The Synergistic Effect of Co-Treatment of Methyl Jasmonate and Cyclodextrins on Pterocarpan Production in Sophora flavescens Cell Cultures
Abstract
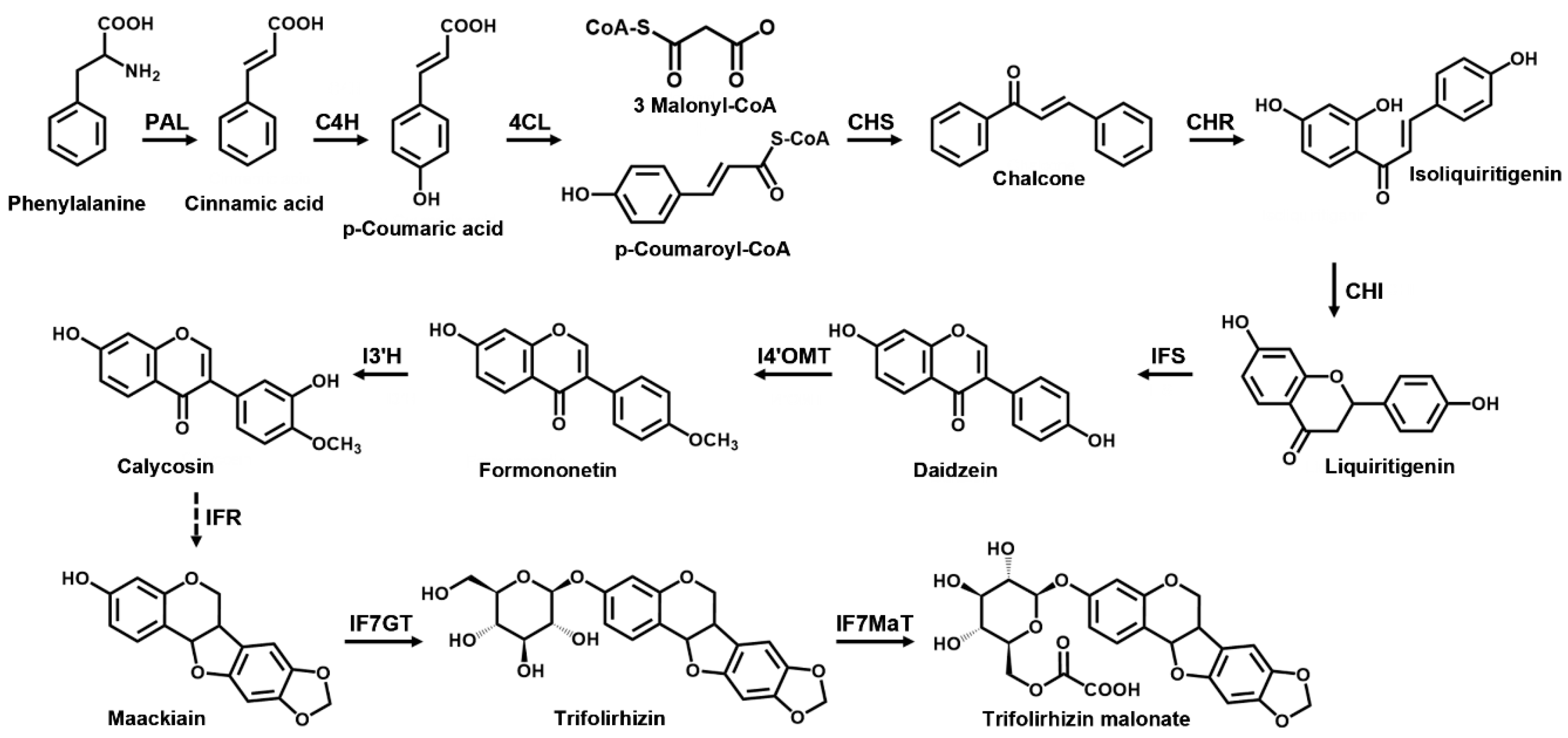
:1. Introduction
2. Results and Discussion
2.1. Establishment and Optimization of the Cell Suspension Cultures of S. flavescens
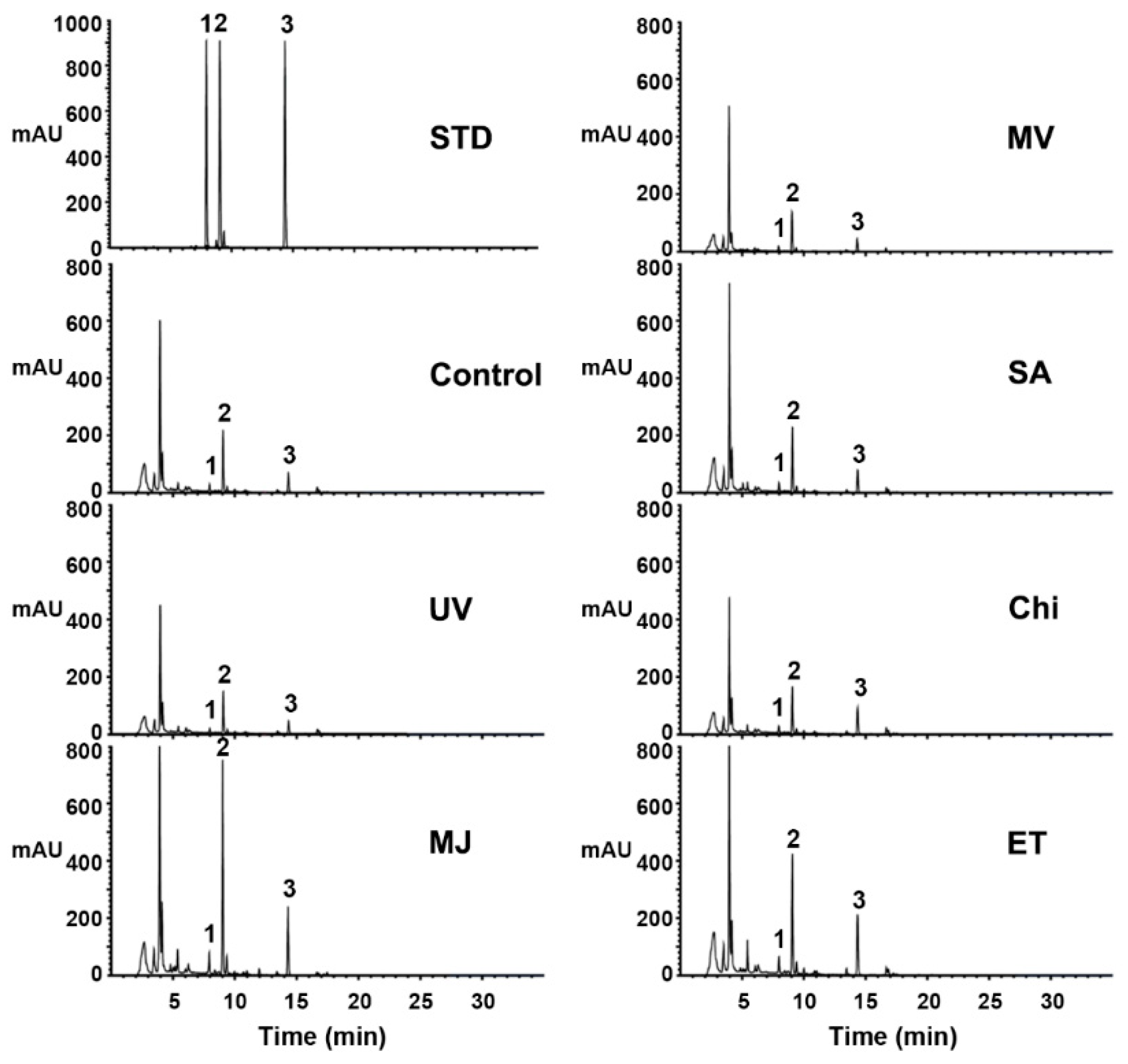
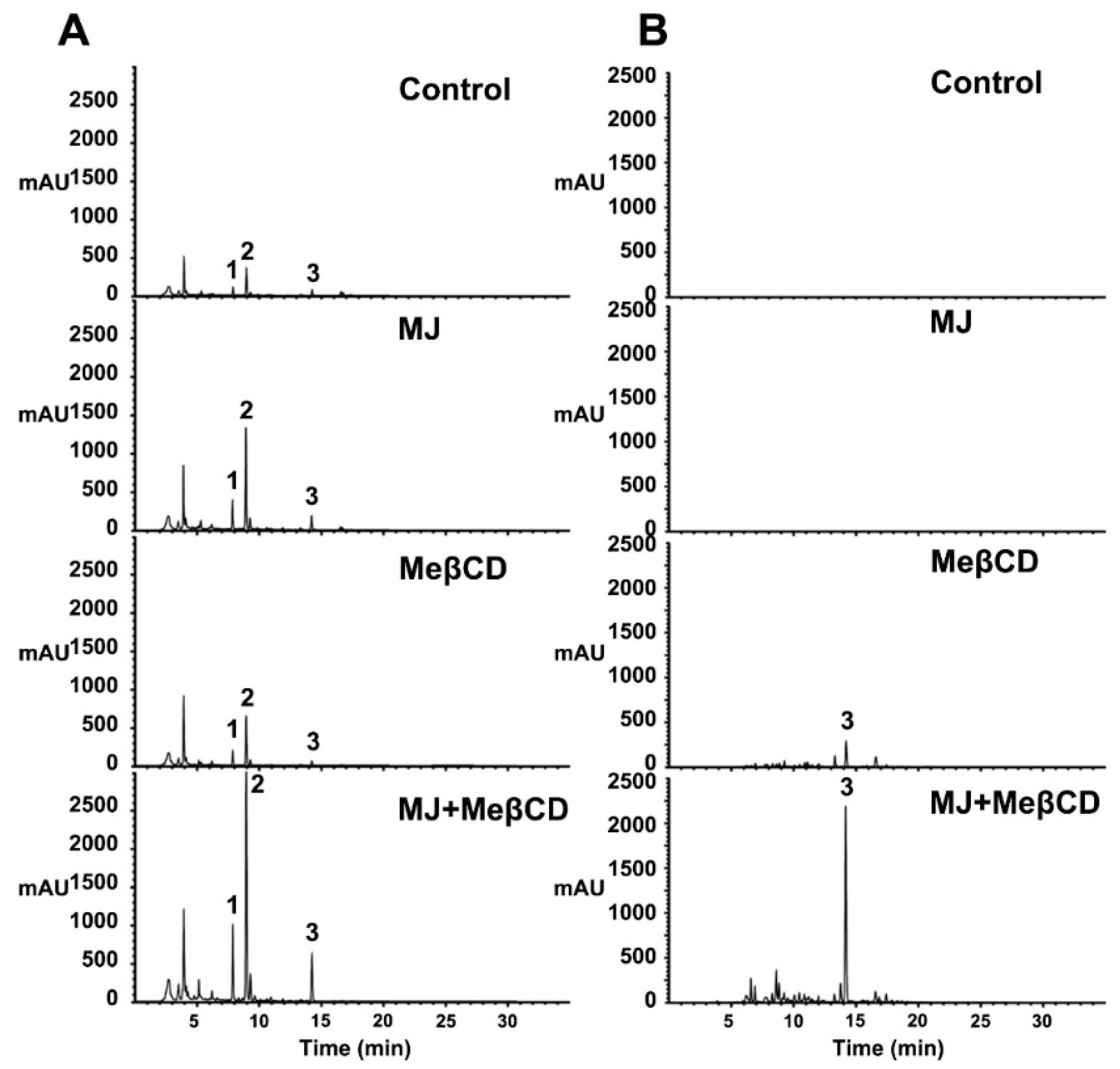
2.2. Effects of Various Elicitors on Pterocarpan Accumulation in Cell Suspension Cultures of S. flavescens
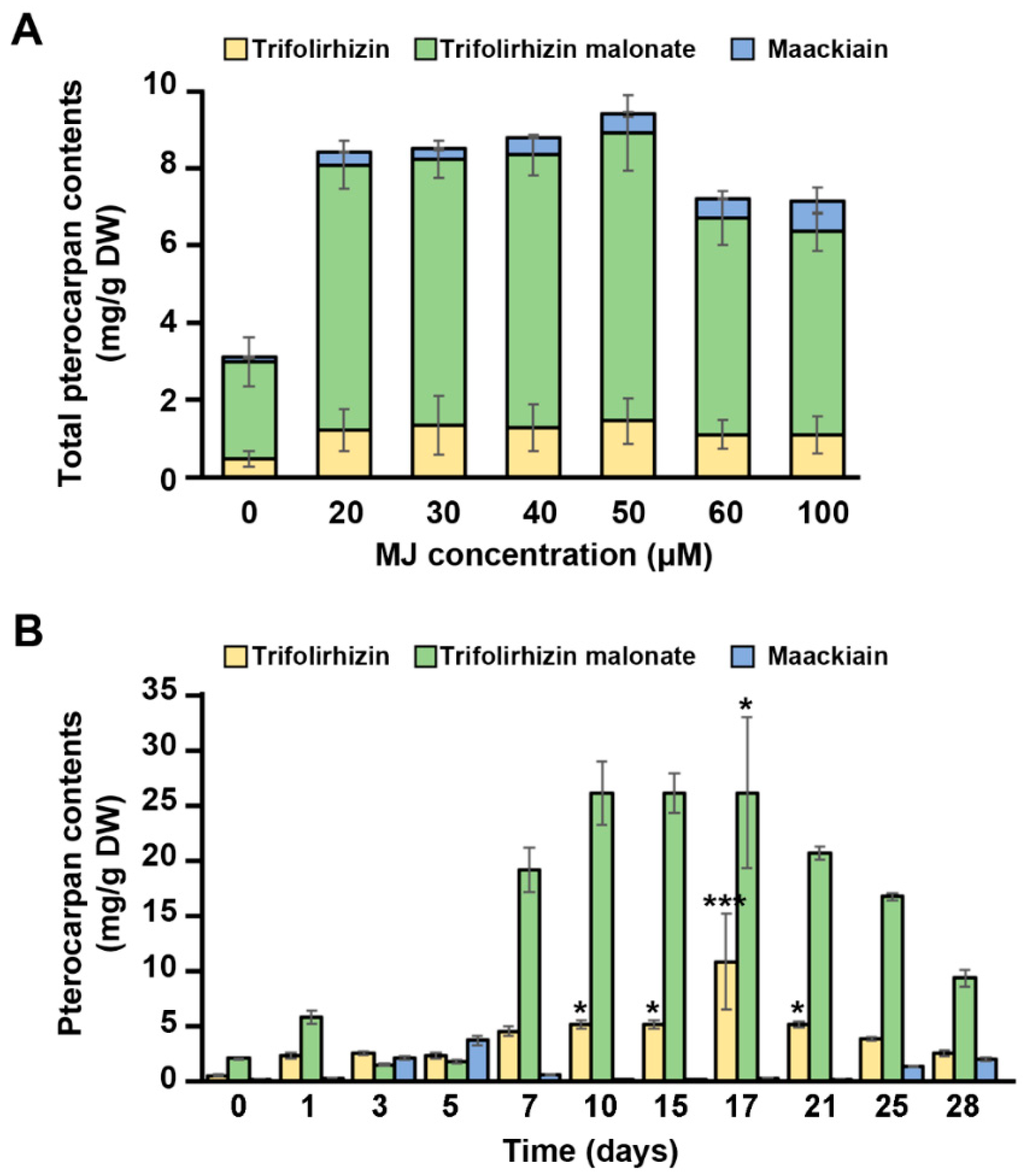
2.3. Effects of Methyl Jasmonate on Pterocarpan Contents in Cell Suspension Cultures of S. flavescens
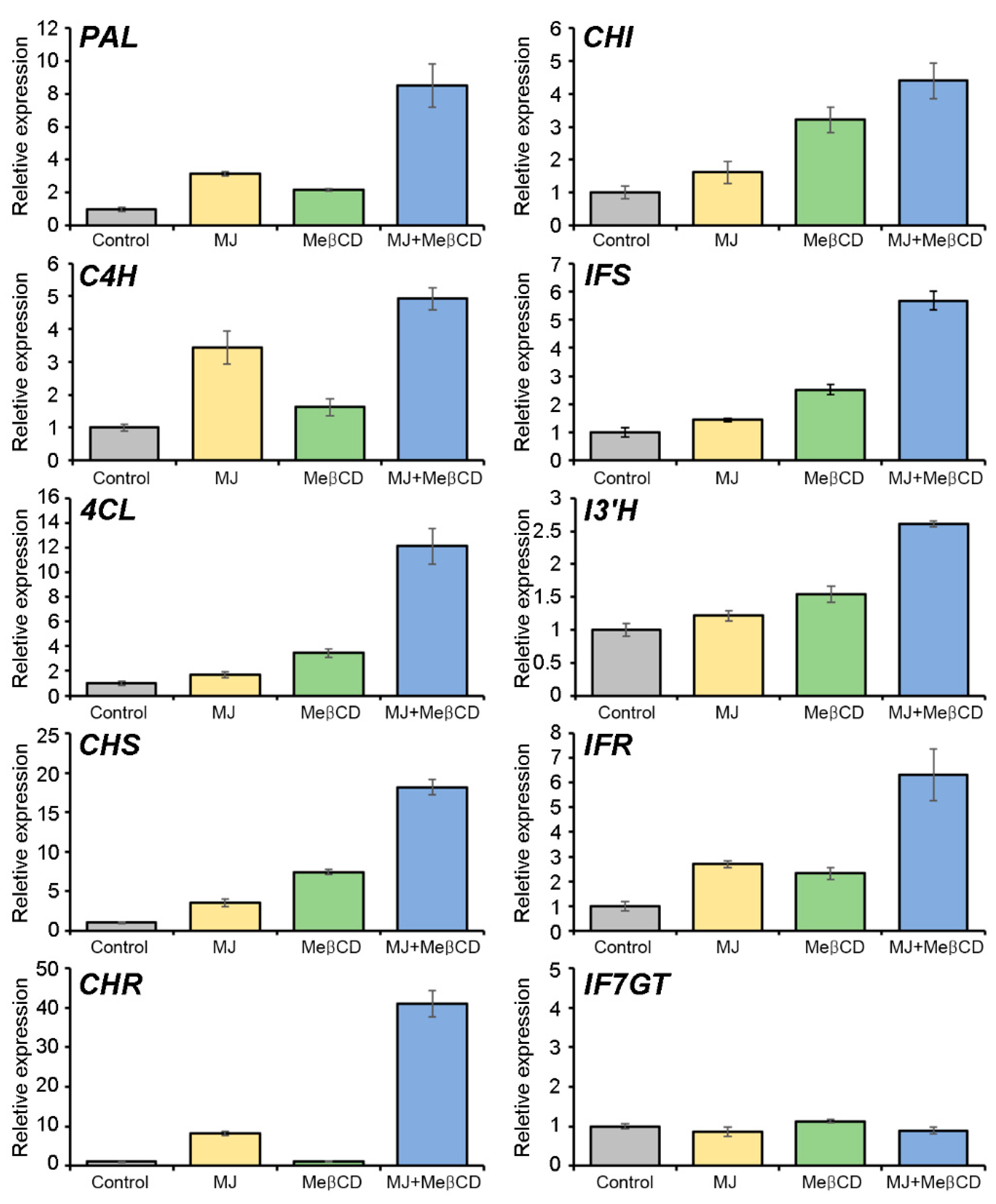
2.4. Transcriptional Activation of the Pterocarpan Biosynthetic Genes by Methyl Jasmonate and Methyl-β-Cyclodextrin Elicitation
2.5. Cyclodextrin Effectively Induces Maackiain Production in Culture Medium of the MJ-treated Cells
3. Materials and Methods
3.1. Callus Induction and Cell Cultures
3.2. Elicitation of the S. flavescens Cell Cultures
3.3. RNA Extraction and Quantitative RT-PCR (qRT-PCR) Analysis
3.4. Statistical Analysis
3.5. Quantification of Pterocarpans by High-performance Liquid Chromatography (HPLC)
3.6. Recycling Preparative HPLC and NMR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yazaki, K. Natural Products and Metabolites. In Handbook of Plant Biotechnology; Wiley: Chichester, UK, 2004; Volume 2, pp. 811–857. [Google Scholar]
