
- Moldovan, N.; Al-Ebraheem, A.; Lobo, L.; Park, R.; Farquharson, M.J.; Bock, N.A. Altered transition metal homeostasis in the cuprizone model of demyelination. NeuroToxicology 2015, 48, 1–8. [Google Scholar] [CrossRef]
- Todorich, B.; Zhang, X.; Seaman, W.E.; Connor, J.R.; Slagle-Webb, B.; Slagle-Webb, B. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J. Neurochem. 2008, 107, 1495–1505. [Google Scholar] [CrossRef] [Green Version]
- Hulet, S.; Heyliger, S.; Powers, S.; Connor, J. Oligodendrocyte progenitor cells internalize ferritin via clathrin-dependent receptor mediated endocytosis. J. Neurosci. 2000, 61, 52–60. [Google Scholar] [CrossRef]
- Adams, P.C.; Powell, L.W.; Halliday, J.W. Isolation of a human hepatic ferritin receptor. Hepatology 1988, 8, 719–721. [Google Scholar] [CrossRef]
- Adamo, A.; Paez, P.; Cabrera, O.E.; Wolfson, M.; Franco, P.; Pasquini, J.; Soto, E.; Paez, P. Remyelination after cuprizone-induced demyelination in the rat is stimulated by apotransferrin. Exp. Neurol. 2006, 198, 519–529. [Google Scholar] [CrossRef]
- Franco, P.G.; Pasquini, L.A.; Perez, M.J.; Rosato-Siri, M.V.; Silvestroff, L.; Pasquini, J.M. Paving the way for adequate myelination: The contribution of galectin-3, transferrin and iron. FEBS Lett. 2015, 589, 3388–3395. [Google Scholar] [CrossRef] [Green Version]
- Taraboletti, A.; Walker, T.; Avila, R.; Huang, H.; Caporoso, J.; Manandhar, E.; Leeper, T.C.; Modarelli, D.A.; Medicetty, S.; Shriver, L.P. Cuprizone Intoxication Induces Cell Intrinsic Alterations in Oligodendrocyte Metabolism Independent of Copper Chelation. Biochemistry 2017, 56, 1518–1528. [Google Scholar] [CrossRef]
- Vela, D. The Dual Role of Hepcidin in Brain Iron Load and Inflammation. Front. Neurosci. 2018, 12, 740. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Pandur, E.; Nagy, J.; Poór, VS.; Sarnyai, A.; Huszár, A.; Miseta, A.; Sipos, K. Alpha-1 antitrypsin binds preprohepcidin intracellularly and prohepcidin in the serum. FEBS J. 2009, 276, 2012–2021. [Google Scholar] [CrossRef]
- Gagliardo, B.; Kubat, N.; Faye, A.; Jaouen, M.; Durel, B.; Deschemin, J.-C.; Canonne-Hergaux, F.; Sari, M.-A.; Vaulont, S. Pro-hepcidin is unable to degrade the iron exporter ferroportin unless maturated by a furin-dependent process. J. Hepatol. 2009, 50, 394–401. [Google Scholar] [CrossRef]
- Sonnweber, T.; Nachbaur, D.; Schroll, A.; Nairz, M.; Seifert, M.; Demetz, E.; Haschka, D.; Mitterstiller, A.-M.; Kleinsasser, A.; Burtscher, M.; et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut 2014, 63, 1951–1959. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Nemeth, E. New insights into iron regulation and erythropoiesis. Curr. Opin. Hematol. 2015, 22, 199–205. [Google Scholar] [CrossRef] [Green Version]
- De Domenico, I.; Ward, DM.; Kaplan, J. Hepcidin regulation: Ironing out the details. J. Clin. Invest. 2014, 117, 1755–1758. [Google Scholar] [CrossRef]
- Muckenthaler, M.U. Fine Tuning of Hepcidin Expression by Positive and Negative Regulators. Cell Metab. 2008, 8, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Verga Falzacappa, M.V.; Vujic Spasic, M.; Kessler, R.; Stolte, J.; Hentze, M.W.; Muckenthaler, M.U. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 2007, 109, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Varga, E.; Pandur, E.; Abraham, H.; Horváth, A.; Ács, P.; Komoly, S.; Miseta, A.; Sipos, K. Cuprizone Administration Alters the Iron Metabolism in the Mouse Model of Multiple Sclerosis. Cell. Mol. Neurobiol. 2018, 38, 1081–1097. [Google Scholar] [CrossRef]
- Kipp, M.; Clarner, T.; Dang, J.; Copray, S.; Beyer, C. The cuprizone animal model: New insights into an old story. Acta Neuropathol. 2009, 118, 723–736. [Google Scholar] [CrossRef]
- Gudi, V.; Gingele, S.; Skripuletz, T.; Stangel, M. Glial response during cuprizone-induced de- and remyelination in the CNS: Lessons learned. Front. Cell. Neurosci. 2014, 8, 73. [Google Scholar] [CrossRef]
- Doan, V.; Kleindienst, A.M.; McMahon, E.J.; Long, B.R.; Matsushima, G.K.; Taylor, L.C. Abbreviated exposure to cuprizone is sufficient to induce demyelination and oligodendrocyte loss. J. Neurosci. Res. 2013, 91, 363–373. [Google Scholar] [CrossRef]
- Acs, P.; Komoly, S. Selective ultrastructural vulnerability in the cuprizone-induced experimental demyelination. Ideggyogy. Sz. 2012, 65, 266–270. [Google Scholar]
