GO/Bi2S3 Doped PVDF/TPU Nanofiber Membrane with Enhanced Photothermal Performance
Abstract
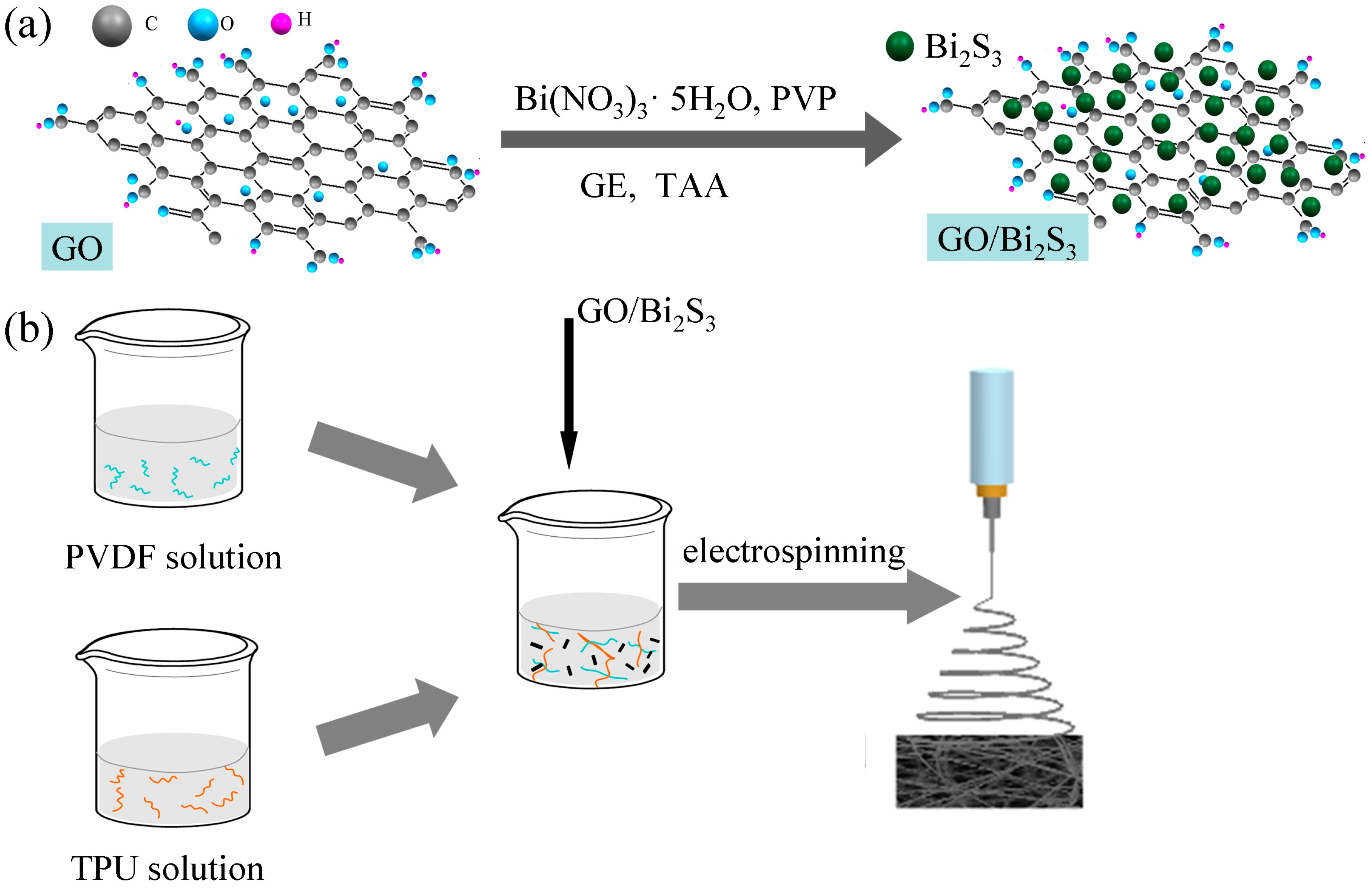
:1. Introduction
2. Results and Discussion
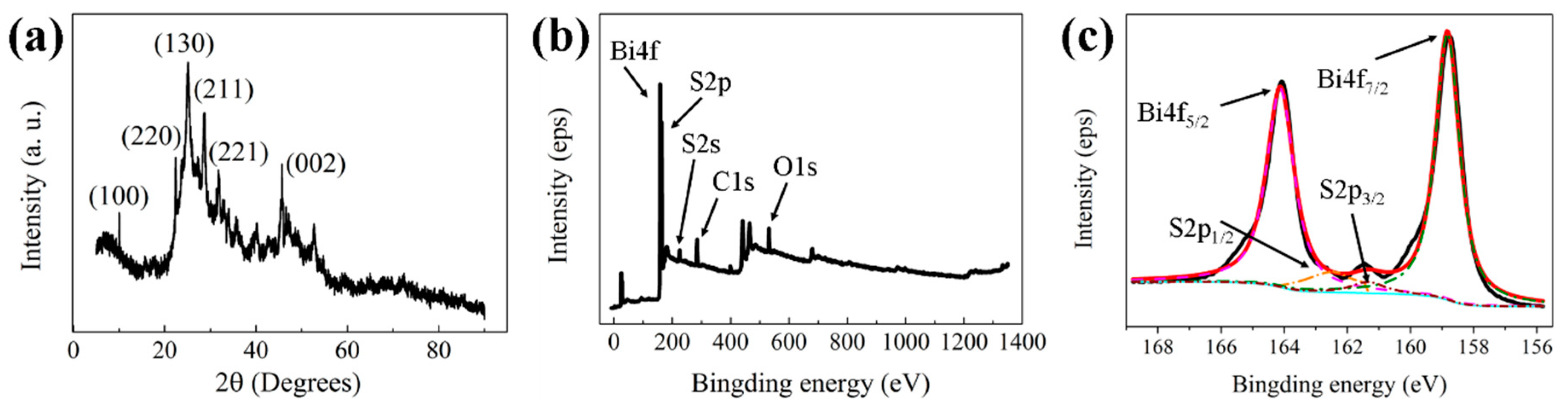
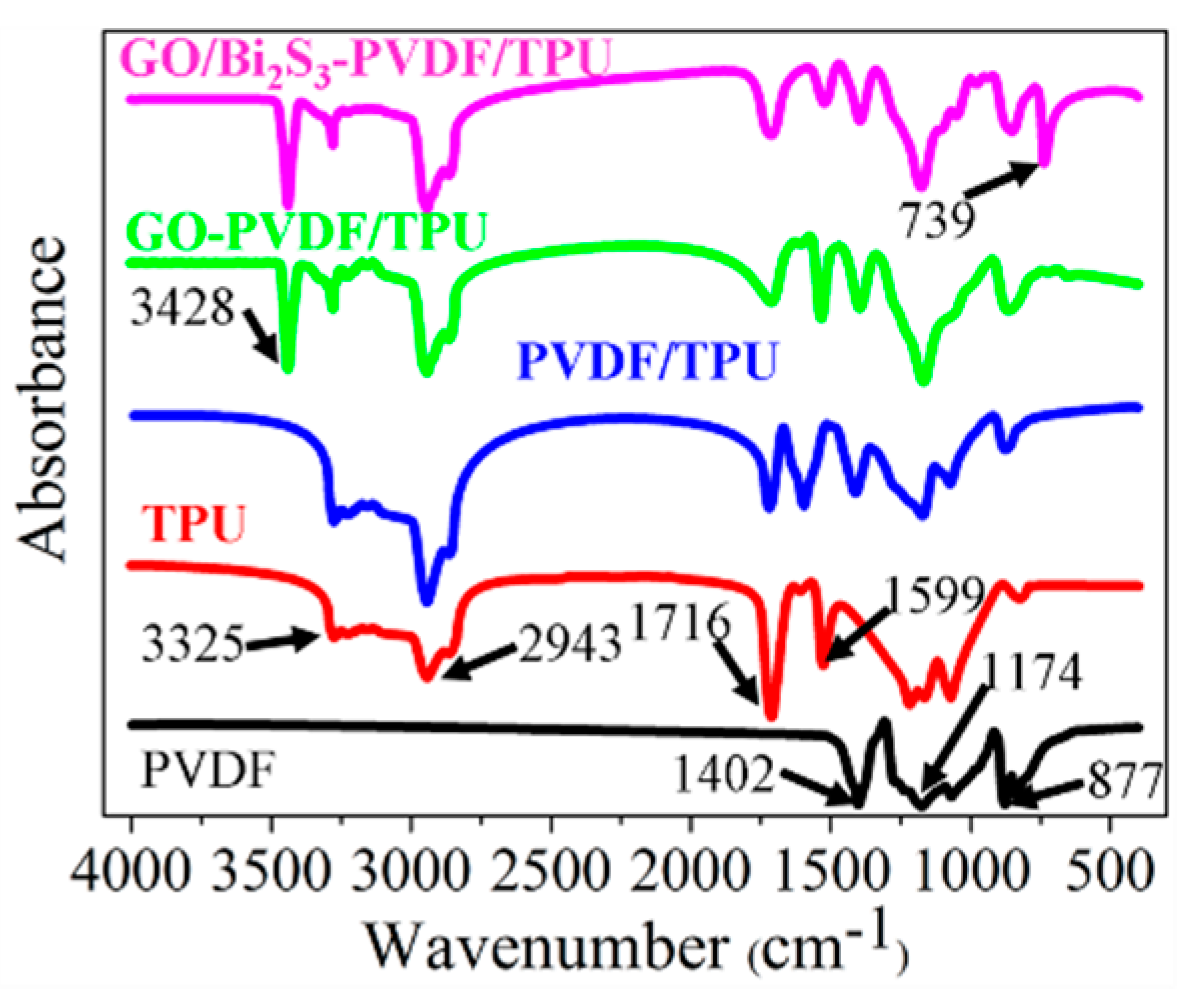
2.1. Morphology and Composition
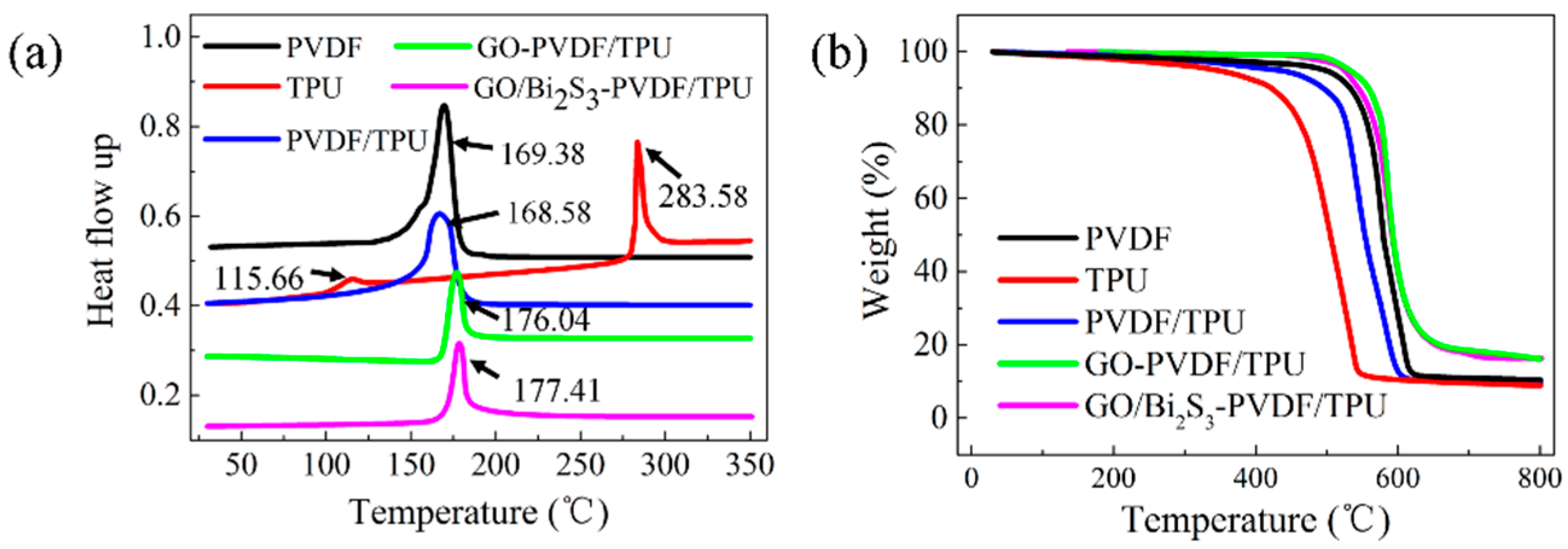
2.2. Thermal Property
2.3. Mechanical Property
2.4. Light Absorption Performance
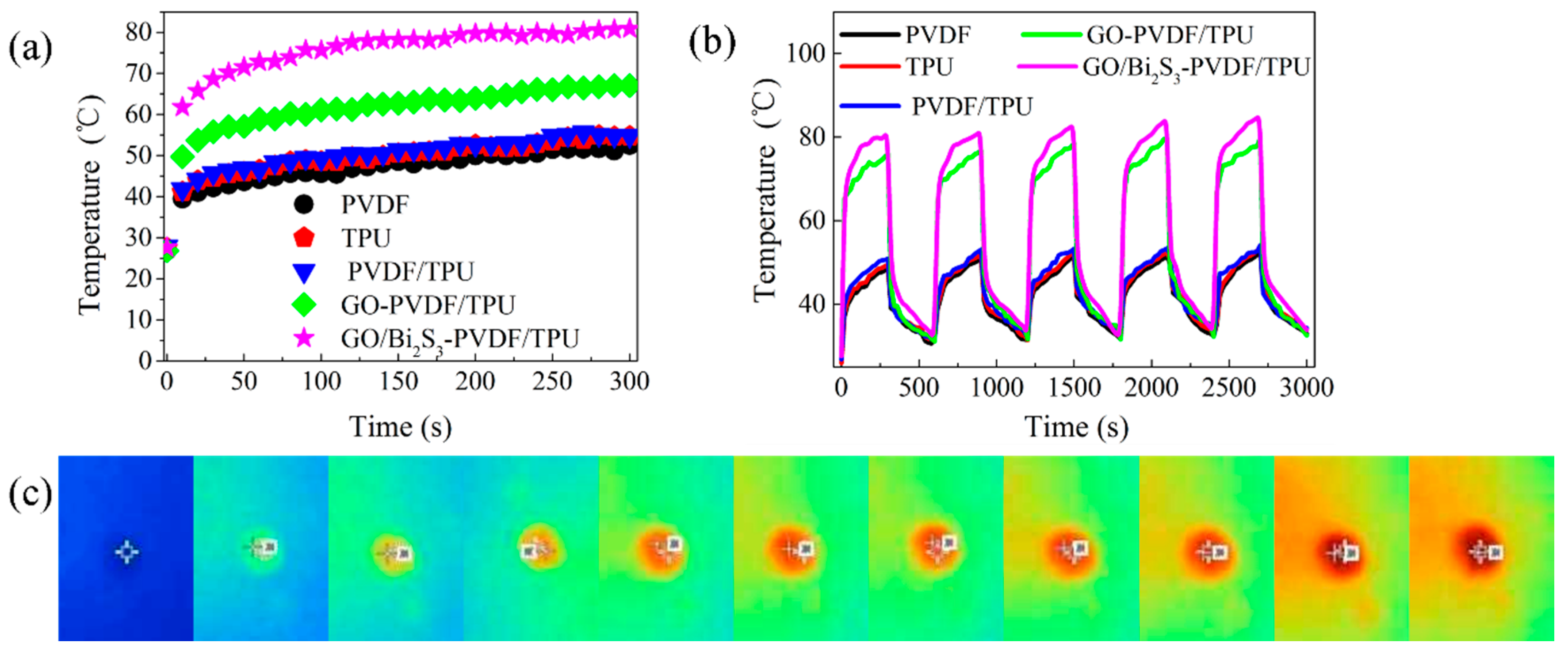
2.5. Photothermal Conversion Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO Nanoparticles
3.3. Synthesis of GO/Bi2S3
3.4. Fabrication of Nanofiber Membranes
3.5. Characterization
3.6. Photothermal Conversion Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, L.; Gao, M.; Peh, C.; Wang, X.; Ho, G. Self-Contained Monolithic Carbon Sponges for Solar-Driven Interfacial Water Evaporation Distillation and Electricity Generation. Adv. Energy Mater. 2018, 8, 1702149. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.; Ho, G. Solar-driven photothermal nanostructured materials designs and prerequisites for evaporation and catalysis applications. Mater. Horiz. 2018, 5, 323–343. [Google Scholar] [CrossRef]
- Chang, C.; Yang, C.; Liu, Y.; Tao, P.; Song, C.; Shang, W.; Wu, J.; Deng, T. Efficient Solar-Thermal Energy Harvest Driven by Interfacial Plasmonic Heating-Assisted Evaporation. ACS Appl. Mater. Interfaces 2016, 8, 23412–23418. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhu, L.; Peh, C.; Ho, G. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Han, Y.; Zhang, J.; Cui, F.; Zhao, Y.; Li, X.; Wang, W. Multifunctional CuO nanowire mesh for highly efficient solar evaporation and water purification. Acs. Sustain. Chem. Eng. 2019, 7, 5476–5485. [Google Scholar] [CrossRef]