- Croteau, R. Natural products (secondary metabolites). In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2002; pp. 1250–1318. [Google Scholar]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Bae, K. Medicinal Plants of Korea; Bae, K., Ed.; Kyo-Hak Pub: Seoul, Korea, 2000. [Google Scholar]
- Lee, J.; Jung, J.-E.; Son, S.-H.; Kim, H.-B.; Noh, Y.-H.; Min, S.R.; Park, K.-H.; Kim, D.-S.; Park, S.U.; Lee, H.-S.; et al. Profiling of the Major Phenolic Compounds and Their Biosynthesis Genes in Sophora flavescens Aiton. Sci. World J. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, N.; Kuboyama, T.; Kazuma, K.; Konno, K.; Tohda, C. The Extract of Roots of Sophora flavescens Enhances the Recovery of Motor Function by Axonal Growth in Mice with a Spinal Cord Injury. Front. Pharmacol. 2016, 6, 4822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-González, L.; Hernández-Cervantes, C.; Alvarez-Corral, M.; Muñoz-Dorado, M.; Rodríguez-García, I. Synthesis of Pterocarpans. Nat. Prod. Commun. 2011, 6, 537–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvam, C.; Jordan, B.C.; Prakash, S.; Mutisya, D.; Thilagavathi, R. Pterocarpan scaffold: A natural lead molecule with diverse pharmacological properties. Eur. J. Med. Chem. 2017, 128, 219–236. [Google Scholar] [CrossRef]
- Ruan, Y. Flavonoids Stimulate Spore Germination in Fusarium solani Pathogenic on Legumes in a Manner Sensitive to Inhibitors of cAMP-Dependent Protein Kinase. Mol. Plant Microbe Interact. 1995, 8, 929. [Google Scholar] [CrossRef]
- Aoki, T.; Akashi, T.; Ayabe, S.-I. Flavonoids of Leguminous Plants: Structure, Biological Activity, and Biosynthesis. J. Plant Res. 2000, 113, 475–488. [Google Scholar] [CrossRef]
