A Modified Protocol of Diethylnitrosamine Administration in Mice to Model Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
2.1. General Observations
2.2. Liver Tumors
2.3. Assessment of HCC
2.4. Biochemical Markers
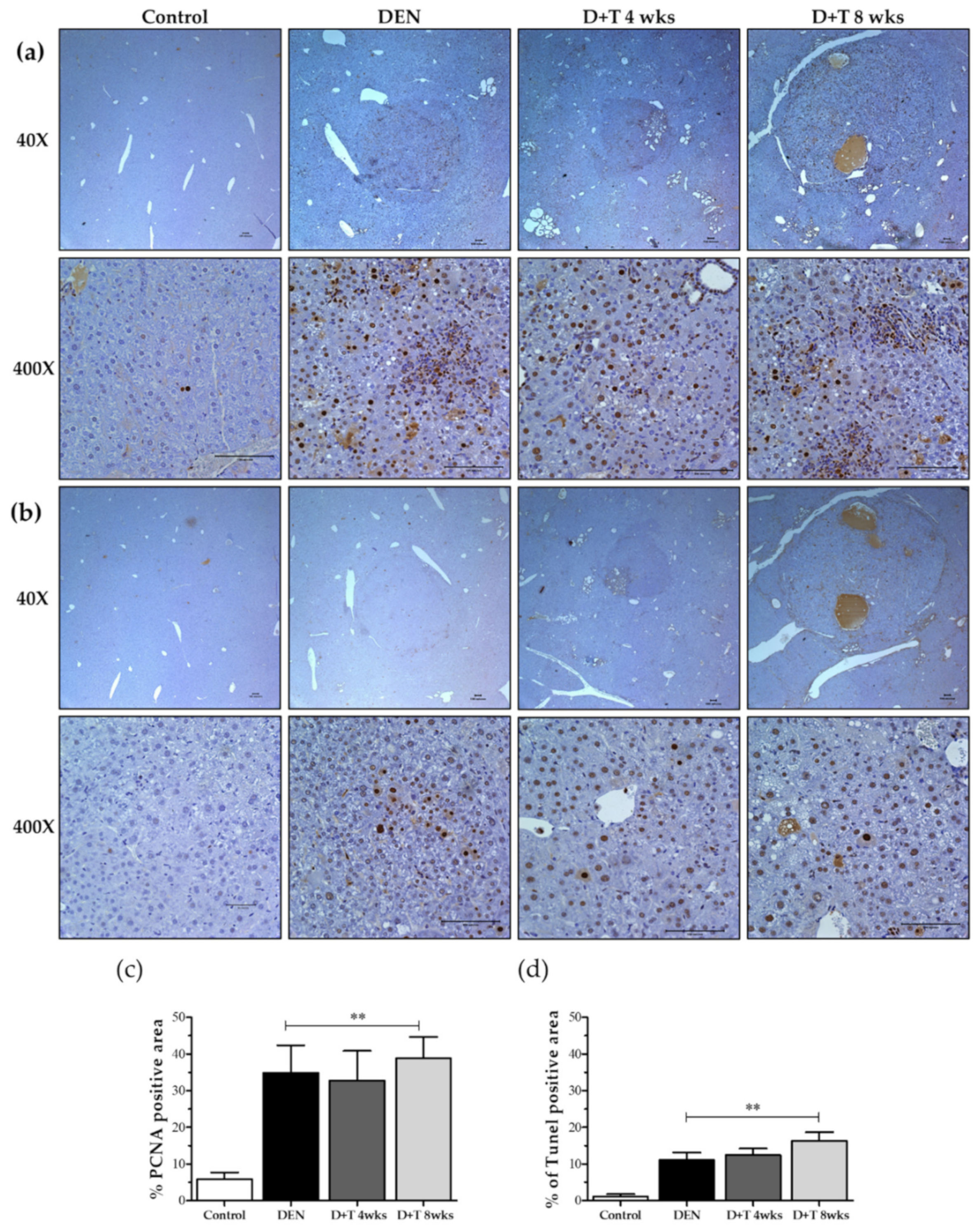
2.5. Hepatocellular Death and Proliferation
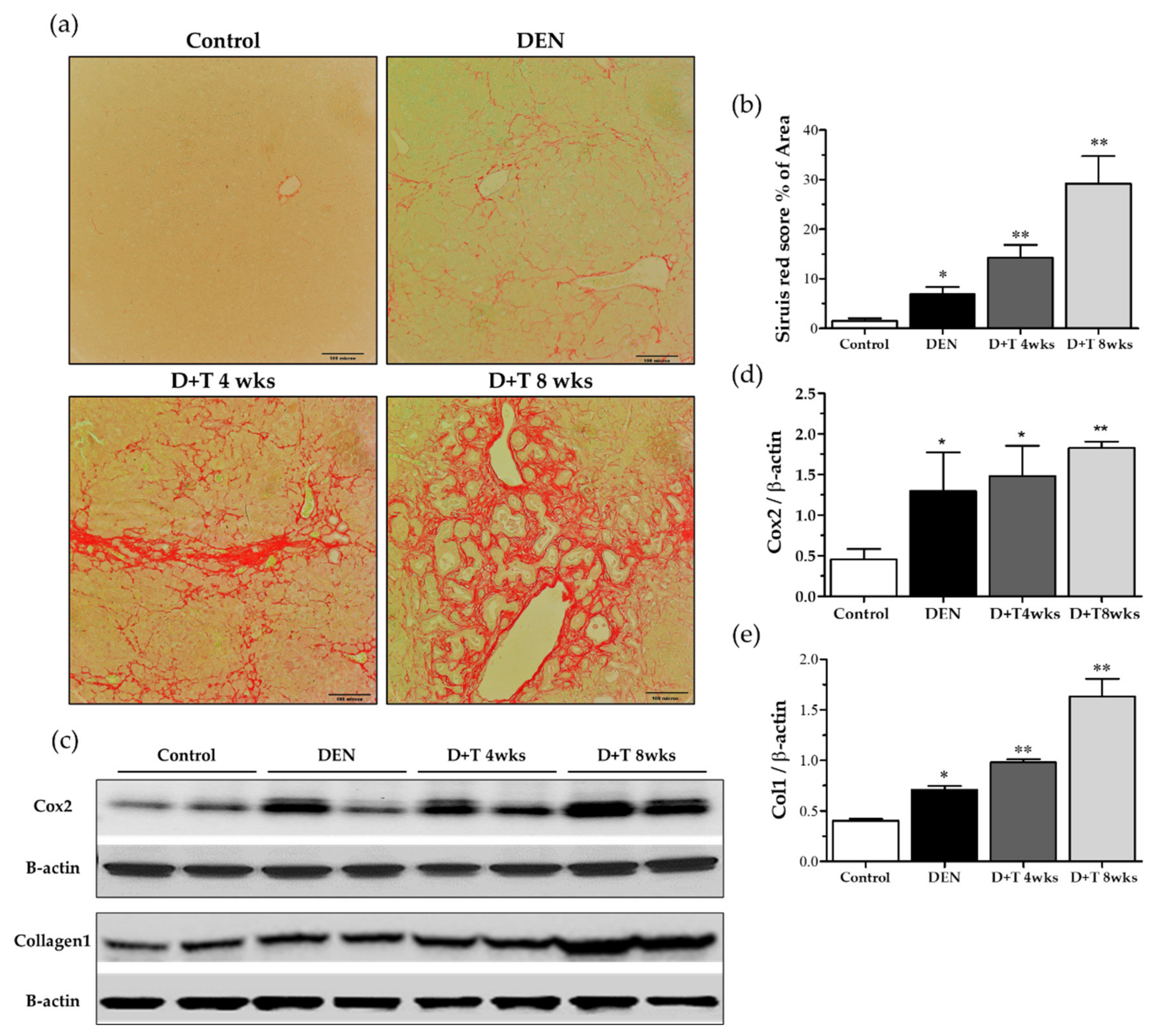
2.6. Fibrogenesis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal
4.3. Experimental Design
4.4. Histopathological Examination
4.5. Blood Chemistry
4.6. Immunohistochemistry
4.7. Quantification of Fibrosis
4.8. Protein Extraction and Western Blot Analysis
4.9. Data Analysis and Statistics
5. Conclusion
6. Patent
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| DEN | Diethylnitrosamine |
| TAA | Thioacetamide |
| BW | Bodyweight |
| SD | Standard deviation |
| mg | Milligram |
| Kg | Kilogram |
| wks | Weeks |
| D+T | DEN+TAA |
| H&E | Hematoxylin and eosin |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| PCNA | Proliferating cell nuclear antigen |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| Col1 | Collagen 1 |
References
- Yang, J.D.; Roberts, L.R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Singh, P.P.; Roberts, L.R.; Sanchez, W. Chemopreventive strategies in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Alkofer, B.; Lepennec, V.; Chiche, L. Hepatocellular cancer in the non-cirrhotic liver. J. Visc. Surg. 2011, 148, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Chun, Y.S.; Poon, R.T.; Schwartz, M.E.; Yao, F.Y.; Marsh, J.W.; Bhoori, S.; Lee, S.-G. Liver transplantation for hepatocellular carcinoma. Ann. Surg. Oncol. 2008, 15, 1001–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Minicis, S.; Kisseleva, T.; Francis, H.; Baroni, G.S.; Benedetti, A.; Brenner, D.; Alvaro, D.; Alpini, G.; Marzioni, M. Liver carcinogenesis: Rodent models of hepatocarcinoma and cholangiocarcinoma. Dig. Liver Dis. 2013, 45, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Cheng, A.S.; Tsang, D.P.; Li, M.S.; Go, M.Y.; Cheung, Y.S.; Zhao, G.J.; Ng, S.S.; Lin, M.C.; Yu, J.; et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor-dependent hepatocarcinogenesis. J. Clin. Investig. 2011, 121, 3159–3175. [Google Scholar] [CrossRef] [Green Version]