- Ren, H.; Tang, M.; Guan, B.; Wang, K.; Yang, J.; Wang, F.; Wang, M.; Shan, J.; Chen, Z.; Wei, D. Hierarchical graphene foam for efficient omnidirectional solar–thermal energy conversion. Adv. Mater. 2017, 29, 1702590. [Google Scholar] [CrossRef]
- Hao, D.; Yang, Y.; Xu, B.; Cai, Z. Bifunctional fabric with photothermal effect and photocatalysis for highly efficient clean water generation. ACS Sustain. Chem. Eng. 2018, 6, 10789–10797. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Zhu, Z.; Hou, H.; Zuo, J.; Cui, F.; Liu, D.; Wang, W. A mechanically durable, sustained corrosion-resistant photothermal nanofiber membrane for highly efficient solar distillation. J. Mater. Chem. A 2019, 7, 22296–22306. [Google Scholar] [CrossRef]
- Hohyun, L.; Hyoung-Joon, J.; Hyo, W. Chemical and physical reinforcement behavior of dialdehyde nanocellulose in PVA composite film: A comparison of nanofiber and nanocrystal. Carbohyd. Polym. 2020, 232, 115771. [Google Scholar]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as efficient solar steam-generation devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef]
- Dou, R.; Du, Z.; Bao, T.; Dong, X.; Zheng, X.; Yu, M.; Yin, W.; Dong, B.; Yan, L.; Gu, Z. The polyvinylpyrrolidone functionalized rGO/Bi2S3 nanocomposite as a near-infrared light-responsive nanovehicle for chemo-photothermal therapy of cancer. Nanoscale 2016, 8, 11531–11542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Ma, D.; Zhang, S.; Wang, W.; Chen, W.; Huang, S.; Yu, K. PEGylated Cu3BiS3 hollow nanospheres as a new photothermal agent for 980 nm-laser-driven photothermochemotherapy and a contrast agent for X-ray computed tomography imaging. Nanoscale 2016, 8, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, H.; Shi, B.; Guo, L.; Zhang, Y.; An, X.; Zhang, H.; Yang, S. Hydrophilic Cu3BiS3 nanoparticles for computed tomography imaging and photothermal therapy. Part. Part. Syst. Charact. 2015, 32, 668–679. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Wang, X.; Zhu, J.; Yang, Y.; Tian, J.; Cui, W.; Ge, C.; Li, Y.; Pan, Y. Facile synthesis of magnetic core–shell nanocomposites for MRI and CT bimodal imaging. J. Mater. Chem. B 2015, 3, 6905–6910. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Huang, W.; Li, L.; Jin, B.; Li, H.; Zhai, T. Submillimeter 2D Bi2Se3 Flakes toward High-Performance Infrared Photodetection at Optical Communication Wavelength. Adv. Funct. Mater. 2018, 28, 1802707. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H. Novel Bismuth-Based Nanomaterials Used for Cancer Diagnosis and Therapy. Chem. Eur. J. 2018, 24, 17405–17418. [Google Scholar] [CrossRef]
- Yang, S.; Li, Z.; Wang, Y.; Fan, X.; Miao, Z.; Hu, Y.; Li, Z.; Sun, Y.; Besenbacher, F.; Yu, M. Multifunctional Bi@PPy-PEG core–shell nanohybrids for dual-modal imaging and photothermal therapy. ACS Appl. Mater. Inter. 2018, 10, 1605–1615. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Hu, X.; Yao, J.; Chen, Z.; Hao, M.; Tian, W.; Huang, Z.; Li, F. Multilayer Nanofiber Composite Separator for Lithium-Ion Batteries with High Safety. Polymers 2019, 11, 1671. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yang, W.; Li, T. Preparation of graphene oxide/polyvinglidene fluoride composite filtration membrane and its filtrtion performance. J. Text. Res. 2019, 40, 32–37. [Google Scholar]
- Sun, H.; Zhang, X.; He, Y.; Zhang, D.; Feng, X.; Zhao, Y.; Chen, L. Preparation of PVDF-g-PAA-PAMAM membrane for efficient removal of copper ions. Chem. Eng. Sci. 2019, 209, 115186. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Qin, L.; Li, X.; Meng, Q.; Shen, C.; Zhang, G. Enhanced MPBR with polyvinylpyrrolidone-graphene oxide/PVDF hollow fiber membrane for efficient ammonia nitrogen wastewater treatment and high-density Chlorella cultivation. Chem. Eng. J. 2020, 379, 122368. [Google Scholar] [CrossRef]
- Song, S.; Xia, S.; Liu, Y.; Lv, X.; Sun, S. Effect of Na+ MMT-ionic liquid synergy on electroactive, mechanical, dielectric and energy storage properties of transparent PVDF-based nanocomposites. Chem. Eng. J. 2020, 384, 123365. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, H.; Wang, C.; Zhang, S.; Zhang, L.; Liu, X.; Liu, F.; Zhu, X.; Rohani, S.; Ching, C. A novel PVDF/PFSA-g-GO ultrafiltration membrane with enhanced permeation and antifouling performances. Sep. Purif. Technol. 2020, 233, 116038. [Google Scholar] [CrossRef]
