In Search of a Phosphorus Dendrimer-Based Carrier of Rose Bengal: Tyramine Linker Limits Fluorescent and Phototoxic Properties of a Photosensitizer
Abstract
:1. Introduction
2. Results
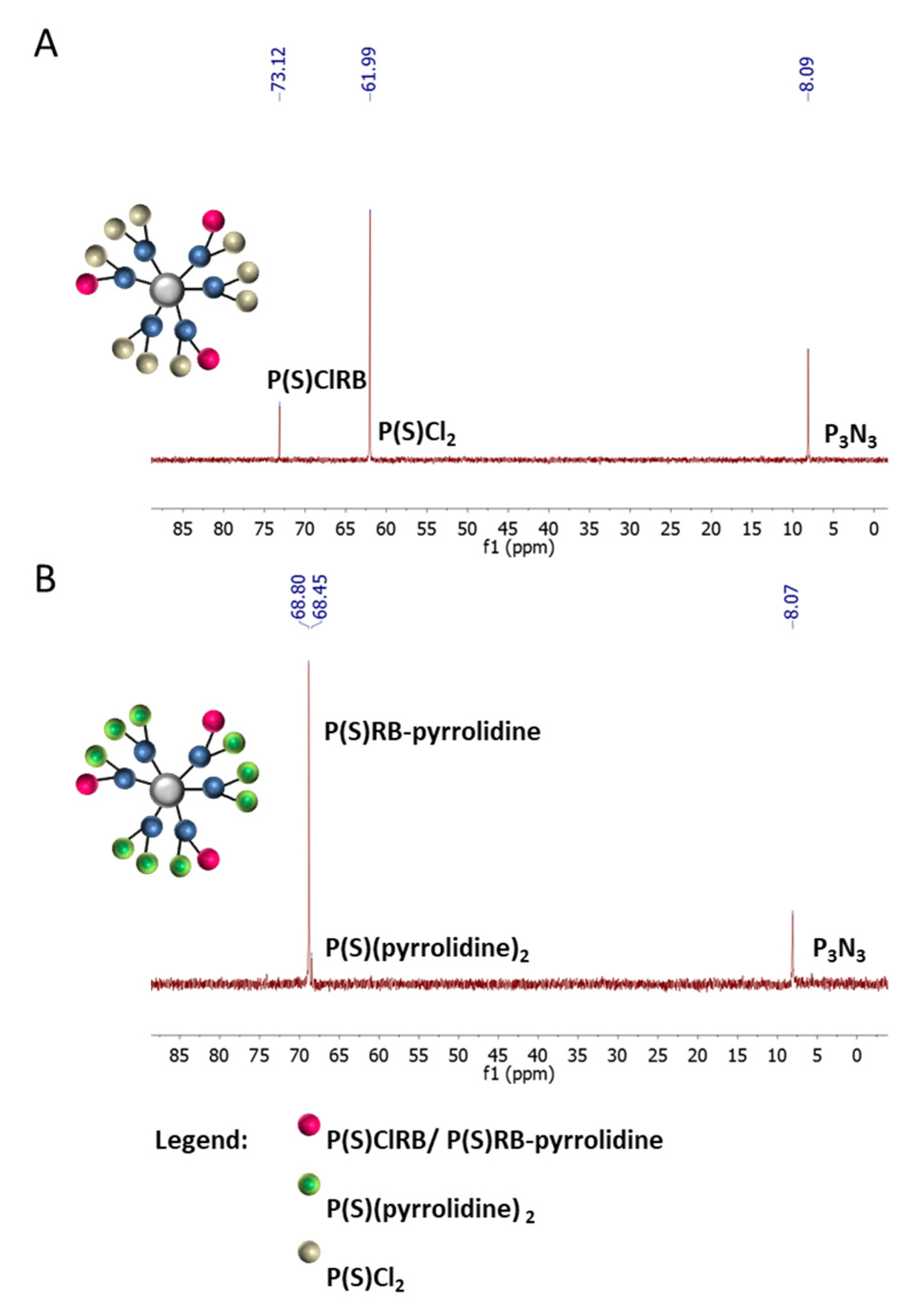
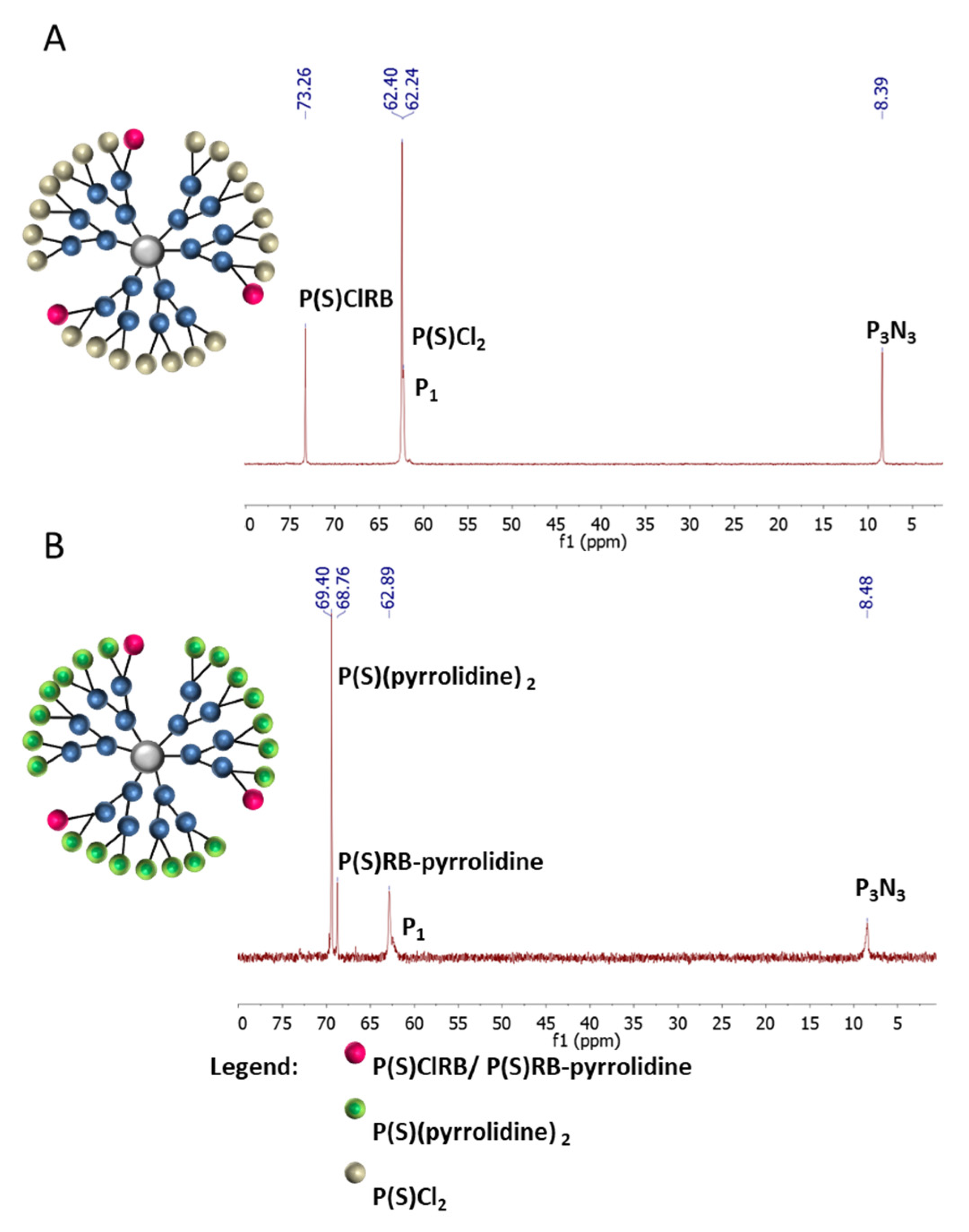
