Analysis of Spatial Distribution and Prognostic Value of Different Pan Cytokeratin Immunostaining Intensities in Breast Tumor Tissue Sections
Abstract
:1. Introduction
2. Results
2.1. The Prognostic Model for Distant Metastasis Risk
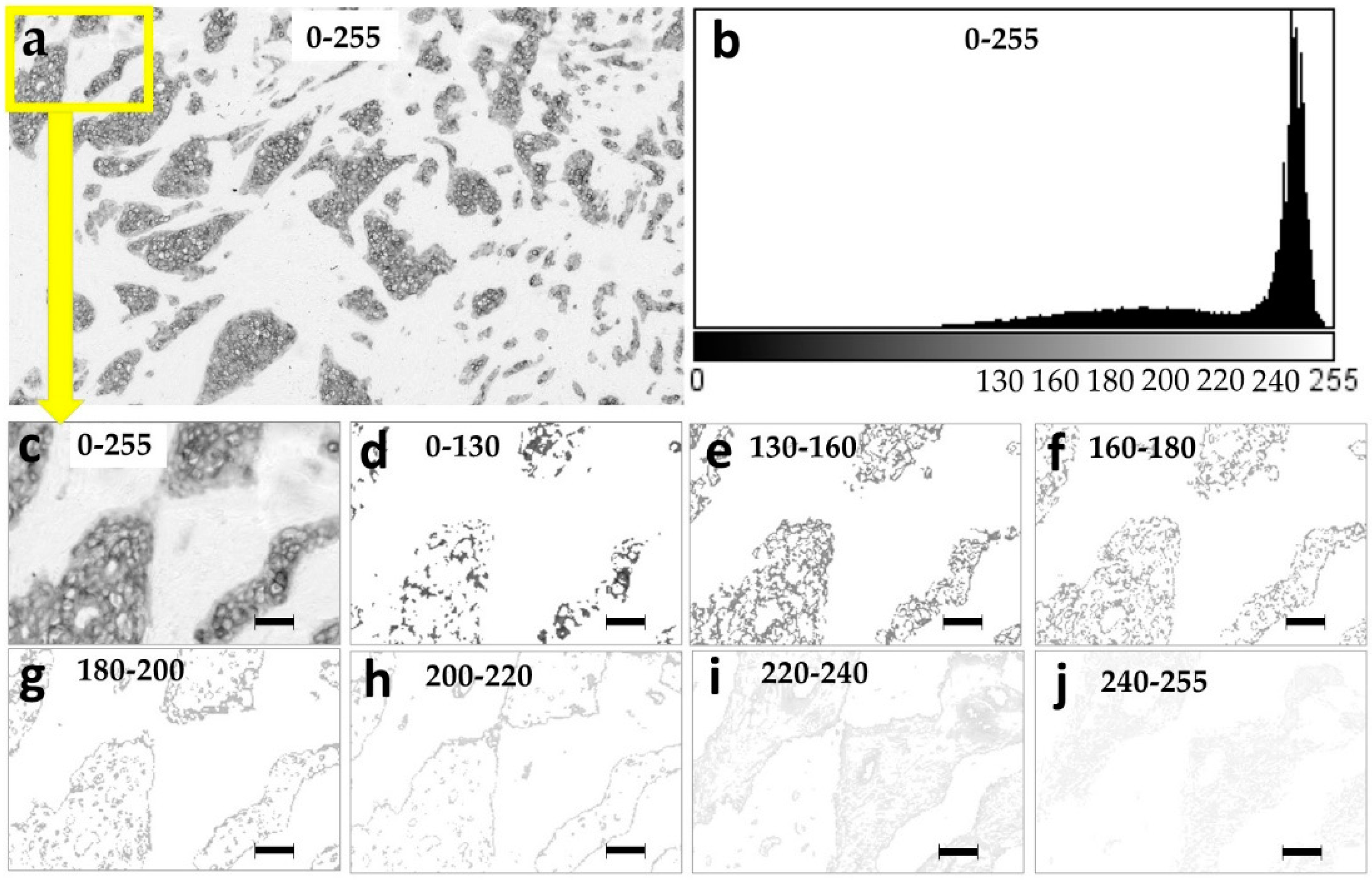
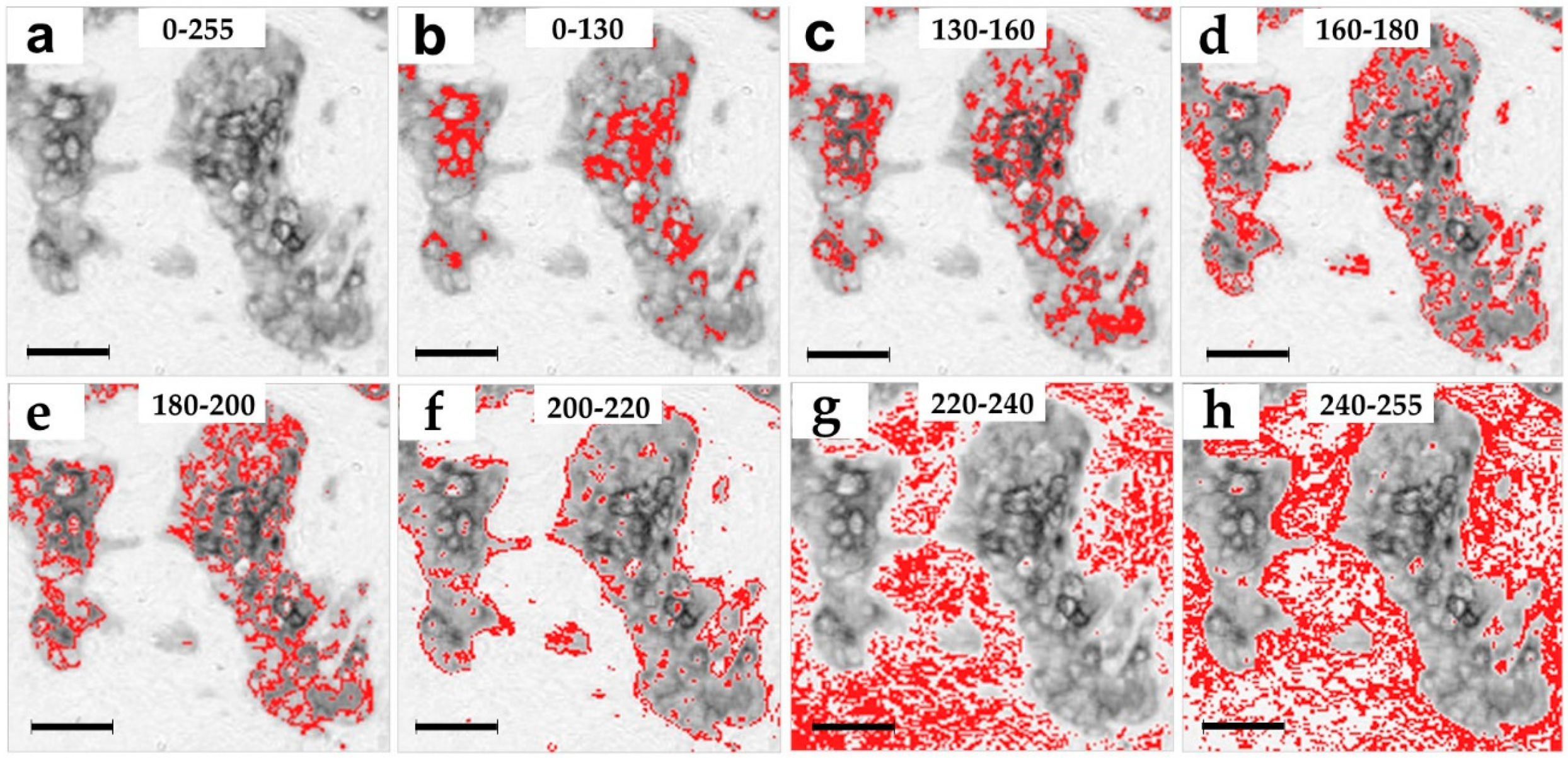
2.2. Spatial Distribution of the Pan Cytokeratin Immunostaining Intensities
2.3. Prognostic Evaluation of the Distinct Pan Cytokeratin Staining Intensities
2.4. Prognostic Independence of the Features Calculated in Narrow Ranges of Pan Cytokeratin Immunostaining Intensities
3. Discussion
4. Materials and Methods
4.1. Ethical Approval Statement
4.2. Patient Group
4.3. Study Workflow
4.4. Preparation of Tumor Tissue Sections
4.5. Selection of Tissue Sections
4.6. Immunostaining
4.7. Image Acquisition
4.8. Image Selection
4.9. Stain Decomposition
4.10. Creation of the Images with Narrow Pixel Intensity Ranges
4.11. Fractal Analysis
4.12. GLCM Analysis
4.13. Calculation of Skewness, Kurtosis, IntDen, RawIntDen and Area of the Grey Level Range
4.14. Prognostic Evaluation
4.15. Validation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASM | Angular Second Moment |
| IDM | Inverse Difference Moment |
| IntDen | Integrated Density, the product of Area and Mean Gray Value |
| RawIntDen | The sum of the pixel values in the image |
| GLCM | Gray-Level Co-Occurrence Matrix |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| Y-INT | Intersection with Y-axis |
| DB | Box fractal dimension |
| Λ | Lacunarity |
| AUC | Area under the ROC curve |
| ROC | Receiver operating characteristic |
| TNM | Staging system based on tumor size, lymph node spread, and metastasis. |
References
- Dillekas, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.P.; Rose, C.J.; Waterton, J.C.; Carano, R.A.; Parker, G.J.; Jackson, A. Imaging intratumor heterogeneity: Role in therapy response, resistance, and clinical outcome. Clin. Cancer Res. 2015, 21, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Franco, P.; De Rose, F.; De Santis, M.C.; Pasinetti, N.; Lancellotta, V.; Meduri, B.; Meattini, I.; Clinical Oncology Breast Cancer Group Investigators. Omission of postoperative radiation after breast conserving surgery: A progressive paradigm shift towards precision medicine. Clin. Transl. Radiat. Oncol. 2020, 21, 112–119. [Google Scholar] [CrossRef]
