The Regenerative Effect of Portal Vein Injection of Liver Organoids by Retrorsine/Partial Hepatectomy in Rats
Abstract
:1. Introduction
2. Results
2.1. Liver Organoids Made from ED14 Fetal Liver Cells
2.2. Liver Organoid Transplantation Accelerates Liver Regeneration in the Retrorsine/Partial Hepatectomy Model
2.3. Liver Organoids Reconstructed Normal Liver Structure and Inhibit Precancerous Lesion Derived by Chronic Injury from Retrorsine/Partial Hepatectomy
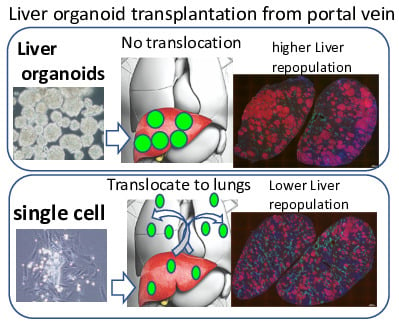
2.4. Trans-Portal Injection of Human Induced Pluripotent Stem Cell Liver Organoids Do Not Translocate Outside Liver
2.5. Human Induced Pluripotent Stem Cell Liver Organoids Attached and Reconstituted in the Immunodeficient Rat Liver
3. Discussion
4. Materials and Methods
4.1. Animals and Retrorsine Administration
4.2. Isolation of Fetal Liver Cells and Liver Organoid Formation
4.3. Liver Organoid and Monolayer Cell Transplantation to the Retrorsine/Partial Hepatectomy Liver
4.4. Liver Repopulation with the Transplanted Liver Organoids
4.5. Human Induced Pluripotent Stem Cell-Derived Liver Organoid Formation
4.6. Histochemistry and Immunohistochemistry
4.7. RT-PCRs
4.8. Serological Analyses
4.9. Study Approval
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Best, D.H.; Coleman, W.B. Bile duct destruction by 4,4’-diaminodiphenylmethane does not block the small hepatocyte-like progenitor cell response in retrorsine-exposed rats. Hepatology 2007, 46, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Horsen, S.P.; McCowan, T.C.; Goertzen, T.C.; Warkentin, P.I.; Cai, H.B.; Strom, S.C.; Fox, I.J. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics 2003, 111, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, R.D.; Mitry, R.R.; Dhawan, A. Current status of hepatocyte transplantation. Transplantation 2012, 93, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Enosawa, S.; Horikawa, R.; Yamamoto, A.; Sakamoto, S.; Shigeta, T.; Nosaka, S.; Fujimoto, J.; Tanoue, A.; Nakamura, K.; Umezawa, A.; et al. Hepatocyte transplantation using a living donor reduced graft in a baby with ornithine transcarbamylase deficiency: A novel source of hepatocytes. Liver Transpl. 2014, 20, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Dabeva, M.D.; Petkov, P.M.; Sandhu, J.; Oren, R.; Laconi, E.; Hurston, E.; Shafritz, D.A. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am. J. Pathol. 2000, 156, 2017–2031. [Google Scholar] [CrossRef] [Green Version]
- Oertel, M.; Menthena, A.; Dabeva, M.D.; Shafritz, D.A. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology 2006, 130, 507–520. [Google Scholar] [CrossRef]
- Menthena, A.; Koehler, C.; Sandhu, J.S.; Yovchev, M.I.; Hurston, E.; Shafritz, D.A.; Oertel, M. Activin A, p15INK4b signaling, and cell competition promote stem/progenitor cell repopulation of livers in aging rats. Gastroenterology 2011, 140, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Takebe, T.; Zhang, R.R.; Koike, H.; Kimura, M.; Yoshizawa, E.; Enomura, M.; Koike, N.; Sekine, K.; Taniguchi, H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 2014, 9, 396–409. [Google Scholar] [CrossRef]
- Takebe, T.; Enomura, M.; Yoshizawa, E.; Kimura, M.; Koike, H.; Ueno, Y.; Matsuzaki, T.; Yamazaki, T.; Toyohara, T.; Osafune, K.; et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem. Cell. 2015, 16, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Baccarani, U.; Adani, G.L.; Sanna, A.; Avellini, C.; Sainz-Barriga, M.; Lorenzin, D.; Montanaro, D.; Gasparini, D.; Risaliti, A.; Donini, A.; et al. Portal vein thrombosis after intraportal hepatocytes transplantation in a liver transplant recipient. Transpl. Int. 2005, 18, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Najimi, M.; Defresne, F.; Sokal, E.M. Concise review: Updated advances and current challenges in cell therapy for inborn liver metabolic defects. Stem Cells Transl. Med. 2016, 5, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Stephenne, X.; Nicastro, E.; Eeckhoudt, S.; Hermans, C.; Nyabi, O.; Lombard, C.; Najimi, M.; Sokal, E. Bivalirudin in combination with heparin to control mesenchymal cell procoagulant activity. PLoS ONE 2012, 7, e42819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, P.; Chen, L.; Wang, Y.; Wang, Z.; Zhang, B. The effects of organoid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials 2015, 41, 15–25. [Google Scholar] [CrossRef]
