Early OA Stage Like Response Occurs after Dynamic Stretching of Human Synovial Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Cell Culture Experimental Setup
- •
- 1st group (controls): synovial fibroblasts under standard cell culture conditions for a total of 48 h;
- •
- 2nd group: synovial fibroblasts exposed to isotropic static tension of 16% for 48 h. For static tension custom-made isotropic silicon stamps were introduced from the bottom into the flexible surface of the bioflex plates, leading to a surface increase of 16%.
- •
- 1st group (controls): synovial fibroblasts under standard cell culture conditions for a total of 28 h (consisting of 24 h preincubation and 4 h under normal cell culture conditions);
- •
- 2nd group: synovial fibroblasts exposed to cyclic tension for 4 h (10% tension at a frequency of 1 Hz). For the cyclic tension set-ups short-term high-frequency and dynamic cyclic tensile stretching, a custom-made, cyclic cell-stretching machine consisting of a 6-well fitting slot and 6 stamps, which are simultaneously elongated and retracted according to a previously compiled script, was used. By elongation of the stamps with defined height, operator-defined stretching of the flexible surface with a defined frequency can be obtained.
- •
- 1st group (controls): synovial fibroblasts incubated under standard cell culture conditions for a total of 72 h;
- •
- 2nd group: synovial fibroblasts exposed to a modest stretching (SM, Figure 1c) protocol consisting of cyclic tension for 16 h (2% tension by a frequency of 0.2 Hz) two times including a break of 8 h between the two tension setups;
- •