- VanEtten, H.D.; Matthews, P.S.; Mercer, E.H. (+)-Maackiain and (+)-medicarpin as phytoalexins in Sophora Japonica and identification of the (−) isomers by biotransformation. Phytochemistry 1983, 22, 2291–2295. [Google Scholar] [CrossRef]
- Ingham, J.L. Notizen: Phytoalexin Production by Flowers of Garden Pea (Pisum sativum). Z. Naturforschung C 1979, 34, 296–298. [Google Scholar] [CrossRef]
- Strange, R.N.; Ingham, J.L.; Cole, D.L.; Cavill, M.E.; Edwards, C.; Cooksey, C.J.; Garrattd, P.J. Isolation of the Phytoalexin Medicarpin from Leaflets of Arachis hypogaea and Related Species of the Tribe Aeschynomeneae. Z. Naturforschung C 1985, 40, 313–316. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Ralston, L.; Subramanian, S.; Matsuno, M.; Yu, O. Partial Reconstruction of Flavonoid and Isoflavonoid Biosynthesis in Yeast Using Soybean Type I and Type II Chalcone Isomerases. Plant Physiol. 2005, 137, 1375–1388. [Google Scholar] [CrossRef] [Green Version]
- Shimada, N.; Aoki, T.; Sato, S.; Nakamura, Y.; Tabata, S.; Ayabe, S.-I. A Cluster of Genes Encodes the Two Types of Chalcone Isomerase Involved in the Biosynthesis of General Flavonoids and Legume-Specific 5-Deoxy(iso)flavonoids in Lotus japonicus1. Plant Physiol. 2003, 131, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z. Diterpene Glycosides as Natural Solubilizers. U.S. Patent Application PCT/USA2009, 13 April 2009. [Google Scholar]