- He, W.; Li, Z.; Chen, S.; Yang, G.; Yang, Z.; Liu, P.; Yan, Q. Energetic metastable n-Al@ PVDF/EMOF composite nanofibers with improved combustion performances. Chem. Eng. J. 2020, 383, 123146. [Google Scholar] [CrossRef]
- Zhou, A.; Jia, R.; Wang, Y.; Sun, S.; Xin, X.; Wang, M.; Zhao, Q.; Zhu, H. Abatement of sulfadiazine in water under a modified ultrafiltration membrane (PVDF-PVP-TiO2-dopamine) filtration-photocatalysis system. Sep. Purif. Technol. 2020, 234, 116099. [Google Scholar] [CrossRef]
- Fallahiarezoudar, E.; Ahmadipourroudposht, M.; Idris, A.; Yusof, N. Optimization and development of Maghemite (γ-Fe2O3) filled poly-L-lactic acid (PLLA)/thermoplastic polyurethane (TPU) electrospun nanofibers using Taguchi orthogonal array for tissue engineering heart valve. Mater. Sci. Eng. C 2017, 76, 616–627. [Google Scholar] [CrossRef]
- Beniwal, A. Novel TPU/Fe2O3 and TPU/Fe2O3/PPy nanocomposites synthesized using electrospun nanofibers investigated for analyte sensing applications at room temperature. Sensor Actuat. B—Chem. 2020, 304, 127384. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Ma, Y.; Yang, W. Improved performance of lithium ion battery separator enabled by co-electrospinnig polyimide/poly (vinylidene fluoride-co-hexafluoropropylene) and the incorporation of TiO2-(2-hydroxyethyl methacrylate). J. Power Sources 2015, 273, 1127–1135. [Google Scholar] [CrossRef]
- Ince, G.; Alpern, R.; Amare, D.; Crawford, J.; Dolan, B.; Jones, S.; Kobylarz, R.; Reveley, M.; Cebe, P. Impact of nanosilicates on poly (vinylidene fluoride) crystal polymorphism: Part 1. Melt-crystallization at high supercooling. Polymer 2010, 51, 1485–1493. [Google Scholar] [CrossRef]