- 3rd group: synovial fibroblasts exposed to a mixed stretching protocol (SM/SA, Figure 1d) consisting of cyclic tension for 16 h (four repetitions of 2 h 2% tension by a frequency of 0.2 Hz and 2 h 15% tension by a frequency of 0.5 Hz), two times, including a break of 8 h between the two tension setups;
- •
- 4th group: synovial fibroblasts exposed to an advanced stretching protocol (SA, Figure 1e) consisting of cyclic tension for 16 h (15% tension by a frequency of 0.5 Hz), two times, including a break of 8 h between the two tension setups.
2.2. Assessment of Cell Number
2.3. Assessment of Cytotoxicity via LDH Assay
2.4. RNA Isolation and cDNA Synthesis
2.5. Real-Time Quantitative RT-qPCR and Relative Gene Expression
2.6. Assessment of Total Collagen Content in Cell Culture Supernatant
2.7. Determination of Hyaluronic Acid Fragment Content in Cell Culture Supernatant
2.8. Statistical Analysis
3. Results
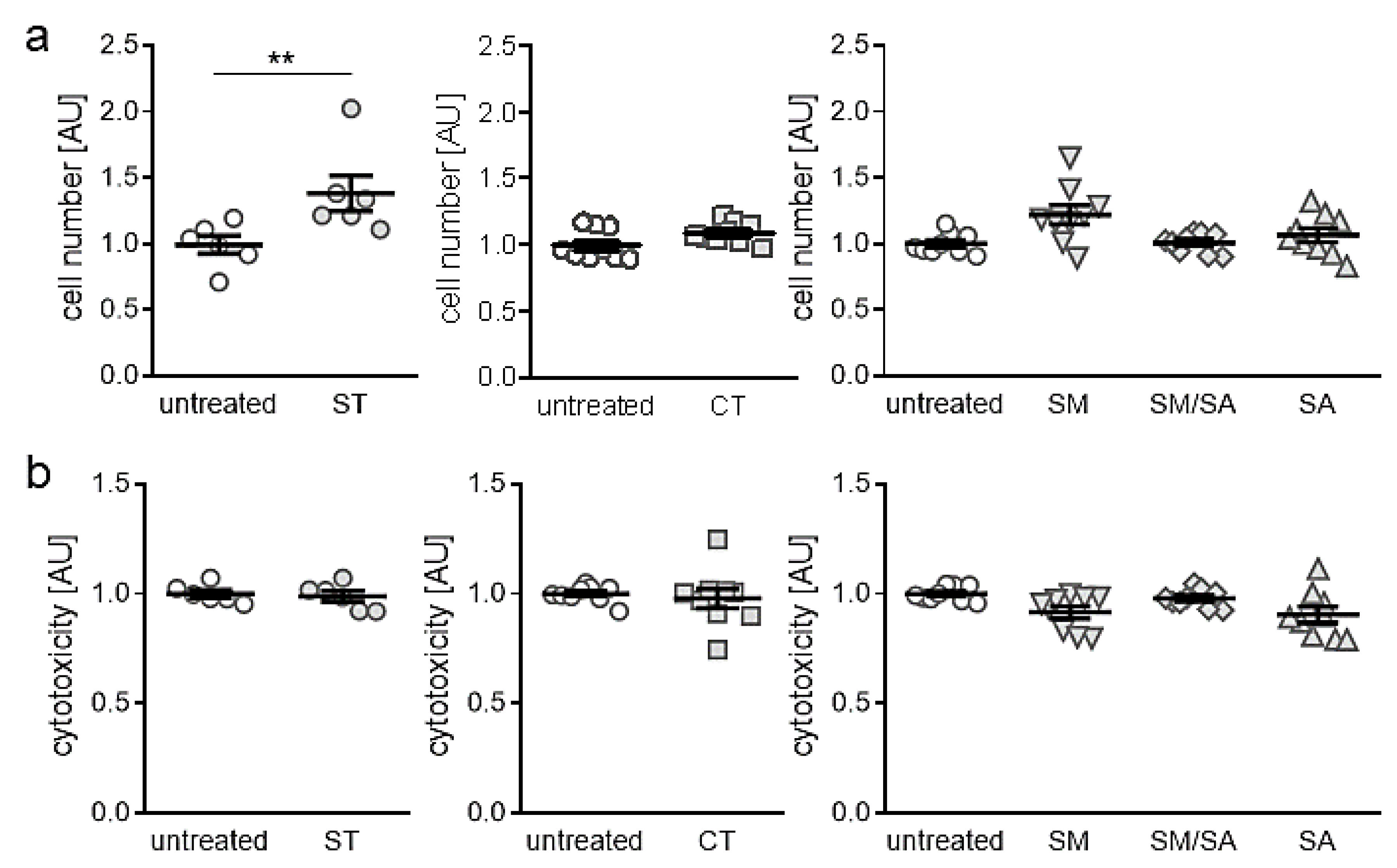