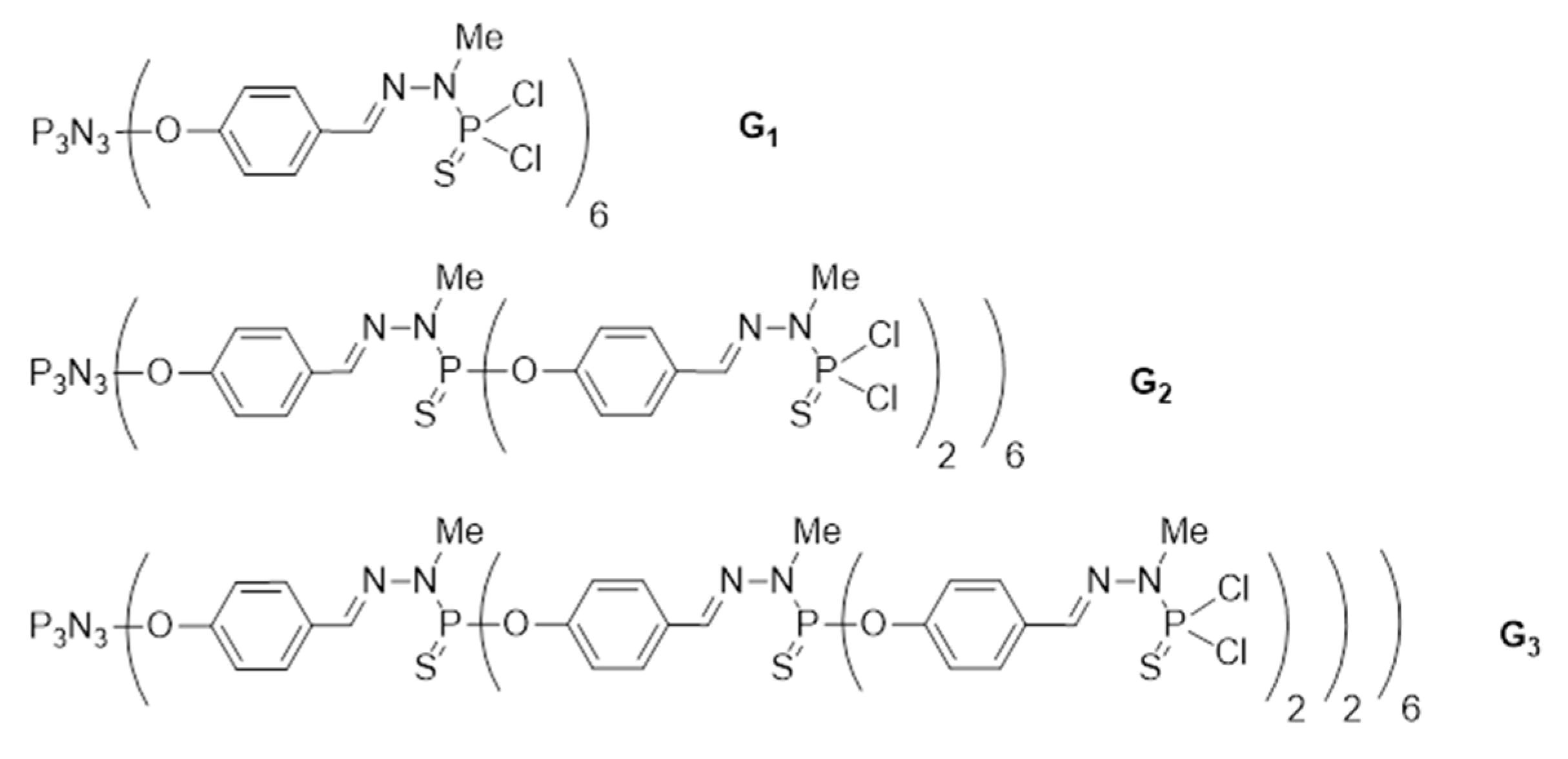
2.1. Synthesis and Characterization of Conjugates
2.2. Spectroscopy Studies
2.3. Size and Zeta Potential Measurements
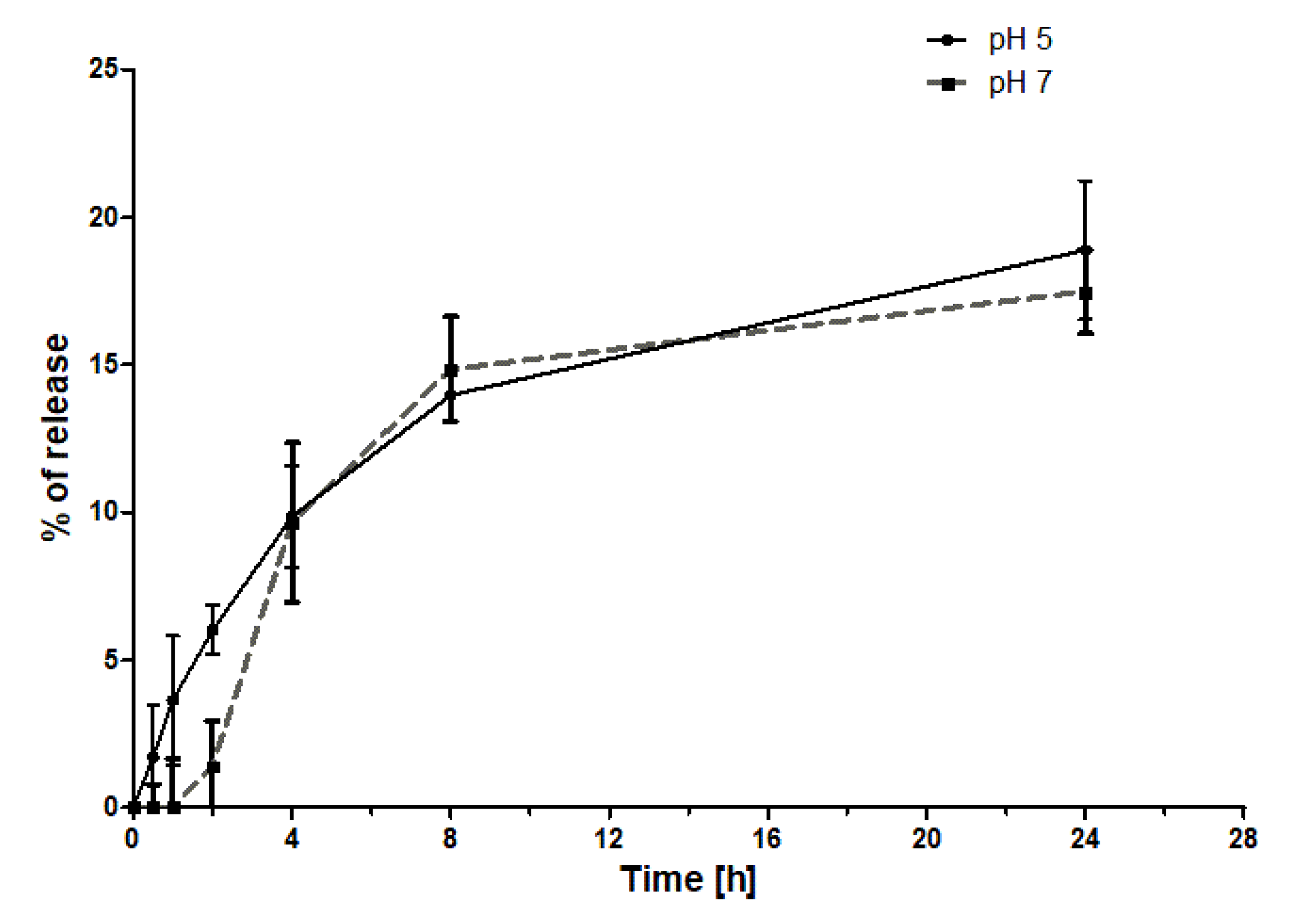
2.4. Drug Release Studies
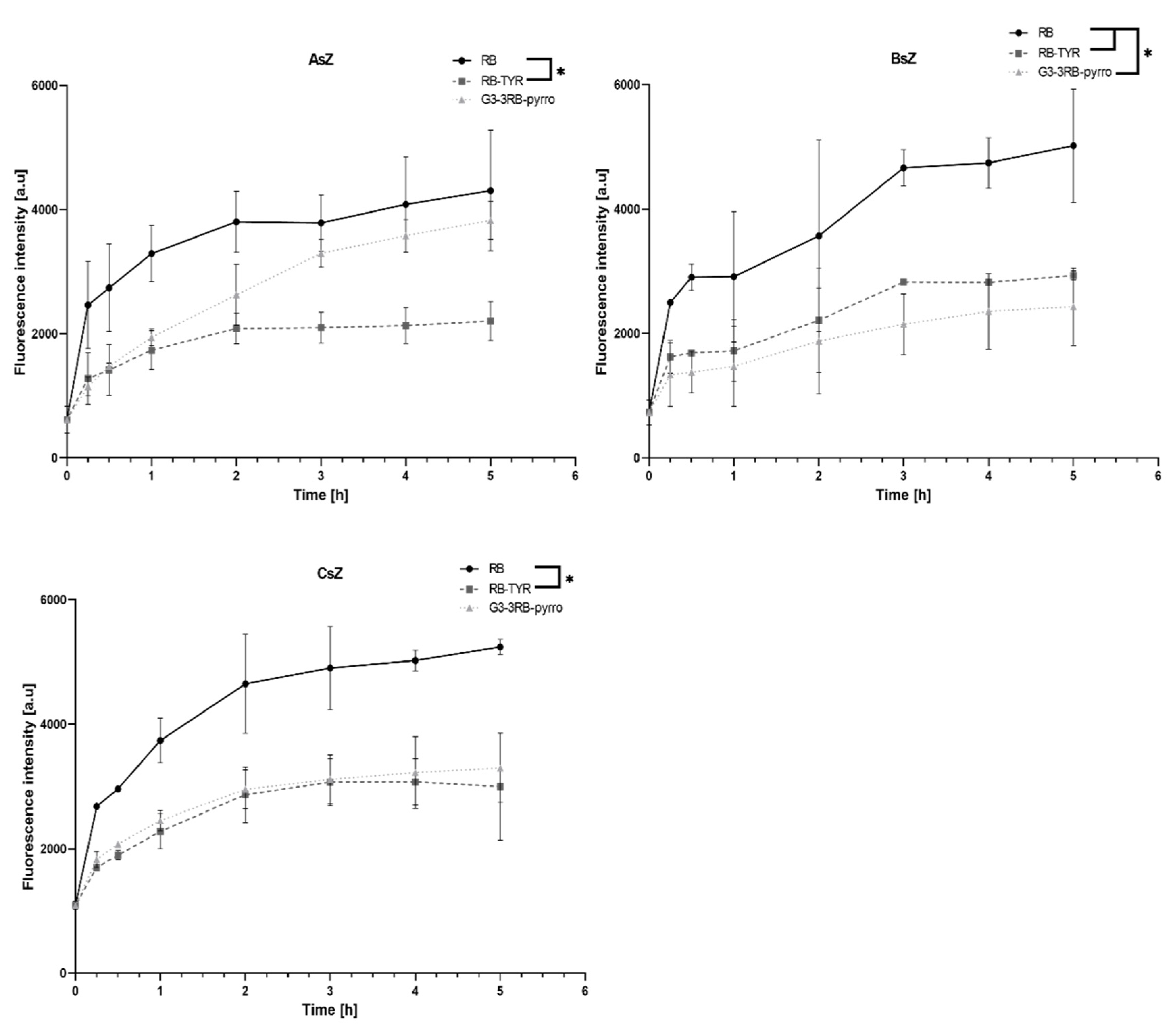