- Elston, C.W.; Gresham, G.A.; Rao, G.S.; Zebro, T.; Haybittle, J.L.; Houghton, J.; Kearney, G. The cancer research campaign (King’s/Cambridge trial for early breast cancer: Clinico-pathological aspects. Br. J. Cancer 1982, 45, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Schnitt, S.J. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod. Pathol. 2010, 23 (Suppl. 2), S60–S64. [Google Scholar] [CrossRef] [Green Version]
- Carlson, B. Oncotype DX Test Offers Guidance For Women Debating Chemotherapy. Biotechnol. Healthc. 2006, 3, 12–14. [Google Scholar]
- Orucevic, A.; Chen, J.; McLoughlin, J.M.; Heidel, R.E.; Panella, T.; Bell, J. Is the TNM staging system for breast cancer still relevant in the era of biomarkers and emerging personalized medicine for breast cancer—An institution’s 10-year experience. Breast J. 2015, 21, 147–154. [Google Scholar] [CrossRef]
- Al-Thoubaity, F.K. Molecular classification of breast cancer: A retrospective cohort study. Ann. Med. Surg. (Lond.) 2020, 49, 44–48. [Google Scholar] [CrossRef]
- Buyse, M.; Loi, S.; van’t Veer, L.; Viale, G.; Delorenzi, M.; Glas, A.M.; d’Assignies, M.S.; Bergh, J.; Lidereau, R.; Ellis, P.; et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J. Natl. Cancer Inst. 2006, 98, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, J.M.; Hveem, T.; Pretorius, M.; Oukrif, D.; Nielsen, B.; Albregtsen, F.; Lovat, L.B.; Novelli, M.R.; Danielsen, H.E. Comparison of nuclear texture analysis and image cytometric DNA analysis for the assessment of dysplasia in Barrett’s oesophagus. Br. J. Cancer 2011, 105, 1218–1223. [Google Scholar] [CrossRef] [Green Version]
- Laurinavicius, A.; Laurinaviciene, A.; Dasevicius, D.; Elie, N.; Plancoulaine, B.; Bor, C.; Herlin, P. Digital image analysis in pathology: Benefits and obligation. Anal. Cell Pathol. 2012, 35, 75–78. [Google Scholar] [CrossRef]
- Angell, H.K.; Gray, N.; Womack, C.; Pritchard, D.I.; Wilkinson, R.W.; Cumberbatch, M. Digital pattern recognition-based image analysis quantifies immune infiltrates in distinct tissue regions of colorectal cancer and identifies a metastatic phenotype. Br. J. Cancer 2013, 109, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Laurinavicius, A.; Plancoulaine, B.; Laurinaviciene, A.; Herlin, P.; Meskauskas, R.; Baltrusaityte, I.; Besusparis, J.; Dasevi Ius, D.; Elie, N.; Iqbal, Y.; et al. A methodology to