3.1. Effects of Different Mhanical Strain Protocols on Cell Number and Cytotoxicity
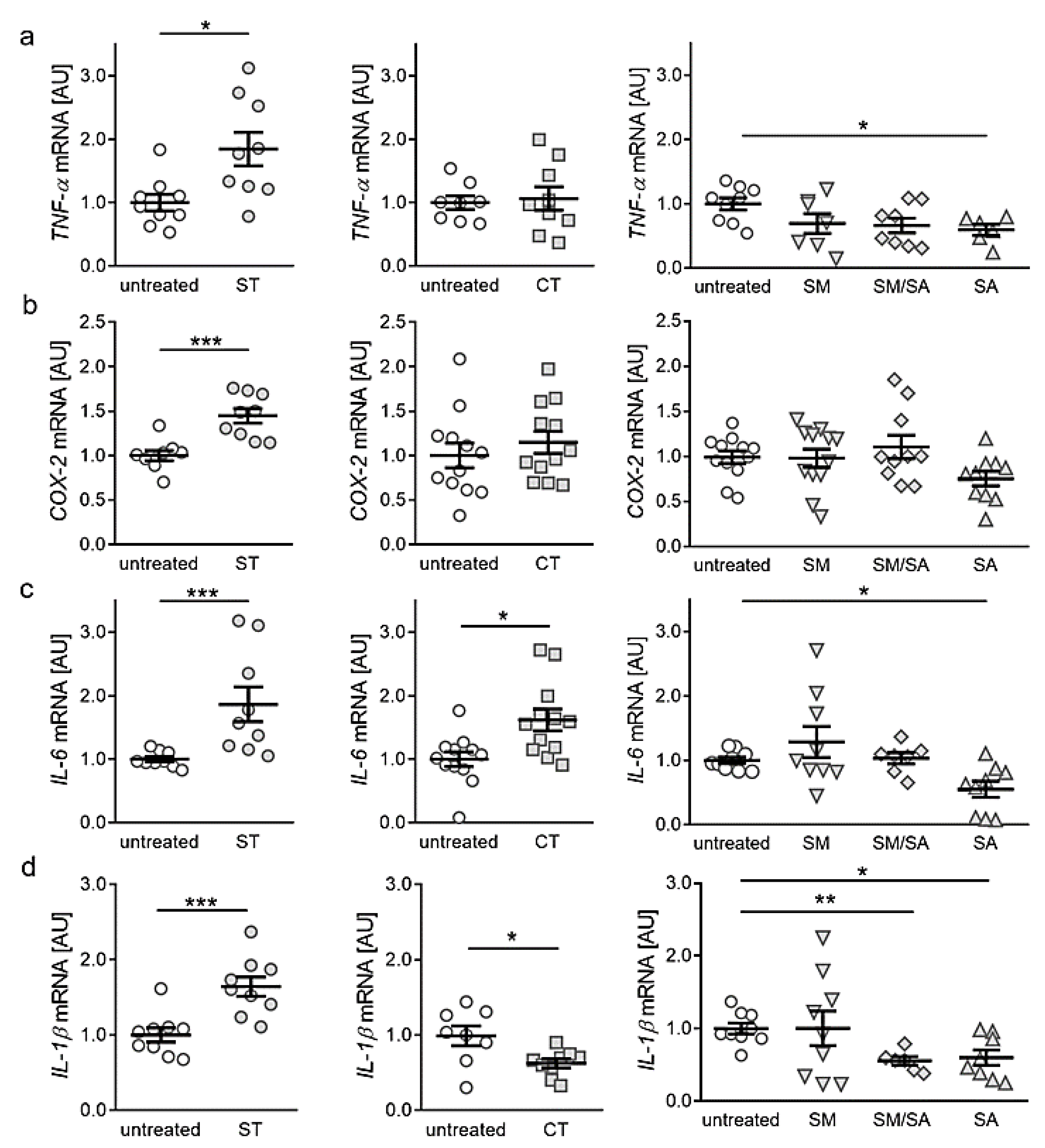
3.2. Effects of Various Tensile Strain Protocols on the Expression of Proinflammatory Genes
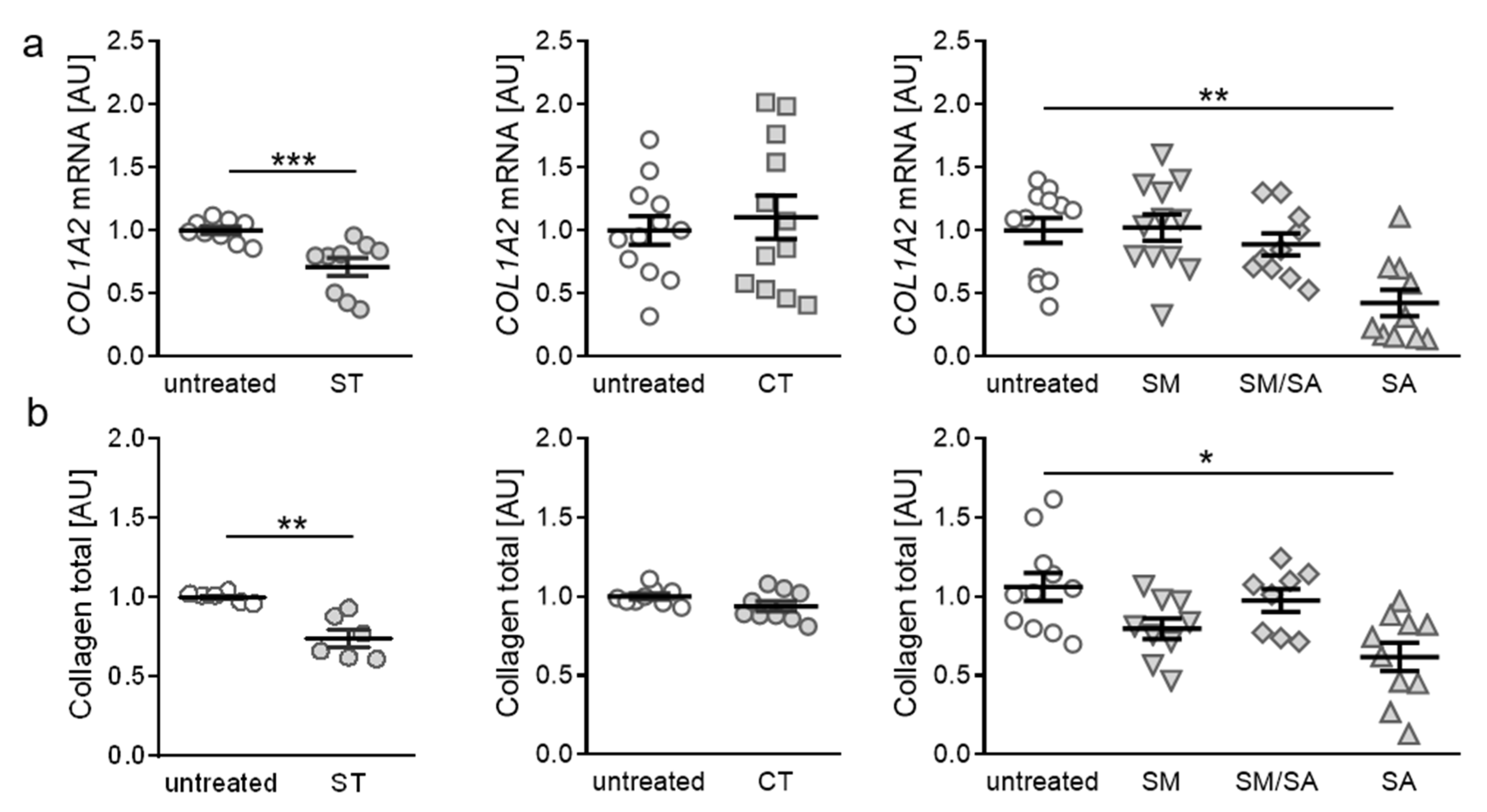
3.3. Effects of Various Tensile Strain Protocols on Collagen Synthesis
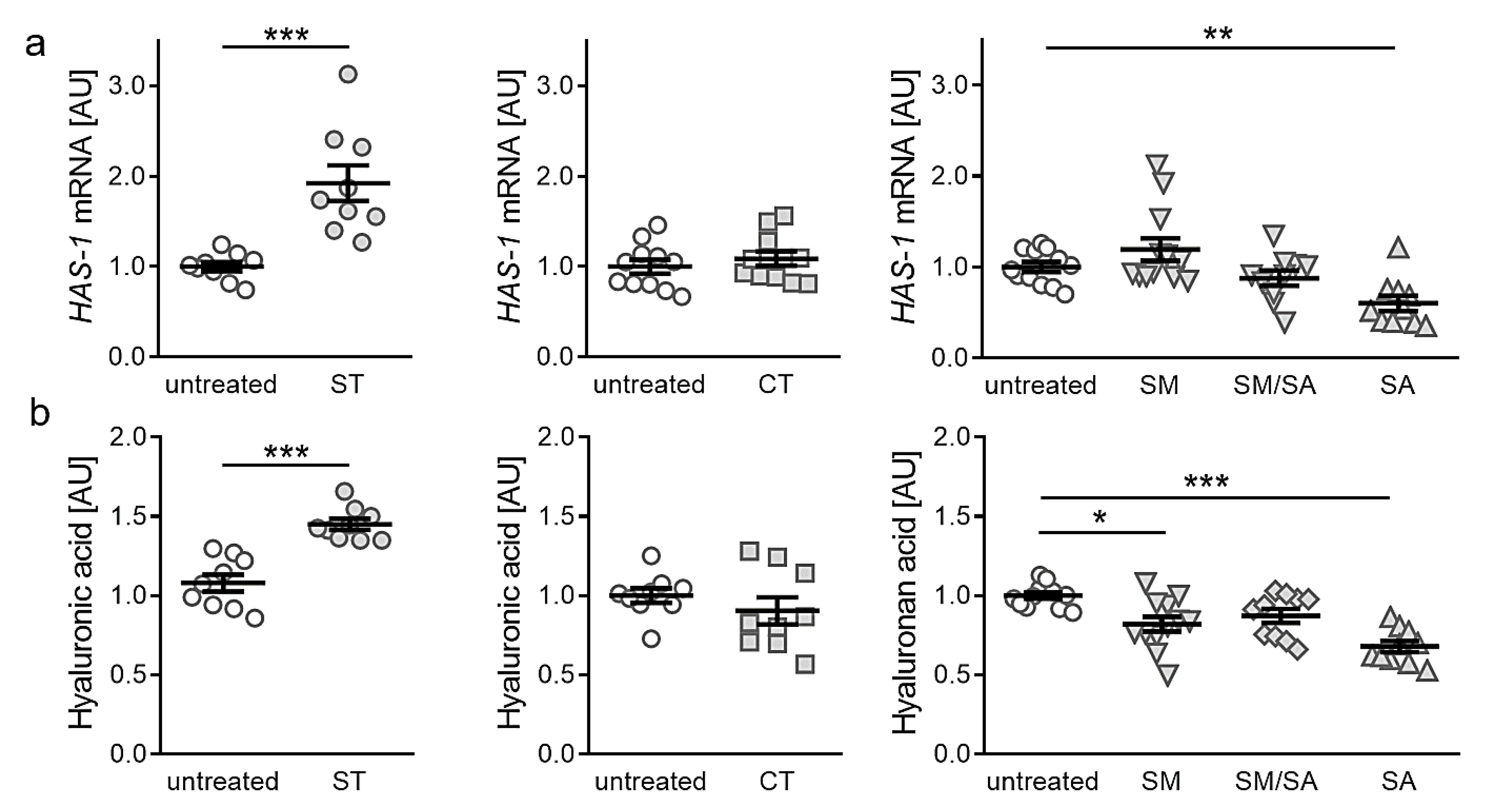
3.4. Effects of Different Tensile Strain Protocols on Hyaluronic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Healt Organization (WHO) Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium. The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ. Tech. Rep. Ser. 2003, 919, 1–218. [Google Scholar]
- Sen, R.; Hurley, J.A. StatPearls Osteoarthritis. In StatPearls Treasure Island; StatPearls. Available online: https://pubmed.ncbi.nlm.nih.gov/29493951/ (accessed on 30 January 2020).