- El, A.; Arrakhiz, F.; Vaudreuil, S.; Essassi, E.; Qaiss, A. Piezoelectric β-polymorph formation and properties enhancement in graphene oxide–PVDF nanocomposite films. Appl. Surf. Sci. 2012, 258, 7668–7677. [Google Scholar]
- Han, D.; Yan, L.; Chen, W.; Li, W. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohyd. Polym. 2011, 83, 653–658. [Google Scholar] [CrossRef]
- Almond, D.; Patel, P.; Patel, P. Photothermal science and techniques; Springer Science & Business Media: Berlin, Germany, 1996. [Google Scholar]
- Hessel, C.; Pattani, V.; Rasch, M.; Panthani, M.; Koo, B.; Tunnell, J.; Korgel, B. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastai, Y.; Polarz, S.; Antonietti, M. Silica-Carbon Nanocomposites-A New Concept for the Design of Solar Absorbers. Adv. Funct. Mater. 2002, 12, 197–202. [Google Scholar] [CrossRef]
- Amri, A.; Jiang, Z.; Pryor Trevor, Y.; Djordjevic, S. Developments in the synthesis of flat plate solar selective absorber materials via sol-gel methods: A review. Renew. Sustain. Energy Rev. 2014, 36, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, L.; Wang, P. Self-floating carbon nanotube membrane on macroporous silica substrate for highly efficient solar-driven interfacial water evaporation. ACS Sustain. Chem. Eng. 2016, 4, 1223–1230. [Google Scholar] [CrossRef] [Green Version]




| Membrane | Compositions | Average Fiber Diameter (nm) | Average Pore Size (ìm) | Porosity (%) |
|---|---|---|---|---|