ensure and improve accuracy of Ki67 labelling index estimation by automated digital image analysis in breast cancer tissue. Breast Cancer Res. 2014, 16, R35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pribic, J.; Vasiljevic, J.; Kanjer, K.; Konstantinovic, Z.N.; Milosevic, N.T.; Vukosavljevic, D.N.; Radulovic, M. Fractal dimension and lacunarity of tumor microscopic images as prognostic indicators of clinical outcome in early breast cancer. Biomark. Med. 2015, 9, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Aeffner, F.; Zarella, M.D.; Buchbinder, N.; Bui, M.M.; Goodman, M.R.; Hartman, D.J.; Lujan, G.M.; Molani, M.A.; Parwani, A.V.; Lillard, K.; et al. Introduction to Digital Image Analysis in Whole-slide Imaging: A White Paper from the Digital Pathology Association. J. Pathol. Inform. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Jarvinen, M.J.; Nelson, W.G.; Huang, J.W.; Woodcock-Mitchell, J.; Sun, T.T. Correlation of specific keratins with different types of epithelial differentiation: Monoclonal antibody studies. Cell 1982, 30, 361–372. [Google Scholar] [CrossRef]
- Vranes, V.; Rajkovic, N.; Li, X.; Plataniotis, K.N.; Todorovic Rakovic, N.; Milovanovic, J.; Kanjer, K.; Radulovic, M.; Milosevic, N.T. Size and Shape Filtering of Malignant Cell Clusters within Breast Tumors Identifies Scattered Individual Epithelial Cells as the Most Valuable Histomorphological Clue in the Prognosis of Distant Metastasis Risk. Cancers 2019, 11, 1615. [Google Scholar] [CrossRef] [Green Version]
- Rajkovic, N.; Li, X.; Plataniotis, K.N.; Kanjer, K.; Radulovic, M.; Milosevic, N.T. The Pan-Cytokeratin Staining Intensity and Fractal Computational Analysis of Breast Tumor Malignant Growth Patterns Prognosticate the Occurrence of Distant Metastasis. Front. Oncol. 2018, 8, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambasco, M.; Eliasziw, M.; Magliocco, A.M. Morphologic complexity of epithelial architecture for predicting invasive breast cancer survival. J. Transl. Med. 2010, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Tambasco, M.; Magliocco, A.M. Relationship between tumor grade and computed architectural complexity in breast cancer specimens. Hum. Pathol. 2008, 39, 740–746. [Google Scholar] [CrossRef]