- Felson, D.T.; Niu, J.; Neogi, T.; Goggins, J.; Nevitt, M.C.; Roemer, F.; Torner, J.; Lewis, C.E.; Guermazi, A. MOST Investigators Group Synovitis and the risk of knee osteoarthritis: The Most Study. Osteoarthr. Cartil. 2015, 24, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Roemer, F.W.; Guermazi, A.; Felson, D.T.; Niu, J.; Nevitt, M.C.; Crema, M.D.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Zhang, Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: The Most study. Ann. Rheum. Dis. 2011, 70, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; de Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. BioMed Res. Int. 2019, 2019, 6390182–6390218. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.; Spear, M. Evidence for plical support of the patella. J. Anat. 2017, 231, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Belluzzi, E.; Olivotto, E.; Toso, G.; Cigolotti, A.; Pozzuoli, A.; Biz, C.; Trisolino, G.; Ruggieri, P.; Grigolo, B.; Ramonda, R.; et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect. Tissue Res. 2018, 60, 136–145. [Google Scholar] [CrossRef]
- Eymard, F.; Chevalier, X. Inflammation of the infrapatellar fat pad. Jt. Bone Spine 2016, 83, 389–393. [Google Scholar] [CrossRef]
- Favero, M.; El-Hadi, H.; Belluzzi, E.; Granzotto, M.; Porzionato, A.; Sarasin, G.; Rambaldo, A.; Iacobellis, C.; Cigolotti, A.; Fontanella, C.G.; et al. Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology 2017, 56, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-C.; Hsu, C.-J.; Chen, H.-T.; Tsou, H.-K.; Chuang, S.-M.; Tang, C.-H. CTGF Increases IL-6 Expression in Human Synovial Fibroblasts through Integrin-Dependent Signaling Pathway. PLoS ONE 2012, 7, e51097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.-H.; Tang, C.-H.; Hsu, C.-J.; Hou, S.-M.; Liu, J.-F. CCN4 induces IL-6 production through αvβ5 receptor, PI3K, Akt, and NF-κB singling pathway in human synovial fibroblasts. Arthritis Res. Ther. 2013, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnokutsky, S.; Attur, M.; Palmer, G.; Samuels, J.; Abramson, S.B. Current concepts in the pathogenesis of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauner, M.; Sipos, W.; Pietschmann, P. Osteoimmunology. Int. Arch. Allergy Immunol. 2007, 143, 31–48. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-? B signalling pathway in osteoarthritis. Int. J. Biochem. Cell Boil. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, P.-P.; Guo, C.; Zhu, F.; Konstantopoulos, K.; Wang, Z.-Y. Fluid shear stress-induced osteoarthritis: Roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J. 2013, 27, 4664–4677. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Singh, G.K.; Zhang, F.; Wang, P.; Liu, W.; Zhong, L.; Yang, L. Comparative Study of Normal and Rheumatoid Arthritis Fibroblast-Like Synoviocytes Proliferation under Cyclic Mechanical Stretch: Role of Prostaglandin E2. Connect. Tissue Res. 2011, 53, 246–254. [Google Scholar] [CrossRef]
- Wang, P.; You, X.; Yan, Y.; Singh, G.K.; Li, X.; Zhou, W.; Liu, W.; Zhang, F.; Lv, Y.; Yang, L. Cyclic mechanical stretch downregulates IL-1β-induced COX-2 expression and PGE2production in rheumatoid arthritis fibroblast-like synoviocytes. Connect. Tissue Res. 2010, 52, 190–197. [Google Scholar] [CrossRef]
- Morisugi, T.; Tanaka, Y.; Kawakami, T.; Kirita, T. Mechanical stretch enhances NF-kappaB-dependent gene expression and poly (ADP-ribose) synthesis in synovial cells. J. Biochem. 2010, 147, 633–644. [Google Scholar] [CrossRef]