- Koch, K.S.; Maeda, S.; He, G.; Karin, M.; Leffert, H.L. Targeted deletion of hepatocyte Ikkbeta confers growth advantages. Biochem. Biophys. Res. Commun. 2009, 380, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Hacker, H.J.; Mtiro, H.; Bannasch, P.; Vesselinovitch, S.D. Histochemical profile of mouse hepatocellular adenomas and carcinomas induced by a single dose of diethylnitrosamine. Cancer Res. 1991, 51, 1952–1958. [Google Scholar] [PubMed]
- Kamino, H.; Yamazaki, Y.; Saito, K.; Takizawa, D.; Kakizaki, S.; Moore, R.; Negishi, M. Nuclear receptor CAR-regulated expression of the FAM84A gene during the development of mouse liver tumors. Int. J. Oncol. 2011, 38, 1511–1520. [Google Scholar] [CrossRef]
- Lee, J.S.; Chu, I.S.; Mikaelyan, A.; Calvisi, D.F.; Heo, J.; Reddy, J.K.; Thorgeirsson, S.S. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat. Genet. 2004, 36, 1306–1311. [Google Scholar] [CrossRef]
- Wang, M.; Halasi, M.; Kabirov, K.; Banerjee, A.; Landolfi, J.; Lyubimov, A.V.; Gartel, A.L. Combination treatment with bortezomib and thiostrepton is effective against tumor formation in mouse models of DEN/PB-induced liver carcinogenesis. Cell Cycle 2012, 11, 3370–3372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, X.; Long, L.; Yang, C.; Lu, Y.; Cheng, M. Maotai ameliorates diethylnitrosamine-initiated hepatocellular carcinoma formation in mice. PLoS ONE 2014, 9, e93599. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Wang, J.; Yu, Y.; Zhen, C.; Gu, J.; Liu, W.; Wen, Y.; Chen, L.; Gao, Y.; Xia, Q.; et al. DJ-1 promotes development of DEN-induced hepatocellular carcinoma and proliferation of liver cancer cells. Oncotarget 2017, 8, 8499–8511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawanishi, S.; Hiraku, Y.; Murata, M.; Oikawa, S. The role of metals in site-specific DNA damage with reference to carcinogenesis. Free Radic. Biol. Med. 2002, 32, 822–832. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Verna, L.; Whysner, J.; Williams, G.M. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 1996, 71, 57–81. [Google Scholar] [CrossRef]
- Inokuchi, S.; Aoyama, T.; Miura, K.; Osterreicher, C.H.; Kodama, Y.; Miyai, K.; Akira, S.; Brenner, D.A.; Seki, E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 844–849. [Google Scholar] [CrossRef] [Green Version]
- Aydinlik, H.; Nguyen, T.D.; Moennikes, O.; Buchmann, A.; Schwarz, M. Selective pressure during tumor promotion by phenobarbital leads to clonal outgrowth of beta-catenin-mutated mouse liver tumors. Oncogene 2001, 20, 7812–7816. [Google Scholar] [CrossRef] [Green Version]