- Ferreyra, M.L.F.; Rius, S.; Fernie, A.R. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Takahashi, H.; Nakamura, M.; Bunsupa, S.; Yoshimoto, N.; Yamamoto, H.; Suzuki, H.; Shibata, D.; Yamazaki, M.; Saito, K. Transcriptome Analysis of Nine Tissues to Discover Genes Involved in the Biosynthesis of Active Ingredients in Sophora flavescens. Boil. Pharm. Bull. 2015, 38, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, C.C.; Zaheer, M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: Recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 126, 1–18. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Hussain, S.; Fareed, S.; Ansari, S.; Rahman, A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Jeong, Y.J.; An, C.H.; Park, S.-C.; Pyun, J.W.; Lee, J.-Y.; Kim, S.W.; Kim, H.-S.; Kim, H.; Jeong, J.C.; Kim, C.Y. Methyl Jasmonate Increases Isoflavone Production in Soybean Cell Cultures by Activating Structural Genes Involved in Isoflavonoid Biosynthesis. J. Agric. Food Chem. 2018, 66, 4099–4105. [Google Scholar] [CrossRef]
- Mulabagal, V.; Tsay, H.-S. Plant Cell Cultures an Alternative and Efficient Source for the Production of Biologically Important Secondary Metabolites. J. Appl. Sci. Eng. Technol. 2004, 2, 29–48. [Google Scholar]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical Applications of Cyclodextrins. 2. In Vivo Drug Delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef] [PubMed]
- Bru-Martinez, R.; Sellés, S.; Casado-Vela, J.; Belchi-Navarro, S.; Pedreño, M. Modified Cyclodextrins Are Chemically Defined Glucan Inducers of Defense Responses in Grapevine Cell Cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martínez-Zapater, J.M.; Bru, R.; Pedreño, M.A. Synergistic effect of methyl jasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef] [Green Version]