- Nazet, U.; Schröder, A.; Spanier, G.; Wolf, M.; Proff, P.; Kirschneck, C. Simplified method for applying static isotropic tensile strain in cell culture experiments with identification of valid RT-qPCR reference genes for PDL fibroblasts. Eur. J. Orthod. 2019, 52. [Google Scholar] [CrossRef]
- Muschter, D.; Beiderbeck, A.-S.; Späth, T.; Kirschneck, C.; Schröder, A.; Grässel, S. Sensory Neuropeptides and their Receptors Participate in Mechano-Regulation of Murine Macrophages. Int. J. Mol. Sci. 2019, 20, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohberger, B.; Kaltenegger, H.; Weigl, L.; Mann, A.; Kullich, W.; Stuendl, N.; Leithner, A.; Steinecker-Frohnwieser, B. Mechanical exposure and diacerein treatment modulates integrin-FAK-MAPKs mechanotransduction in human osteoarthritis chondrocytes. Cell. Signal. 2019, 56, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Kanamoto, T.; Kita, K.; Akamine, Y.; Nakamura, N.; Mae, T.; Yoshikawa, H.; Nakata, K. Cyclic compressive loading on 3D tissue of human synovial fibroblasts upregulates prostaglandin E2 via COX-2 production without IL-1β and TNF-α. Bone Jt. Res. 2014, 3, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Nazet, U.; Schröder, A.; Grässel, S.; Muschter, D.; Proff, P.; Kirschneck, C. Housekeeping gene validation for RT-qPCR studies on synovial fibroblasts derived from healthy and osteoarthritic patients with focus on mechanical loading. PLoS ONE 2019, 14, e0225790. [Google Scholar] [CrossRef]
- Kirschneck, C.; Proff, P.; Fanghänel, J.; Wolf, M.; Roldán, J.C.; Roemer, P. Reference genes for valid gene expression studies on rat dental, periodontal and alveolar bone tissue by means of RT-qPCR with a focus on orthodontic tooth movement and periodontitis. Ann. Anat. 2016, 204, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Koretsi, V.; Kirschneck, C.; Proff, P.; Roemer, P. Expression of glutathione peroxidase 1 in the spheno-occipital synchondrosis and its role in ROS-induced apoptosis. Eur. J. Orthod. 2014, 37, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Schröder, A.; Küchler, E.C.; Omori, M.A.; Spanier, G.; Proff, P.; Kirschneck, C. Effects of ethanol on human periodontal ligament fibroblasts subjected to static compressive force. Alcohol 2019, 77, 59–70. [Google Scholar] [CrossRef]
- Ospelt, C.; Brentano, F.; Rengel, Y.; Stanczyk, J.; Kolling, C.; Tak, P.P.; Gay, R.E.; Gay, S.; Kyburz, D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008, 58, 3684–3692. [Google Scholar] [CrossRef] [Green Version]
- Lefèvre, S.; Schwarz, M.; Meier, F.; Zimmermann-Geller, B.; Tarner, I.H.; Rickert, M.; Steinmeyer, J.; Sauerbier, M.; Rehart, S.; Müller-Ladner, U.; et al. Disease-Specific Effects of Matrix and Growth Factors on Adhesion and Migration of Rheumatoid Synovial Fibroblasts. J. Immunol. 2017, 198, 4588–4595. [Google Scholar] [CrossRef] [Green Version]
- Meier, F.; Frommer, K.W.; Peters, M.A.; Brentano, F.; Lefèvre, S.; Schröder, D.; Kyburz, D.; Steinmeyer, J.; Rehart, S.; Gay, S.; et al. Visfatin/Pre-B-cell Colony-enhancing Factor (PBEF), a Proinflammatory and Cell Motility-changing Factor in Rheumatoid Arthritis. J. Boil. Chem. 2012, 287, 28378–28385. [Google Scholar] [CrossRef] [Green Version]