- Vujasinovic, T.; Pribic, J.; Kanjer, K.; Milosevic, N.T.; Tomasevic, Z.; Milovanovic, Z.; Nikolic-Vukosavljevic, D.; Radulovic, M. Gray-Level Co-Occurrence Matrix Texture Analysis of Breast Tumor Images in Prognosis of Distant Metastasis Risk. Microsc. Microanal. 2015, 21, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Sredni, S.T.; Neves, J.I.; de Camargo, B.; Caballero, O.L.; Soares, F.A. Pan-cytokeratin immunoexpression in Wilms’ tumors: A simple approach for understanding tumor epithelial differentiation. Sao Paulo Med. J. 2004, 122, 181–183. [Google Scholar] [CrossRef]
- Xu, Q.; Yuan, J.P.; Chen, Y.Y.; Zhang, H.Y.; Wang, L.W.; Xiong, B. Prognostic Significance of the Tumor-Stromal Ratio in Invasive Breast Cancer and a Proposal of a New Ts-TNM Staging System. J. Oncol. 2020, 2020, 9050631. [Google Scholar] [CrossRef] [PubMed]
- Gujam, F.J.; Edwards, J.; Mohammed, Z.M.; Going, J.J.; McMillan, D.C. The relationship between the tumour stroma percentage, clinicopathological characteristics and outcome in patients with operable ductal breast cancer. Br. J. Cancer 2014, 111, 157–165. [Google Scholar] [CrossRef]
- Hansen, T.F.; Kjaer-Frifeldt, S.; Lindebjerg, J.; Rafaelsen, S.R.; Jensen, L.H.; Jakobsen, A.; Sorensen, F.B. Tumor-stroma ratio predicts recurrence in patients with colon cancer treated with neoadjuvant chemotherapy. Acta Oncol. 2018, 57, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Galon, J.; Pages, F.; Tartour, E.; Sautes-Fridman, C.; Kroemer, G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011, 71, 5601–5605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Richards, C.H.; McMillan, D.C.; Horgan, P.G.; Roxburgh, C.S. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann. Oncol. 2014, 25, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Rye, I.H.; Trinh, A.; Saetersdal, A.B.; Nebdal, D.; Lingjaerde, O.C.; Almendro, V.; Polyak, K.; Borresen-Dale, A.L.; Helland, A.; Markowetz, F.; et al. Intratumor heterogeneity defines treatment-resistant HER2+ breast tumors. Mol. Oncol. 2018, 12, 1838–1855. [Google Scholar] [CrossRef] [Green Version]
- Beca, F.; Polyak, K. Intratumor Heterogeneity in Breast Cancer. Adv. Exp. Med. Biol. 2016, 882, 169–189. [Google Scholar] [CrossRef]
- Gerdes, M.J.; Gokmen-Polar, Y.; Sui, Y.; Pang, A.S.; LaPlante, N.; Harris, A.L.; Tan, P.H.; Ginty, F.; Badve, S.S. Single-cell heterogeneity in ductal carcinoma in situ of breast. Mod. Pathol. 2018, 31, 406–417. [Google Scholar] [CrossRef]