- Uchida, S.; Itaka, K.; Nomoto, T.; Endo, T.; Matsumoto, Y.; Ishii, T.; Kataoka, K. An injectable organoid system with genetic modification for cell transplantation therapy. Biomaterials 2014, 35, 2499–2506. [Google Scholar] [CrossRef]
- Gabriel, E.; Schievenbusch, S.; Kolossov, E.; Hengstler, J.G.; Rotshteyn, T.; Bohlen, H.; Hengstler, J.G.; Hescheler, J.; Drobinskaya, I. Differentiation and selection of hepatocyte precursors in suspension organoid culture of transgenic murine embryonic stem cells. PLoS ONE 2012, 7, e44912. [Google Scholar] [CrossRef]
- Nicolas, C.T.; Hickey, R.D.; Allen, K.L.; Du, Z.; Guthman, R.M.; Kaiser, R.A.; Amiot, B.; Bansal, A.; Pandey, M.K.; Suksanpaisan, L.; et al. Hepatocyte organoids as an alternative to single cells for transplantation after ex vivo gene therapy in mice and pig models. Surgery 2018, 164, 473–481. [Google Scholar] [CrossRef]
- Chojkier, M. Hepatic sinusoidal-obstruction syndrome: Toxicity of pyrrolizidine alkaloids. J. Hepatol. 2003, 39, 437–446. [Google Scholar] [CrossRef]
- Laconi, E.; Oren, R.; Mukhopadhyay, D.K.; Hurston, E.; Laconi, S.; Pani, P.; Dabeva, M.D.; Shafritz, D.A. Long term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am. J. Pathol. 1998, 153, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Helmy, A. Review article: Updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment. Pharmacol. Ther. 2006, 23, 11–25. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Sun, Y.; Guo, X.; Huang, W.; Peng, Y.; Zheng, J. Comparative Study of Hepatotoxicity of Pyrrolizidine Alkaloids Retrorsine and Monocrotaline. Chem. Res. Toxicol. 2017, 30, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Clouston, A.D.; Forbes, S.J. Links Between Hepatic Fibrosis, Ductular Reaction, and Progenitor Cell Expansion. Gastroenterology 2014, 146, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Loi, R.; Perra, A.; Factor, V.M.; Ledda-Columbano, G.M.; Columbano, A.; Thorgeirsson, S.S. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology 2010, 51, 1401–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rókusz, A.; Veres, D.; Szücs, A.; Bugyik, E.; Mózes, M.; Paku, S.; Nagy, P.; Dezso, K. Ductular reaction correlates with fibrogenesis but does not contribute to liver regeneration in experimental fibrosis models. PLoS ONE 2017, 12, e0176518. [Google Scholar] [CrossRef]
- Muraca, M.; Neri, D.; Parenti, A.; Feltracco, P.; Granato, A.; Vilei, M.T.; Ferraresso, C.; Ballarin, R.; Zanusso, G.E.; Giron, G.; et al. Intraportal hepatocyte transplantation in the pig: Hemodynamic and histopathological study. Transplantation 2002, 73, 890–896. [Google Scholar] [CrossRef]
- Enosawa, S.; Yuan, W.; Douzen, M.; Nakazawa, A.; Omasa, T.; Fukuda, A.; Sakamoto, S.; Shigeta, T.; Kasahara, M. Consideration of a safe protocol for hepatocyte transplantation using infantile pigs. Cell Med. 2012, 3, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Bilir, B.M.; Guinette, D.; Karrer, F.; Kumpe, D.A.; Krysl, J.; Stephens, J.; McGavran, L.; Ostrowska, A.; Durham, J. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000, 6, 32–40. [Google Scholar] [CrossRef]
- Rajvanshi, P.; Kerr, A.; Bhargava, K.K.; Burk, R.D.; Gupta, S. Studies of liver repopulation using dipeptidyl peptidase IV deficient rats. Hepatology 1996, 23, 482–496. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koid o, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver organoids Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchida, T.; Murata, S.; Matsuki, K.; Mori, A.; Matsuo, M.; Mikami, S.; Okamoto, S.; Ueno, Y.; Tadokoro, T.; Zheng, Y.-W.; et al. The Regenerative Effect of Portal Vein Injection of Liver Organoids by Retrorsine/Partial Hepatectomy in Rats. Int. J. Mol. Sci. 2020, 21, 178. https://doi.org/10.3390/ijms21010178
Tsuchida T, Murata S, Matsuki K, Mori A, Matsuo M, Mikami S, Okamoto S, Ueno Y, Tadokoro T, Zheng Y-W, et al. The Regenerative Effect of Portal Vein Injection of Liver Organoids by Retrorsine/Partial Hepatectomy in Rats. International Journal of Molecular Sciences. 2020; 21(1):178. https://doi.org/10.3390/ijms21010178
Chicago/Turabian StyleTsuchida, Tomonori, Soichiro Murata, Koichiro Matsuki, Akihiro Mori, Megumi Matsuo, Satoshi Mikami, Satoshi Okamoto, Yasuharu Ueno, Tomomi Tadokoro, Yun-Wen Zheng, and et al. 2020. "The Regenerative Effect of Portal Vein Injection of Liver Organoids by Retrorsine/Partial Hepatectomy in Rats" International Journal of Molecular Sciences 21, no. 1: 178. https://doi.org/10.3390/ijms21010178