2.5. Cellular Uptake Assay
2.6. Singlet Oxygen Generation
2.7. Phototoxicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Drug–Dendrimer Conjugates—General Information
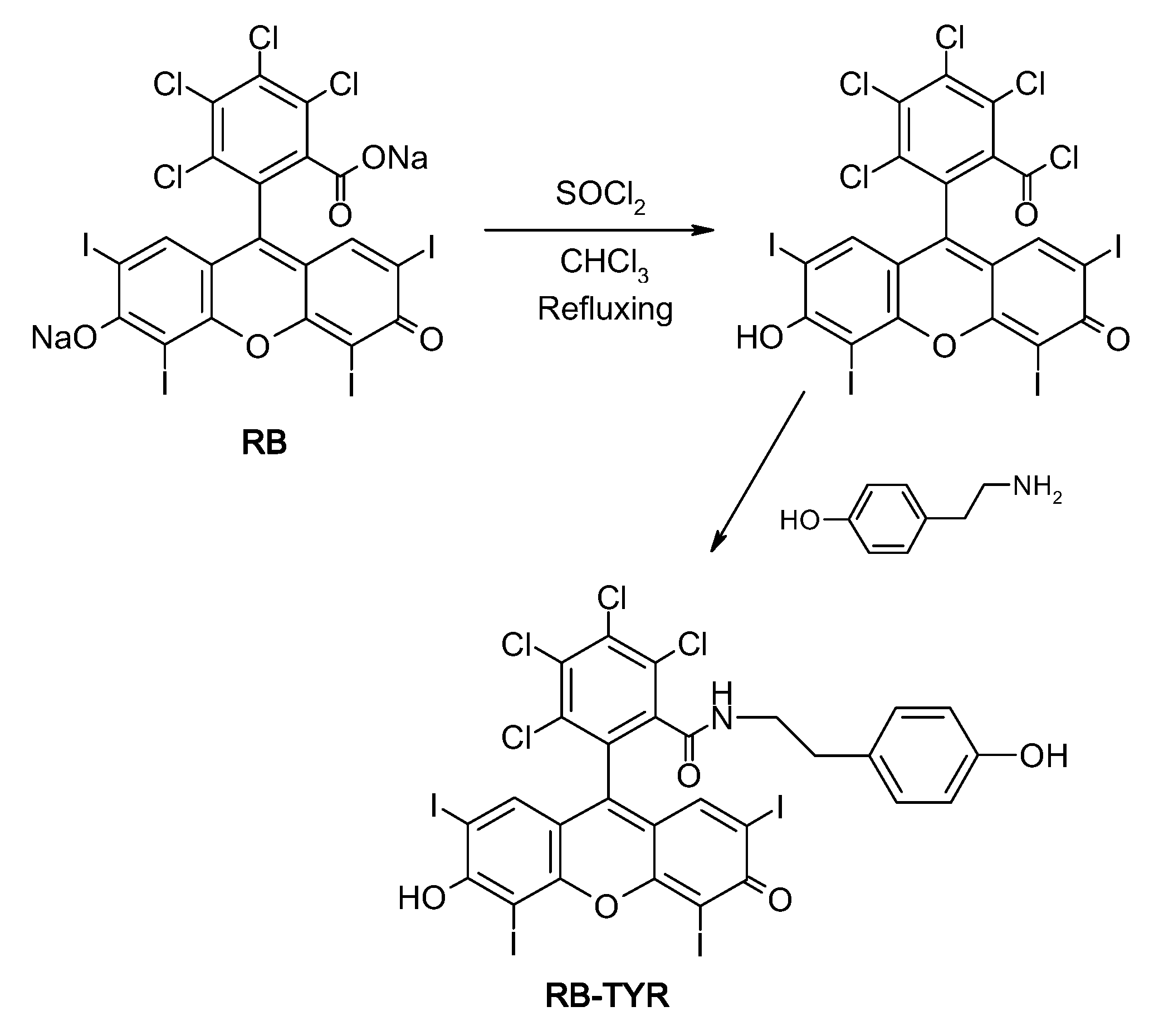
4.2.2. Synthesis of the Rose Bengal Acid Chloride