- Liang, F.; Cao, W.; Wang, Y.; Li, L.; Zhang, G.; Wang, Z. The prognostic value of tumor budding in invasive breast cancer. Pathol. Res. Pract. 2013, 209, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Alhaiek, A.A.; Amadi, S.; Qattan, A.T.; Crawford, M.; Radulovic, M.; Godovac-Zimmermann, J. Systematic nucleo-cytoplasmic trafficking of proteins following exposure of MCF7 breast cancer cells to estradiol. J. Proteome Res. 2014, 13, 1112–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rykala, J.; Przybylowska, K.; Majsterek, I.; Pasz-Walczak, G.; Sygut, A.; Dziki, A.; Kruk-Jeromin, J. Angiogenesis markers quantification in breast cancer and their correlation with clinicopathological prognostic variables. Pathol. Oncol. Res. 2011, 17, 809–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brankovic-Magic, M.V.; Nikolic-Vukosavljevic, D.B.; Neskovic-Konstantinovic, Z.B.; Kanjer, K.S.; Spuzic, I.V. Variations in the content of steroid receptors in breast cancer. Comparison between primary tumors and metastatic lesions. Acta Oncol. 1992, 31, 629–633. [Google Scholar] [CrossRef]
- Li, X.; Plataniotis, K.N. A Complete Color Normalization Approach to Histopathology Images Using Color Cues Computed From Saturation-Weighted Statistics. IEEE Trans. Biomed. Eng. 2015, 62, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, N.; Kolarevic, D.; Kanjer, K.; Milosevic, N.T.; Nikolic-Vukosavljevic, D.; Radulovic, M. Comparison of Monofractal, Multifractal and gray level Co-occurrence matrix algorithms in analysis of Breast tumor microscopic images for prognosis of distant metastasis risk. Biomed. Microdevices 2016, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, G.J.; Radulovic, M.; Sopta, J.P.; Nikitovic, M.; Milosevic, N.T. Fractal and Gray Level Cooccurrence Matrix Computational Analysis of Primary Osteosarcoma Magnetic Resonance Images Predicts the Chemotherapy Response. Front. Oncol. 2017, 7, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]

| Classification | AUC a/p–Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | ||||||||||
| Grey Level Ranges | Specific | Non-Spec | ||||||||
| 0–255 | 0–130 | 130–160 | 160–180 | 180–200 | 200–220 | 220–240 | 240–255 | 0–220 | 220–255 | |
| GLCM features | ||||||||||
| ASM | 0.77/0.000 * | 0.61/0.14 | 0.66/0.03 * | 0.68/0.01 * | 0.64/0.06 | 0.52/0.70 | 0.36/0.04 * | 0.45/0.53 | 0.68/0.01 * | 0.36/0.04 * |