- Imaoka, S.; Osada, M.; Minamiyama, Y.; Yukimura, T.; Toyokuni, S.; Takemura, S.; Hiroi, T.; Funae, Y. Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Cancer Lett. 2004, 203, 117–125. [Google Scholar] [CrossRef]
- Loeppen, S.; Schneider, D.; Gaunitz, F.; Gebhardt, R.; Kurek, R.; Buchmann, A.; Schwarz, M. Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002, 62, 5685–5688. [Google Scholar] [PubMed]
- Watson, R.E.; Goodman, J.I. Effects of phenobarbital on DNA methylation in GC-rich regions of hepatic DNA from mice that exhibit different levels of susceptibility to liver tumorigenesis. Toxicol. Sci. 2002, 68, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awuah, P.K.; Rhieu, B.H.; Singh, S.; Misse, A.; Monga, S.P. beta-Catenin loss in hepatocytes promotes hepatocellular cancer after diethylnitrosamine and phenobarbital administration to mice. PLoS ONE 2012, 7, e39771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesselinovitch, S.D.; Rao, K.V.; Mihailovich, N. Neoplastic response of mouse tissues during perinatal age periods and its significance in chemical carcinogenesis. Natl. Cancer Inst. Monogr. 1979, 239–250. [Google Scholar] [PubMed]
- Farber, E.; Solt, D.; Cameron, R.; Laishes, B.; Ogawa, K.; Medline, A. Newer insights into the pathogenesis of liver cancer. Am. J. Pathol. 1977, 89, 477–482. [Google Scholar] [PubMed]
- Klinman, N.R.; Erslev, A.J. Cellular response to partial hepatectomy. Proc. Soc. Exp. Biol. Med. 1963, 112, 338–340. [Google Scholar] [CrossRef]
- Kimura, M.; Fujii, Y.; Yamamoto, R.; Yafune, A.; Hayashi, S.M.; Suzuki, K.; Shibutani, M. Involvement of multiple cell cycle aberrations in early preneoplastic liver cell lesions by tumor promotion with thioacetamide in a two-stage rat hepatocarcinogenesis model. Exp. Toxicol. Pathol. 2013, 65, 979–988. [Google Scholar] [CrossRef]
- Akhtar, T.; Sheikh, N. An overview of thioacetamide-induced hepatotoxicity. Toxin Rev. 2013, 32, 43–46. [Google Scholar] [CrossRef]
- Zaldivar, M.M.; Pauels, K.; von Hundelshausen, P.; Berres, M.L.; Schmitz, P.; Bornemann, J.; Kowalska, M.A.; Gassler, N.; Streetz, K.L.; Weiskirchen, R.; et al. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology 2010, 51, 1345–1353. [Google Scholar] [CrossRef]