- Arredondo, M.; Núñez, M.T. Iron and copper metabolism. Mol. Aspects. Med. 2005, 26, 313–327. [Google Scholar] [CrossRef]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic crossroads of iron and copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Todorich, B.; Pasquini, J.M.; García, C.I.; Paez, P.M.; Connor, J.R. Oligodendrocytes and myelination: The role of iron. Glia 2009, 57, 467–478. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, H.; Van Horssen, J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011, 585, 3715–3723. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, H.; Van Horssen, J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 506–510. [Google Scholar] [CrossRef]
- Tezuka, T.; Tamura, M.; Kondo, M.A.; Sakaue, M.; Okada, K.; Takemoto, K.; Fukunari, A.; Miwa, K.; Ohzeki, H.; Kano, S.-I.; et al. Cuprizone short-term exposure: Astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol. Dis. 2013, 59, 63–68. [Google Scholar] [CrossRef]
- Saab, A.S.; Tzvetanova, I.D.; Nave, K.-A. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr. Opin. Neurobiol. 2013, 23, 1065–1072. [Google Scholar] [CrossRef]
- Hesse, A.; Wagner, M.; Held, J.; Brück, W.; Salinas-Riester, G.; Hao, Z.; Waisman, A.; Kuhlmann, T. In toxic demyelination oligodendroglial cell death occurs early and is FAS independent. Neurobiol. Dis. 2010, 37, 362–369. [Google Scholar] [CrossRef]
- Goldberg, J.; Daniel, M.; Van Heuvel, Y.; Victor, M.; Beyer, C.; Clarner, T.; Kipp, M. Short-Term Cuprizone Feeding Induces Selective Amino Acid Deprivation with Concomitant Activation of an Integrated Stress Response in Oligodendrocytes. Cell. Mol. Neurobiol. 2013, 33, 1087–1098. [Google Scholar] [CrossRef]
- Lane, D.; Merlot, A.; Huang, M.-H.; Bae, D.-H.; Jansson, P.; Sahni, S.; Kalinowski, D.; Richardson, D.; Kalinowski, D. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta. Mol. Basis. Dis. 2015, 1853, 1130–1144. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Musallam, K.M.; Taher, A.T.; Rivella, S. Ineffective Erythropoiesis: Anemia and Iron Overload. Hematol. Clin. 2018, 32, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Wallace, D.F.; Subramaniam, V.N. Hepcidin: Regulation of the master iron regulator. Biosci. Rep. 2015, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamashita, T. The roles of RGMa-neogenin signaling in inflammation and angiogenesis. Inflamm. Regen. 2017, 37, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, N.; Wahedi, M.; Zhang, A.-S.; Maxson, J.E.; Zhang, R.H.; Enns, C.A. Neogenin Facilitates the Induction of Hepcidin Expression by Hemojuvelin in the Liver. J. Boil. Chem. 2016, 291, 12322–12335. [Google Scholar] [CrossRef]
- Silvestri, L.; Nai, A.; Pagani, A.; Camaschella, C. The extrahepatic role of TFR2 in iron homeostasis. Front. Pharmacol. 2014, 5, 93. [Google Scholar] [CrossRef]
- Heidari, M.; Johnstone, D.M.; Bassett, B.; Graham, R.M.; Chua, A.C.G.; House, M.J.; Collingwood, J.F.; Bettencourt, C.; Houlden, H.; Ryten, M.; et al. Brain iron accumulation affects myelin-related molecular systems implicated in a rare neurogenetic disease family with neuropsychiatric features. Mol. Psychiatry 2016, 21, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J.; Wilcox, C.L.; Widness, J.A. Microanalysis of non-heme iron in animal tissues. J. Biochem. Biophys. Methods 2004, 58, 239–251. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandur, E.; Pap, R.; Varga, E.; Jánosa, G.; Komoly, S.; Fórizs, J.; Sipos, K. Relationship of Iron Metabolism and Short-Term Cuprizone Treatment of C57BL/6 Mice. Int. J. Mol. Sci. 2019, 20, 2257. https://doi.org/10.3390/ijms20092257
Pandur E, Pap R, Varga E, Jánosa G, Komoly S, Fórizs J, Sipos K. Relationship of Iron Metabolism and Short-Term Cuprizone Treatment of C57BL/6 Mice. International Journal of Molecular Sciences. 2019; 20(9):2257. https://doi.org/10.3390/ijms20092257
Chicago/Turabian StylePandur, Edina, Ramóna Pap, Edit Varga, Gergely Jánosa, Sámuel Komoly, Judit Fórizs, and Katalin Sipos. 2019. "Relationship of Iron Metabolism and Short-Term Cuprizone Treatment of C57BL/6 Mice" International Journal of Molecular Sciences 20, no. 9: 2257. https://doi.org/10.3390/ijms20092257