| 0.66–0.87 | 0.44–0.70 | 0.56–0.81 | 0.56–0.81 | 0.50–0.77 | 0.38–0.67 | 0.22–0.46 | 0.38–0.59 | 0.57–0.78 | 0.24–0.47 | |
| Contrast | 0.39/0.13 | 0.38/0.08 | 0.35/0.04 * | 0.31/0.01 * | 0.34/0.03 * | 0.42/0.28 | 0.45/0.47 | 0.54/0.55 | 0.41/0.22 | 0.42/0.28 |
| 0.28–0.53 | 0.24–0.54 | 0.19–0.43 | 0.19–0.43 | 0.22–0.48 | 0.29–0.56 | 0.33–0.58 | 0.42–0.68 | 0.30–0.53 | 0.30–0.55 | |
| Correlation | 0.60/0.17 | 0.61/0.14 | 0.66/0.02 * | 0.71/0.004 * | 0.71/0.004 * | 0.64/0.06 | 0.56/0.43 | 0.70/0.007 * | 0.59/0.21 | 0.56/0.37 |
| 0.43–0.75 | 0.43–0.74 | 0.60–0.82 | 0.60–0.82 | 0.58–0.83 | 0.52–0.76 | 0.43–0.69 | 0.58–0.81 | 0.44–0.74 | 0.43–0.70 | |
| IDM | 0.75/0.000 * | 0.61/0.14 | 0.66/0.03 * | 0.68/0.01 * | 0.64/0.06 | 0.53/0.70 | 0.41/0.20 | 0.48/0.75 | 0.68/0.01 * | 0.41/0.21 |
| 0.64–0.85 | 0.44–0.74 | 0.56–0.81 | 0.56–0.81 | 0.50–0.77 | 0.39–0.67 | 0.27–0.51 | 0.34–0.61 | 0.57–0.79 | 0.29–0.53 | |
| Entropy | 0.28/0.002 * | 0.40/0.17 | 0.34/0.03 * | 0.32/0.01 * | 0.37/0.06 | 0.48/0.81 | 0.65/0.04 * | 0.54/0.59 | 0.32/0.01 * | 0.61/0.12 |
| 0.19–0.40 | 0.27–0.57 | 0.20–0.45 | 0.20–0.45 | 0.34–0.50 | 0.34–0.63 | 0.56–0.79 | 0.41–0.67 | 0.21–0.44 | 0.49–0.73 | |
| Fractal features | ||||||||||
| DB | 0.37/0.07 | 0.38/0.09 | 0.35/0.04 * | 0.32/0.01 * | 0.31/0.008 * | 0.35/0.04 * | 0.53/0.65 | 0.56/0.37 | 0.38/0.08 | 0.49/0.90 |
| 0.24–0.50 | 0.24–0.52 | 0.22–0.48 | 0.19–0.45 | 0.19–0.43 | 0.23–0.47 | 0.41–0.66 | 0.44–0.69 | 0.25–0.51 | 0.34–0.62 | |
| SE for DB | 0.49/0.90 | 0.63/0.07 | 0.65/0.04 * | 0.64/0.05 * | 0.63/0.08 | 0.55/0.48 | 0.40/0.17 | 0.49/0.85 | 0.50/0.95 | 0.47/0.65 |
| 0.33–0.65 | 0.47–0.74 | 0.53–0.77 | 0.54–0.79 | 0.49–0.76 | 0.42–0.68 | 0.27–0.54 | 0.36–0.62 | 0.34–0.66 | 0.33–0.61 | |
| Λ | 0.70/0.006 * | 0.63/0.06 | 0.67/0.02 * | 0.69/0.008 * | 0.69/0.008 * | 0.65/0.03 * | 0.43/0.32 | 0.45/0.45 | 0.69/0.007 * | 0.46/0.57 |
| 0.57–0.82 | 0.50–0.77 | 0.54–0.79 | 0.56–0.82 | 0.57–0.81 | 0.53–0.77 | 0.31–0.55 | 0.32–0.57 | 0.57–0.81 | 0.33–0.59 | |
| First-order statistics b | ||||||||||
| Area | – | 0.40/0.16 | 0.31/0.006 * | 0.31/0.007 * | 0.40/0.17 | 0.52/0.77 | 0.69/0.009 * | 0.61/0.13 | 0.33/0.02 * | 0.68//0/01 * |
| 0.26–0.55 | 0.18–0.43 | 0.19–0.43 | 0.27–0.53 | 0.38–0.66 | 0.67–0.80 | 0.48–0.74 | 0.20–0.45 | 0.57–0.79 | ||
| Mean | 036/0.05 * | 0.51/0.91 | 0.46/0.58 | 0.36/0.05 * | 0.35/0.03 * | 0.41/0.21 | 0.37/0.007 * | 0.68/0.01 * | 0.57/0.34 | 0.55/0.48 |