- Mueller, K.; Sunami, Y.; Stuetzle, M.; Guldiken, N.; Kucukoglu, O.; Mueller, S.; Kulaksiz, H.; Schwarz, P.; Strnad, P. CHOP-mediated hepcidin suppression modulates hepatic iron load. J. Pathol. 2013, 231, 532–542. [Google Scholar] [CrossRef]
- Tuñón, M.J.; Alvarez, M.; Culebras, J.M.; González-Gallego, J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J. Gastroenterol. 2009, 15, 3086–3098. [Google Scholar] [CrossRef]
- Schnur, J.; Oláh, J.; Szepesi, A.; Nagy, P.; Thorgeirsson, S.S. Thioacetamide-induced hepatic fibrosis in transforming growth factor beta-1 transgenic mice. Eur. J. Gastroenterol. Hepatol. 2004, 16, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.C.; Hamesch, K.; Lunova, M.; Kim, Y.; Weiskirchen, R.; Strnad, P.; Friedman, S.L. Standard operating procedures in experimental liver research: Thioacetamide model in mice and rats. Lab. Anim. 2015, 49, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornek, M.; Raskopf, E.; Guetgemann, I.; Ocker, M.; Gerceker, S.; Gonzalez-Carmona, M.A.; Rabe, C.; Sauerbruch, T.; Schmitz, V. Combination of systemic thioacetamide (TAA) injections and ethanol feeding accelerates hepatic fibrosis in C3H/He mice and is associated with intrahepatic up regulation of MMP-2, VEGF and ICAM-1. J. Hepatol. 2006, 45, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lopategi, A.; Ge, X.; Lu, Y.; Kitamura, N.; Urtasun, R.; Leung, T.M.; Fiel, M.I.; Nieto, N. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut 2014, 63, 1805–1818. [Google Scholar] [CrossRef]
- Henderson, J.M.; Zhang, H.E.; Polak, N.; Gorrell, M.D. Hepatocellular carcinoma: Mouse models and the potential roles of proteases. Cancer Lett. 2017, 387, 106–113. [Google Scholar] [CrossRef]
- Sakurai, T.; Maeda, S.; Chang, L.; Karin, M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc. Natl. Acad. Sci. USA 2006, 103, 10544–10551. [Google Scholar] [CrossRef] [Green Version]
- El-Ashmawy, N.E.; El-Bahrawy, H.A.; Shamloula, M.M.; El-Feky, O.A. Biochemical/metabolic changes associated with hepatocellular carcinoma development in mice. Tumour Biol. 2014, 35, 5459–5466. [Google Scholar] [CrossRef]
- Hsu, S.H.; Wang, B.; Kutay, H.; Bid, H.; Shreve, J.; Zhang, X.; Costinean, S.; Bratasz, A.; Houghton, P.; Ghoshal, K. Hepatic loss of miR-122 predisposes mice to hepatobiliary cyst and hepatocellular carcinoma upon diethylnitrosamine exposure. Am. J. Pathol. 