4.2.3. Synthesis of Rose Bengal–Tyramine
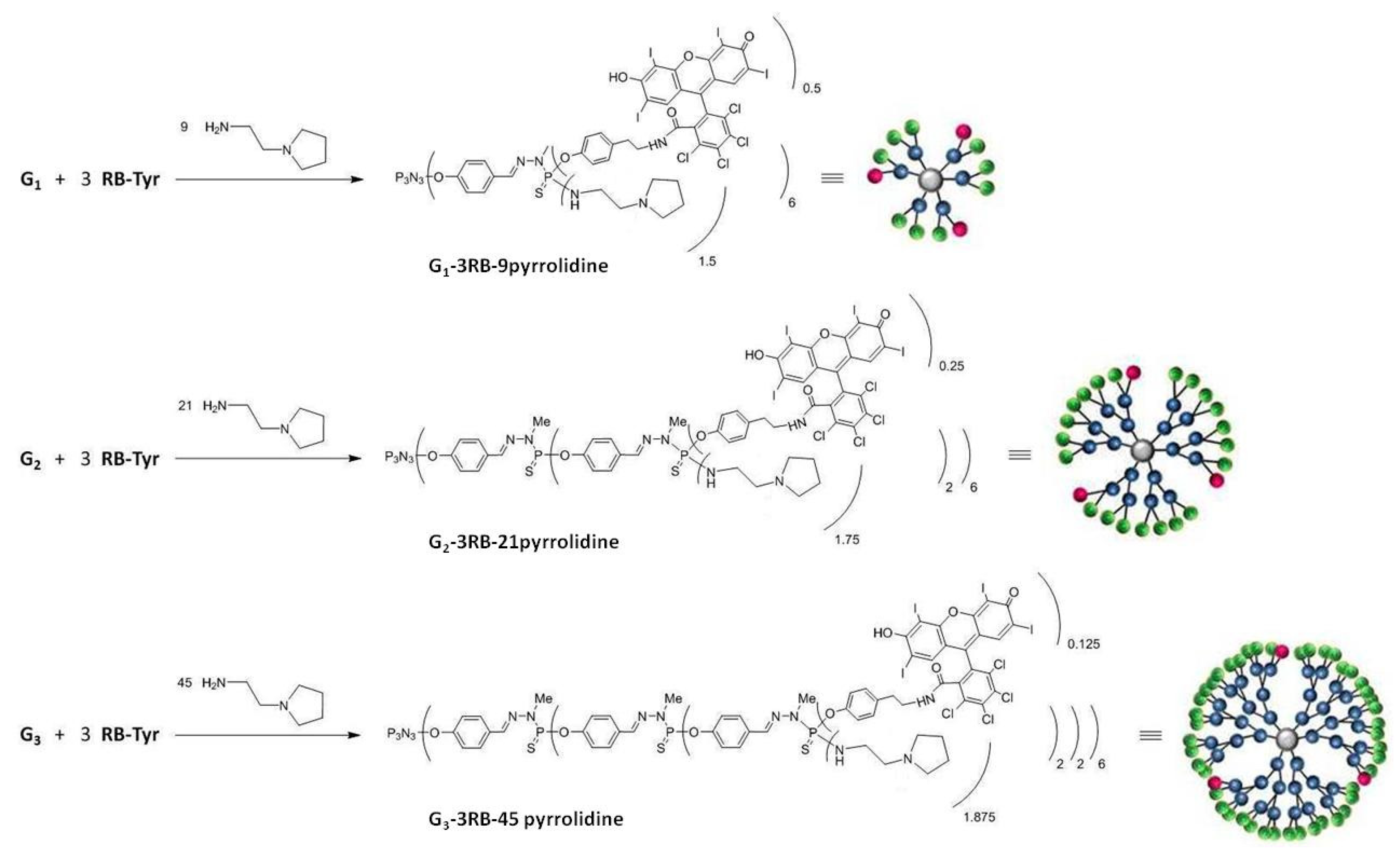
4.2.4. Synthesis of Rose Bengal–G1, G2, G3 Phosphorus Dendrimers—Pyrrolidine Conjugates
Preparation of the PS-Cl2 G1, G2, G3 Dendrimer Conjugates Containing Three Molecules of Rose Bengal
Preparation of the PS-Cl2 G1, G2, G3 Dendrimer Conjugates Containing Three Molecules of Rose Bengal and 9, 21 and 45 Molecules of Pyrrolidine, Respectively
4.2.5. Spectroscopy Studies
4.2.6. Size and Zeta Potential Measurements
4.2.7. Drug Release Studies
4.2.8. Cell Culture
4.2.9. Cellular Uptake Determination
4.2.10. Singlet Oxygen Generation Assay
4.2.11. Cytotoxicity Studies
4.2.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Skin Cancers. Available online: https://www.who.int/uv/faq/skincancer/en/index1.html (accessed on 4 February 2020).