| 0.23–0.49 | 0.27–0.66 | 0.32–0.62 | 0.23–0.50 | 0.22–0.47 | 0.27–0.54 | 0.24–0.50 | 0.56–0.80 | 0.40–0.73 | 0.42–0.69 | |
| Kurtosis | 0.67/0.02 * | 0.50/0.98 | 0.54/0.52 | 0.55/0.52 | 0.59/0.20 | 0.61/0.12 | 0.62/0.10 | 0.69/0.01 * | 0.56/0.68 | 0.64/0.05 * |
| 0.55–0.78 | 0.35–0.64 | 0.31–0.61 | 0.38–0.69 | 0.45–0.72 | 0.48–0.74 | 0.49–0.75 | 0.57–0.81 | 0.37–0.79 | 0.51–0.77 | |
| Pixel Intensity Ranges | ||||||||||
| Analytical method | Original | 0–130 | 130–160 | 160–180 | 180–200 | 200–220 | 220–240 | 240–254 | 0–220 | 220–255 |
| Average AUC values a | ||||||||||
| AUC | 0.66 | 0.60 | 0.64 | 0.67 | 0.65 | 0.57 | 0.62 | 0.59 | 0.62 | 0.58 |
| AUC improvement in the narrow pixel intensity ranges b | ||||||||||
| GLCM | - | 0 | 0.10 | 0.19 | 0.15 | 0 | 0 | 0.10 | 0 | 0 |
| Fractal analysis | - | 0 | 0.18 | 0.20 | 0.20 | 0.02 | 0 | 0 | 0 | 0 |
| First-order statistics | - | 0 | 0 | 0 | 0.01 | 0.0 | 0 | 0.06 | 0 | 0 |
| sum | - | 0 | 0.28 | 0.39 | 0.36 | 0.02 | 0 | 0.16 | 0 | 0 |
| Feature | p-Value a | HR | 95% CI |
|---|---|---|---|
| Tumor size | 0.03 | 1.07 | 1.01–1.15 |
| 0–255 mean | 0.003 | 0.85 | 0.76–0.95 |
| 0–255 entropy | 0.03 | 35.0 | 1.29–944 |
| 160–180 DB | 0.04 | 0.00 | 0.00–0.39 |
| 240–255 kurtosis | 0.002 | 1.02 | 1.01–1.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vranes, V.; Vujasinović, T.; Rajković, N.; Kanjer, K.; Milošević, N.T.; Radulovic, M. Analysis of Spatial Distribution and Prognostic Value of Different Pan Cytokeratin Immunostaining Intensities in Breast Tumor Tissue Sections. Int. J. Mol. Sci. 2020, 21, 4434. https://doi.org/10.3390/ijms21124434
Vranes V, Vujasinović T, Rajković N, Kanjer K, Milošević NT, Radulovic M. Analysis of Spatial Distribution and Prognostic Value of Different Pan Cytokeratin Immunostaining Intensities in Breast Tumor Tissue Sections. International Journal of Molecular Sciences. 2020; 21(12):4434. https://doi.org/10.3390/ijms21124434
Chicago/Turabian StyleVranes, Velicko, Tijana Vujasinović, Nemanja Rajković, Ksenija Kanjer, Nebojša T. Milošević, and Marko Radulovic. 2020. "Analysis of Spatial Distribution and Prognostic Value of Different Pan Cytokeratin Immunostaining Intensities in Breast Tumor Tissue Sections" International Journal of Molecular Sciences 21, no. 12: 4434. https://doi.org/10.3390/ijms21124434