- Porée, B.; Kypriotou, M.; Chadjichristos, C.; Beauchef, G.; Renard, E.; Legendre, F.; Melin, M.; Gueret, S.; Hartmann, D.-J.; Malléin-Gerin, F.; et al. Interleukin-6 (IL-6) and/or Soluble IL-6 Receptor Down-regulation of Human Type II Collagen Gene Expression in Articular Chondrocytes Requires a Decrease of Sp1·Sp3 Ratio and of the Binding Activity of Both Factors to theCOL2A1Promoter. J. Boil. Chem. 2007, 283, 4850–4865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenoufi, H.-L.; Diamant, M.; Rieneck, K.; Lund, B.; Stein, G.S.; Lian, J.B. Increased mRNA expression and protein secretion of interleukin-6 in primary human osteoblasts differentiated in vitro from rheumatoid and osteoarthritic bone. J. Cell. Biochem. 2001, 81, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Satoh, T.; Chiba, J.; Ju, C.; Inoue, K.; Kagawa, J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell. Mol. Ther. 2000, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sakao, K.; Takahashi, K.A.; Arai, Y.; Saito, M.; Honjo, K.; Hiraoka, N.; Asada, H.; Shin-Ya, M.; Imanishi, J.; Mazda, O.; et al. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J. Bone Miner. Metab. 2009, 27, 412–423. [Google Scholar] [CrossRef]
- Saklatvala, J. Tumour necrosis factor α stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef]
- Honorati, M.C.; Cattini, L.; Facchini, A. VEGF Production by Osteoarthritic Chondrocytes Cultured in Micromass and Stimulated by IL-17 and TNF-? Connect. Tissue Res. 2007, 48, 239–245. [Google Scholar] [CrossRef]
- Séguin, C.A.; Bernier, S.M. TNFalpha suppresses link protein and type II collagen expression in chondrocytes: Role of MEK1/2 and NF-kappaB signaling pathways. J. Cell Physiol. 2003, 197, 356–369. [Google Scholar] [CrossRef]
- Lotz, M.; Terkeltaub, R.; Villiger, P.M. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J. Immunol. 1992, 148, 466–473. [Google Scholar]
- Attur, M.G.; Patel, I.R.; Patel, R.N.; Abramson, S.B.; Amin, A.R. Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc. Assoc. Am. Phys. 1998, 110, 65–72. [Google Scholar]
- Melchiorri, C.; Meliconi, R.; Frizziero, L.; Silvestri, T.; Pulsatelli, L.; Mazzetti, I.; Borzì, R.M.; Uguccioni, M.; Facchini, A. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1998, 41, 2165–2174. [Google Scholar]
- Farahat, M.N.; Yanni, G.; Poston, R.; Panayi, G.S. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 1993, 52, 870–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribel-Madsen, S.; Bartels, E.M.; Stockmarr, A.; Borgwardt, A.; Cornett, C.; Danneskiold-Samsøe, B.; Bliddal, H. A Synoviocyte Model for Osteoarthritis and Rheumatoid Arthritis: Response to Ibuprofen, Betamethasone, and Ginger Extract—A Cross-Sectional In Vitro Study. Arthritis 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Pettipher, E.R. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: Evidence for synergistic interactions between cytokines in vivo. Clin. Exp. Immunol. 1989, 75, 306–310. [Google Scholar] [PubMed]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [Green Version]
- Ley, C.; Ekman, S.; Elmén, A.; Nilsson, G.; Eloranta, M.-L. Interleukin-6 and Tumour Necrosis Factor in Synovial Fluid from Horses with Carpal Joint Pathology. J. Veter Med. Ser. A 2007, 54, 346–351. [Google Scholar] [CrossRef]
- Rübenhagen, R.; Schüttrumpf, J.P.; Stürmer, K.M.; Frosch, K.-H. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop. 2012, 83, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Schröder, A.; Nazet, U.; Muschter, D.; Grässel, S.; Proff, P.; Kirschneck, C. Impact of Mechanical Load on the Expression Profile of Synovial Fibroblasts from Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 585. [Google Scholar] [CrossRef] [Green Version]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Bioenerg. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009, 15, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Dann, S.; Spehlmann, M.E.; Hammond, D.C.; Iimura, M.; Hase, K.; Choi, L.J.; Hanson, E.; Eckmann, L. IL-6-Dependent Mucosal Protection Prevents Establishment of a Microbial Niche for Attaching/Effacing Lesion-Forming Enteric Bacterial Pathogens1. J. Immunol. 2008, 180, 6816–6826. [Google Scholar] [CrossRef]