| PVDF | Pristine PVDF electrospun nanofiber | 152 | 1.8 | 70 |
| TPU | Pristine TPU electrospun nanofiber | 425 | 2.3 | 65 |
| PVDF/TPU | Nanofibers were spun by PVDF and TPU mixed solution | 240 | 2.1 | 73 |
| GO-PVDF/TPU | PVDF/TPU electrospun nanofibers loaded with GO NPs | 149 | 1.3 | 83 |
| GO/Bi2S3-PVDF/TPU | PVDF/TPU electrospun nanofibers loaded with GO/Bi2S3 NPs | 129 | 1.2 | 73 |
| Samples | Irradiation Time (s) | Temperature Change (°C) |
|---|---|---|
| PDA/PEI/PPy@PI nanofiber membrane | 3600 | 11.9 |
| CNT-silica bilayered material | 400 | 50 |
| h-G foam | 300 | 33.5 |
| TiO2-PDA/PPy/cotton | 600 | 47.7 |
| GO/Bi2S3-PVDF/TPU | 300 | 53.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Li, Y.; Feng, L.; Hou, Y.; Wang, S.; Yang, B.; Hu, X.; Zhang, W.; Ramakrishna, S. GO/Bi2S3 Doped PVDF/TPU Nanofiber Membrane with Enhanced Photothermal Performance. Int. J. Mol. Sci. 2020, 21, 4224. https://doi.org/10.3390/ijms21124224
Yang W, Li Y, Feng L, Hou Y, Wang S, Yang B, Hu X, Zhang W, Ramakrishna S. GO/Bi2S3 Doped PVDF/TPU Nanofiber Membrane with Enhanced Photothermal Performance. International Journal of Molecular Sciences. 2020; 21(12):4224. https://doi.org/10.3390/ijms21124224
Chicago/Turabian StyleYang, Wenxiu, Yonggui Li, Long Feng, Yimiao Hou, Shuo Wang, Bo Yang, Xuemin Hu, Wei Zhang, and Seeram Ramakrishna. 2020. "GO/Bi2S3 Doped PVDF/TPU Nanofiber Membrane with Enhanced Photothermal Performance" International Journal of Molecular Sciences 21, no. 12: 4224. https://doi.org/10.3390/ijms21124224