- Furuya, T.; Ikuta, A. The Presence of l-Maackiain and Pterocarpin in Callus Tissue of Sophora angustifolia. Chem. Pharm. Bull. 1968, 16, 771. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Ichimura, M.; Tanaka, T.; Iinuma, M.; Mizuno, M. A trifolirhizin malonate from Sophora flavescens var. Angustifolia and its stability. Phytochemistry 1991, 30, 1732–1733. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kawai, S.; Mayumi, J.; Tanaka, T.; Iinuma, M.; Mizuno, M. Prenylated Flavanone Production in Callus Cultures of Sophora favescens var. angustifolia. Z. Naturforschung C 1991, 46c, 172–176. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ichimura, M.; Ishikawa, N.; Tanaka, T.; Iinuma, M.; Mizuno, M. Localization of Prenylated Flavonoids in Sophora flavescens var. angustifolia Plants. Z. Naturforschung C 1992, 47, 535–539. [Google Scholar] [CrossRef]
- Mustafa, N.R.; De Winter, W.; Van Iren, F.; Verpoorte, R. Initiation, growth and cryopreservation of plant cell suspension cultures. Nat. Protoc. 2011, 6, 715–742. [Google Scholar] [CrossRef]
- Shivani, P.A.; Sharma, V.; Kaur, N.; Kaur, N.; Pandey, P.; Tiwari, S. Genome-wide analysis of transcription factors during somatic embryogenesis in banana (Musa spp.) cv. Grand Naine. PLoS ONE 2017, 12, e0182242. [Google Scholar] [CrossRef]
- Nitsri Sangduen, P.K. Histological and scanning electron observations on embryogenic and non-embryogenic calli of aromatic Thai rice (Oryza sativa L. cv. Khao Daw Mali 105). Kasetsart J. 2001, 35, 427–432. [Google Scholar]