- Kirby, J.S.; Miller, C.J. Intralesional chemotherapy for nonmelanoma skin cancer: A practical review. J. Am. Acad. Dermatol. 2010, 63, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.A.; Welch, E.; Leffell, D.J. Management of nonmelanoma skin cancer in 2007. Nat. Clin. Pract. Oncol. 2007, 4, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.M.; Karrer, S. Towards a more specific therapy: Targeting nonmelanoma skin cancer cells. Br. J. Dermatol. 2006, 154, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Vianna Lopez, R.F.; Lange, N.; Guy, R.; Bentley, M.V.L.B. Photodynamic therapy of skin cancer: Controlled drug delivery of 5-ALA and its esters. Adv. Drug Deliv. Rev. 2004, 56, 77–94. [Google Scholar] [CrossRef]
- Ochsner, M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B Biol. 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Granville, D.J.; McManus, B.M.; Hunt, D.W.C. Photodynamic therapy: Shedding light on the biochemical pathways regulating porphyrin-mediated cell death. Histol. Histopathol. 2001, 16, 309–317. [Google Scholar] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Marcucci, F.; Lefoulon, F. Active targeting with particulate drug carriers in tumor therapy: Fundamentals and recent progress. Drug Discov. Today 2004, 9, 219–228. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Nanocarriers in photodynamic therapy—In vitro and in vivo studies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, 1–24. [Google Scholar] [CrossRef]
- Wieder, M.E.; Hone, D.C.; Cook, M.J.; Handsley, M.M.; Gavrilovic, J.; Russell, D.A. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: Cancer therapy using a ‘Trojan horse’. Photochem. Photobiol. Sci. 2006, 5, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Rijcken, C.J.F.; Hofman, J.W.; van Zeeland, F.; Hennink, W.E.; van Nostrum, C.F. Photosensitiser-loaded biodegradable polymeric micelles: Preparation, characterisation and in vitro PDT efficacy. J. Control. Release 2007, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Bergey, E.J.; Oseroff, A.R.; Morgan, J.; Dougherty, T.J.; Prasad, P.N. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: A novel drug-carrier system for photodynamic therapy. J. Am. Chem. Soc. 2003, 125, 7860–7865. [Google Scholar] [CrossRef]
- Pucińska, J.; Podbielska, H. Nanomateriały we wspomaganiu terapii fotodynamicznej. Acta Bio-Optica Inform. Medica. Inżynieria Biomed. 2009, 15, 178–189. [Google Scholar]
- Wilk, K.A.; Zielińska, K.; Pietkiewicz, J.; Skołucka, N.; Choromańska, A.; Rossowska, J.; Garbiec, A.; Saczko, J. Photo-oxidative action in MCF-7 cancer cells induced by hydrophobic cyanines loaded in biodegradable microemulsion-templated nanocapsules. Int. J. Oncol. 2012, 41, 105–116. [Google Scholar] [PubMed] [Green Version]
- Ferenc, M.; Pedziwiatr-Werbicka, E.; Nowak, K.E.; Klajnert, B.; Majoral, J.P.; Bryszewska, M. Phosphorus dendrimers as carriers of siRNA-characterisation of dendriplexes. Molecules 2013, 18, 4451–4466. [Google Scholar] [CrossRef]
- Loup, C.; Zanta, M.A.; Caminade, A.M.; Majoral, J.P.; Meunier, B. Preparation of Water-Soluble Cationic Phosphorus-Containing Dendrimers as DNA Transfecting Agents. Chem.-A Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Dabrzalska, M.; Zablocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. Phosphorus dendrimers and photodynamic therapy. Spectroscopic studies on two dendrimer-photosensitizer complexes: Cationic phosphorus dendrimer with rose bengal and anionic phosphorus dendrimer with methylene blue. Int. J. Pharm. 2015, 492, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Dabrzalska, M.; Janaszewska, A.; Zablocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. Cationic Phosphorus Dendrimer Enhances Photodynamic Activity of Rose Bengal against Basal Cell Carcinoma Cell Lines. Mol. Pharm. 2017, 14, 1821–1830. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis of bowl-shaped dendrimers from generation 1 to generation 8. J. Organomet. Chem. 1997, 529, 51–58. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1 and 2). Trop. J. Pharm. Res. 2013, 12, 255–273. [Google Scholar]