2013, 183, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Bialecki, E.S.; Di Bisceglie, A.M. Diagnosis of hepatocellular carcinoma. HPB 2005, 7, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Shalini, S.; Nikolic, A.; Wilson, C.H.; Puccini, J.; Sladojevic, N.; Finnie, J.; Dorstyn, L.; Kumar, S. Caspase-2 deficiency accelerates chemically induced liver cancer in mice. Cell Death Differ. 2016, 23, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Santos, N.P.; Pereira, I.C.; Pires, M.J.; Lopes, C.; Andrade, R.; Oliveira, M.M.; Colaco, A.; Peixoto, F.; Oliveira, P.A. Histology, bioenergetics and oxidative stress in mouse liver exposed to N-diethylnitrosamine. In Vivo 2012, 26, 921–929. [Google Scholar] [PubMed]
- Shirakami, Y.; Gottesman, M.E.; Blaner, W.S. Diethylnitrosamine-induced hepatocarcinogenesis is suppressed in lecithin:retinol acyltransferase-deficient mice primarily through retinoid actions immediately after carcinogen administration. Carcinogenesis 2012, 33, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teoh, N.C.; Dan, Y.Y.; Swisshelm, K.; Lehman, S.; Wright, J.H.; Haque, J.; Gu, Y.; Fausto, N. Defective DNA strand break repair causes chromosomal instability and accelerates liver carcinogenesis in mice. Hepatology 2008, 47, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Kim, J.Y.; Oh, S.P.; Kang, S.Y.; Kim, B.W.; Wang, H.J.; Song, K.Y.; Kim, H.C.; Lim, I.K. TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology 2008, 47, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Heindryckx, F.; Colle, I.; Van Vlierberghe, H. Experimental mouse models for hepatocellular carcinoma research. Int. J. Exp. Pathol. 2009, 90, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, R.; Nakamura, I.; Hu, C.; Chen, G.; Oseini, A.M.; Seven, E.S.; Miamen, A.G.; Moser, C.D.; Zhou, W.; van Kuppevelt, T.H.; et al. Activation of the transforming growth factor-beta/SMAD transcriptional pathway underlies a novel tumor-promoting role of sulfatase 1 in hepatocellular carcinoma. Hepatology 2015, 61, 1269–1283. [Google Scholar] [CrossRef] [Green Version]
- Najmi, A.K.; Pillai, K.K.; Pal, S.N.; Akhtar, M.; Aqil, M.; Sharma, M. Effect of l-ornithine l-aspartate against thioacetamide-induced hepatic damage in rats. Indian J. Pharmacol. 2010, 42, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low-protein fed rats. Phytother. Res. 2005, 19, 341–345. [Google Scholar] [CrossRef]
- Cervello, M.; Montalto, G. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 5113–5121. [Google Scholar] [CrossRef]