- Al-Khayri, J.M.; Naik, P. Impact of Abiotic Elicitors on In vitro Production of Plant Secondary Metabolites: A Review. J. Adv. Res. Biotechnol. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Tassoni, A.; Fornale, S.; Franceschetti, M.; Musiani, F.; Michael, A.J.; Perry, B.; Bagni, N. Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol. 2005, 166, 895–905. [Google Scholar] [CrossRef]
- Walker, T. Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort). Phytochemistry 2002, 60, 289–293. [Google Scholar] [CrossRef]
- Wang, J.; Qian, J.; Yao, L.; Lu, Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour. Bioprocess. 2015, 2, 975. [Google Scholar] [CrossRef] [Green Version]
- Weidemann, C.; Tenhaken, R.; Höhl, U.; Barz, W. Medicarpin and maackiain 3-O-glucoside-6′-O-malonate conjugates are constitutive compounds in chickpea (Cicer arietinum L.) cell cultures. Plant Cell Rep. 1991, 10, 371–374. [Google Scholar] [CrossRef]
- Jiao, Y.S.; Zhao, Y.; Chen, W.F. Duplications and functional specialization force distinct evolution of isoflavonoid biosynthetic genes in legumes. bioRxiv 2018, 361386. [Google Scholar] [CrossRef]
- Bowsher, C.; Steer, M.; Tobin, A. Plant Biochemistry; Garland Science: New York, NY, USA, 2008. [Google Scholar]
- Zhou, P.; Yang, J.; Zhu, J.; He, S.; Zhang, W.; Yu, R.; Zi, J.; Song, L.; Huang, X. Effects of β-cyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl. Microbiol. Biotechnol. 2015, 99, 7035–7045. [Google Scholar] [CrossRef]