- Posadas, I.; Romero-Castillo, L.; El Brahmi, N.; Manzanares, D.; Mignani, S.; Majoral, J.P.; Ceña, V. Neutral high-generation phosphorus dendrimers inhibit macrophage-mediated inflammatory response in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E7660–E7669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, U.; Asthana, A.; Jain, N.K. Dextran conjugated dendritic nanoconstructs as potential vectors for anti-cancer agent. Biomaterials 2009, 30, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef]
- Leriche, G.; Chisholm, L.; Wagner, A. Cleavable linkers in chemical biology. Bioorganic Med. Chem. 2012, 20, 571–582. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Gorzkiewicz, M.; Klajnert-Maculewicz, B. Chapter X in Dendrimers for Drug Delivery. In Dendrimers as Nanocarriers for Anticancer Drugs; Sharma, A., Keservan, R., Eds.; Apple Academic Press: Palm Bay, Brevard, FL, USA, 2018; pp. 327–374. [Google Scholar]
- Dabrzalska, M.; Benseny-Cases, N.; Barnadas-Rodríguez, R.; Mignani, S.; Zablocka, M.; Majoral, J.P.; Bryszewska, M.; Klajnert-Maculewicz, B.; Cladera, J. Fourier transform infrared spectroscopy (FTIR) characterization of the interaction of anti-cancer photosensitizers with dendrimers. Anal. Bioanal. Chem. 2016, 408, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.L.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.O.; Rolland, O.; Métivier, P.; Bacquet, G.; Fournié, J.J.; Caminade, A.M.; et al. Multiplication of human natural killer cells by nanosized phosphonate-capped dendrimers. Angew. Chemie-Int. Ed. 2007, 46, 2523–2526. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Than, A.; Wang, X.; Xu, S.; Sun, L.; Duan, H.; Xu, C.; Chen, P. Ultrasensitive Profiling of Metabolites Using Tyramine-Functionalized Graphene Quantum Dots. ACS Nano 2016, 10, 3622–3629. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambe, I.H. Ion channels and transporters in the development of drug resistance in cancer cells. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130109. [Google Scholar] [CrossRef] [Green Version]
- Studzian, M.; Szulc, A.; Janaszewska, A.; Appelhans, D.; Pułaski, Ł.; Klajnert-Maculewicz, B. Mechanisms of Internalization of Maltose-Modified Poly(propyleneimine) Glycodendrimers into Leukemic Cell Lines. Biomacromolecules 2017, 18, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Filimon, A.; Sima, L.E.; Appelhans, D.; Voit, B.; Negroiu, G. Internalization and Intracellular Trafficking of Poly(propylene imine) Glycodendrimers with Maltose Shell in Melanoma Cells. Curr. Med. Chem. 2012, 19, 4955–4968. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, J.-H.H.; Liu, Q.; Huang, H.; Chen, M.; Li, K.; Li, C.; Yu, X.-F.F.; Chu, P.K. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials 2014, 35, 1954–1966. [Google Scholar] [CrossRef]







| Hydrodynamic Diameter (nm) | Zeta Potential (mV) | |
|---|---|---|
| G3–3RB | 18.17 ± 4.96 | 44.54 ± 2.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztandera, K.; Marcinkowska, M.; Gorzkiewicz, M.; Janaszewska, A.; Laurent, R.; Zabłocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. In Search of a Phosphorus Dendrimer-Based Carrier of Rose Bengal: Tyramine Linker Limits Fluorescent and Phototoxic Properties of a Photosensitizer. Int. J. Mol. Sci. 2020, 21, 4456. https://doi.org/10.3390/ijms21124456
Sztandera K, Marcinkowska M, Gorzkiewicz M, Janaszewska A, Laurent R, Zabłocka M, Mignani S, Majoral JP, Klajnert-Maculewicz B. In Search of a Phosphorus Dendrimer-Based Carrier of Rose Bengal: Tyramine Linker Limits Fluorescent and Phototoxic Properties of a Photosensitizer. International Journal of Molecular Sciences. 2020; 21(12):4456. https://doi.org/10.3390/ijms21124456
Chicago/Turabian StyleSztandera, Krzysztof, Monika Marcinkowska, Michał Gorzkiewicz, Anna Janaszewska, Regis Laurent, Maria Zabłocka, Serge Mignani, Jean Pierre Majoral, and Barbara Klajnert-Maculewicz. 2020. "In Search of a Phosphorus Dendrimer-Based Carrier of Rose Bengal: Tyramine Linker Limits Fluorescent and Phototoxic Properties of a Photosensitizer" International Journal of Molecular Sciences 21, no. 12: 4456. https://doi.org/10.3390/ijms21124456