- Guerne, P.; Carson, D.; Lotz, M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J. Immunol. 1990, 144, 499–505. [Google Scholar] [PubMed]
- Goldring, M.; Otero, M.; Plumb, D.; Dragomir, C.; Favero, M.; Hachem, K.E.; Hashimoto, K.; Roach, H.; Olivotto, E.; Borzì, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Goldring, M.B. Articular Cartilage Degradation in Osteoarthritis. HSS J. 2012, 8, 7–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2005, 17, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Zhang, M.; Yu, J.; Luo, Z.-P. Local Tensile Stress in the Development of Posttraumatic Osteoarthritis. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miosge, N.; Hartmann, M.; Maelicke, C.; Herken, R. Expression of collagen typI and typeII in consecutive stages of human osteoarthritis. Histochem. Cell Boil. 2004, 122, 229–236. [Google Scholar] [CrossRef]
- Emlen, W.; Niebur, J.; Flanders, G.; Rutledge, J. Measurement of serum hyaluronic acid in patients with rheumatoid arthritis: Correlation with disease activity. J. Rheumatol. 1996, 23, 974–978. [Google Scholar]
- Majeed, M.; McQueen, F.; Yeoman, S.; McLean, L. Relationship between serum hyaluronic acid level and disease activity in early rheumatoid arthritis. Ann. Rheum. Dis. 2004, 63, 1166–1168. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.; Xiao, W.; Li, J.; de La Motte, C.A.; Sandy, J.D.; Plaas, A. Deficiency of hyaluronan synthase 1 (Has1) results in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee joint cartilage damage. Osteoarthr. Cartil. 2015, 23, 1879–1889. [Google Scholar] [CrossRef] [Green Version]

| Gene Symbol | Gene Name | Accession Number | 5′-Forward Primer-3′ | 5′-Reverse Primer-3′ |
|---|---|---|---|---|
| COL1A2 | collagen, type I, α2 | NM_000089.3 | AGAAACACGTCTGGCTAGGAG | GCATGAAGGCAAGTTGGGTAG |
| COX-2 | cyclooxygenase 2 | NM_000963.3 | GAGCAGGCAGATGAAATACCAGTC | TGTCACCATAGAGTGCTTCCAAC |
| EEF1A1 | eukaryotic translation elongation factor 1 | NM_001402.5 | CCTGCCTCTCCAGGATGTCTAC | GGAGCAAAGGTGACCACCATAC |
| HAS-1 | hyaluronan synthase 1 | NM_001523.3 | GAGCCTCTTCGCGTACCTG | CCTCCTGGTAGGCGGAGAT |
| IL-1β | interleukin 1β | NM_000576.3 | TTCGACACATGGGATAACGAGG | TTTTTGCTGTGAGTCCCGGAG |
| IL-6 | interleukin 6 | NM_000600.3 | TGGCAGAAAACAACCTGAACC | CCTCAAACTCCAAAAGACCAGTG |
| RPLP0 | ribosomal protein, large, P0 | NM_001002.3 | GAAACTCTGCATTCTCGCTTCC | GACTCGTTTGTACCCGTTGATG |
| TNF-α | Tumor necrose factor α | NM_000594.3 | GAGGCCAAGCCCTGGTATG | CGGGCCGATTGATCTCAGC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazet, U.; Grässel, S.; Jantsch, J.; Proff, P.; Schröder, A.; Kirschneck, C. Early OA Stage Like Response Occurs after Dynamic Stretching of Human Synovial Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3874. https://doi.org/10.3390/ijms21113874
Nazet U, Grässel S, Jantsch J, Proff P, Schröder A, Kirschneck C. Early OA Stage Like Response Occurs after Dynamic Stretching of Human Synovial Fibroblasts. International Journal of Molecular Sciences. 2020; 21(11):3874. https://doi.org/10.3390/ijms21113874
Chicago/Turabian StyleNazet, Ute, Susanne Grässel, Jonathan Jantsch, Peter Proff, Agnes Schröder, and Christian Kirschneck. 2020. "Early OA Stage Like Response Occurs after Dynamic Stretching of Human Synovial Fibroblasts" International Journal of Molecular Sciences 21, no. 11: 3874. https://doi.org/10.3390/ijms21113874