- Almagro, L.; Gutiérrez, J.; Pedreño, M.A.; Sottomayor, M. Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 119, 543–551. [Google Scholar] [CrossRef]
- Sabater-Jara, A.-B.; Onrubia, M.; Moyano, E.; Bonfill, M.; Palazon, J.; Pedreño, M.; Cusido, R.M. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol. J. 2014, 12, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. In Bioinformatics Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000; Volume 132, pp. 365–386. [Google Scholar]

| Pterocarpan Contents (mg/g Dry Weight (DW)) | |||||
|---|---|---|---|---|---|
| Treatment | Days | Trifolirhizin | Trifolirhizin Malonate | Maackiain | Total |
| Control | 0 | 0.34 ± 0.02 | 1.65 ± 0.05 | 0.02 ± 0.00 | 2.02 |
| 5 | 0.79 ± 0.04 | 1.50 ± 0.04 | 0.06 ± 0.00 | 2.35 | |
| 14 | 0.71 ± 0.16 | 1.56 ± 0.43 | 0.09 ± 0.03 | 2.36 | |
| 25 | 0.68 ± 0.01 | 1.65 ± 0.11 | 0.13 ± 0.01 | 2.46 | |
| 28 | 0.81 ± 0.02 | 1.91 ± 0.08 | 0.08 ± 0.00 | 2.80 | |
| MJ | 0 | 0.51 ± 0.12 | 2.06 ± 0.01 | 0.11 ± 0.04 | 2.69 |
| 1 | 2.35 ± 0.25 | 5.80 ± 0.61 | 0.26 ± 0.03 | 8.41 | |
| 3 | 2.55 ± 0.17 | 1.46 ± 0.09 | 2.14 ± 0.15 | 6.15 | |
| 5 | 2.30 ± 0.25 | 1.76 ± 0.17 | 3.69 ± 0.42 | 7.75 | |
| 7 | 4.54 ± 0.46 | 19.17 ± 2.05 | 0.57 ± 0.06 | 24.28 | |
| 10 | 5.58 ± 0.38 | 26.66 ± 2.89 | 0.14 ± 0.01 | 32.37 | |
| 15 | 5.18 ± 0.39 | 26.13 ± 1.77 | 0.11 ± 0.01 | 31.42 | |
| 17 | 10.81 ± 4.35 | 26.16 ± 6.86 | 0.27 ± 0.01 | 37.24 | |
| 21 | 5.12 ± 0.27 | 20.68 ± 0.62 | 0.15 ± 0.62 | 25.95 | |
| 25 | 3.83 ± 0.15 | 16.74 ± 0.29 | 1.36 ± 0.00 | 21.94 | |
| 28 | 2.49 ± 0.28 | 9.35 ± 0.75 | 2.02 ± 0.15 | 13.86 | |
| Pterocarpan Contents | |||||