- Chen, H.; Cai, W.; Chu, E.S.H.; Tang, J.; Wong, C.C.; Wong, S.H.; Sun, W.; Liang, Q.; Fang, J.; Sun, Z.; et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene 2017, 36, 4415–4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, M.A.; Schöneweiß, M.M.; Sahi, D.; Bahlo, M.; Haugg, A.M.; Kasper, H.U.; Dienes, H.P.; Käferstein, H.; Breuhahn, K.; Schirmacher, P. Cyclooxygenase-2 inhibitors suppress the growth of human hepatocellular carcinoma implants in nude mice. Carcinogenesis 2004, 25, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a000141. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Kamata, H.; Luo, J.L.; Leffert, H.; Karin, M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005, 121, 977–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Thorgeirsson, S.S. Comparative and integrative functional genomics of HCC. Oncogene 2006, 25, 3801–3809. [Google Scholar] [CrossRef] [Green Version]


| Group | Wks of Treatment | Final Body Weight (g) | Absolute Liver Weight (g) | Relative Liver Weight (g) |
|---|---|---|---|---|
| Control | 0 | 34.3 ± 1.9 | 1.08 ± 0.13 | 3.2 ± 0.49 |
| DEN | 8 wks DEN | 21.9 ± 3.2 * | 1.68 ± 0.20 | 7.7 ± 2.08 * |
| DEN+TAA | 8 wks DEN + TAA 4 wks | 22.7 ± 3.1 * | 1.8 ± 0.12 | 8.2 ± 0.94 * |
| DEN+TAA | 8 wks DEN + TAA 8 wks | 22.1±0.6 * | 1.8 ± 0.15 | 8.2 ± 0.58 * |
| Group | Mice # with Tumor /Total # of Mice | Tumor No. | Tumor No./Animal |
|---|---|---|---|
| Control | 0/13 | 0 | 0 |
| DEN | 29/29 | 204 | 7.2 ± 3.42 |
| DEN + TAA 4 weeks | 13/13 | 106 | 8.81 ± 0.94 ns |
| DEN + TAA 8 weeks | 11/11 | 126 | 11.5 ± 2.76 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, A.; Pyao, Y.; Jung, Y.; Lee, J.I.; Lee, W.K. A Modified Protocol of Diethylnitrosamine Administration in Mice to Model Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 5461. https://doi.org/10.3390/ijms21155461
Memon A, Pyao Y, Jung Y, Lee JI, Lee WK. A Modified Protocol of Diethylnitrosamine Administration in Mice to Model Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2020; 21(15):5461. https://doi.org/10.3390/ijms21155461
Chicago/Turabian StyleMemon, Azra, Yuliya Pyao, Yerin Jung, Jung Il Lee, and Woon Kyu Lee. 2020. "A Modified Protocol of Diethylnitrosamine Administration in Mice to Model Hepatocellular Carcinoma" International Journal of Molecular Sciences 21, no. 15: 5461. https://doi.org/10.3390/ijms21155461