|---|---|---|---|---|---|
| Treatment | Trifolirhizin | Trifolirhizin Malonate | Maackiain | Total | |
| Cells (mg/g DW) | Control | 1.25 | 4.56 | 0.31 | 6.13 |
| MJ | 4.30 | 17.37 | 0.75 | 22.42 | |
| MeβCD | 2.21 | 8.42 | 0.24 | 10.86 | |
| MJ + MeβCD | 11.64 | 43.90 | 2.49 | 58.04 | |
| Medium (mg/L) | Control | N.D | N.D | N.D | N.D |
| MJ | N.D | N.D | N.D | N.D | |
| MeβCD | 8.55 | 13.24 | 30.36 | 52.16 | |
| MJ + MeβCD | 16.73 | 71.75 | 222.67 | 311.16 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jeong, Y.J.; Park, S.H.; Park, S.-C.; Lee, S.B.; Lee, J.; Kim, S.W.; Ha, B.-K.; Kim, H.-S.; Kim, H.; et al. The Synergistic Effect of Co-Treatment of Methyl Jasmonate and Cyclodextrins on Pterocarpan Production in Sophora flavescens Cell Cultures. Int. J. Mol. Sci. 2020, 21, 3944. https://doi.org/10.3390/ijms21113944
Kim S, Jeong YJ, Park SH, Park S-C, Lee SB, Lee J, Kim SW, Ha B-K, Kim H-S, Kim H, et al. The Synergistic Effect of Co-Treatment of Methyl Jasmonate and Cyclodextrins on Pterocarpan Production in Sophora flavescens Cell Cultures. International Journal of Molecular Sciences. 2020; 21(11):3944. https://doi.org/10.3390/ijms21113944
Chicago/Turabian StyleKim, Soyoung, Yu Jeong Jeong, Su Hyun Park, Sung-Chul Park, Saet Buyl Lee, Jiyoung Lee, Suk Weon Kim, Bo-Keun Ha, Hyun-Soon Kim, HyeRan Kim, and et al. 2020. "The Synergistic Effect of Co-Treatment of Methyl Jasmonate and Cyclodextrins on Pterocarpan Production in Sophora flavescens Cell Cultures" International Journal of Molecular Sciences 21, no. 11: 3944. https://doi.org/10.3390/ijms21113944






