PD-L1 Expression on Circulating Tumour Cells May Be Predictive of Response to Regorafenib in Patients Diagnosed with Chemorefractory Metastatic Colorectal Cancer
Abstract
1. Introduction
2. Results
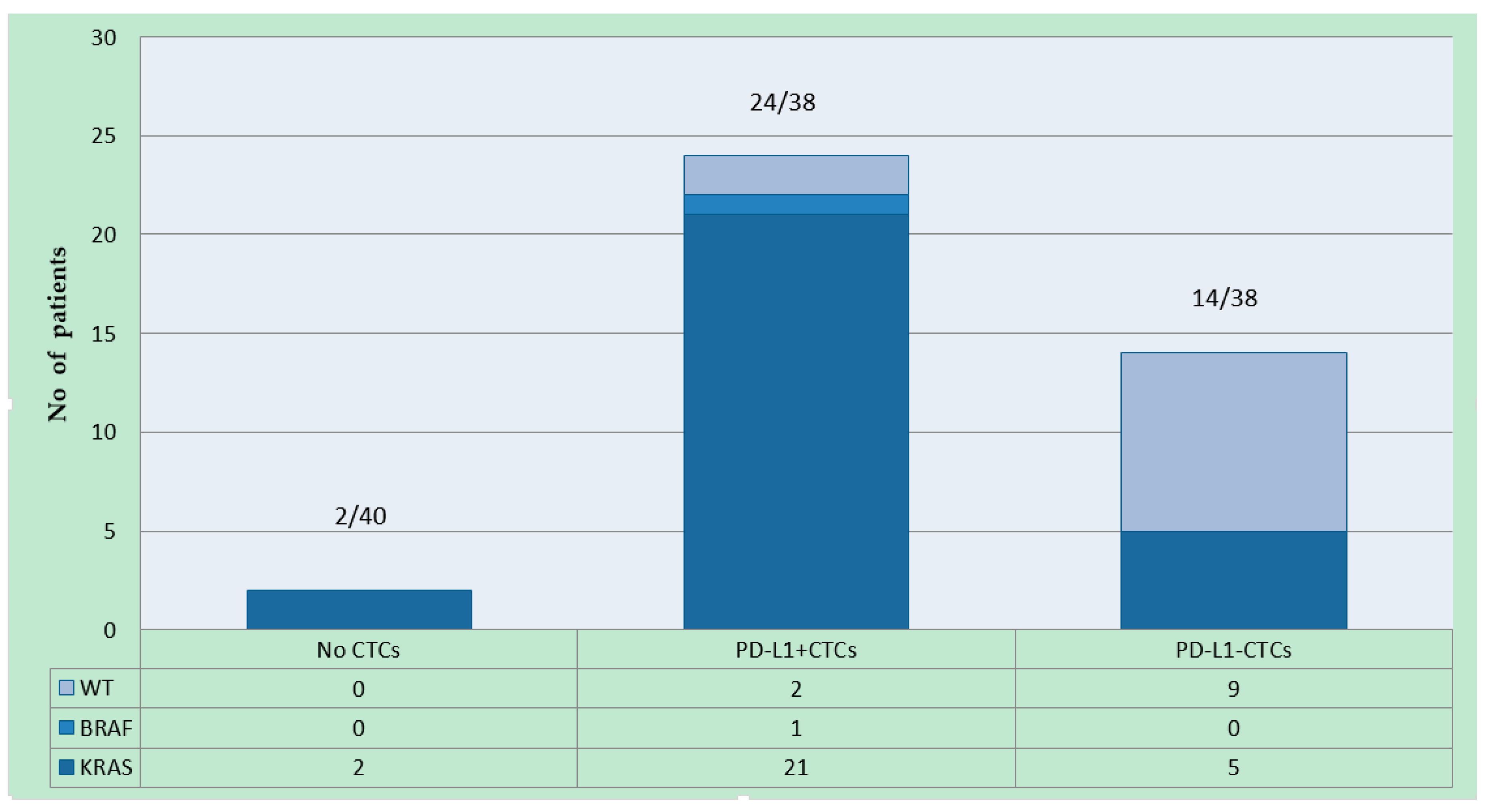
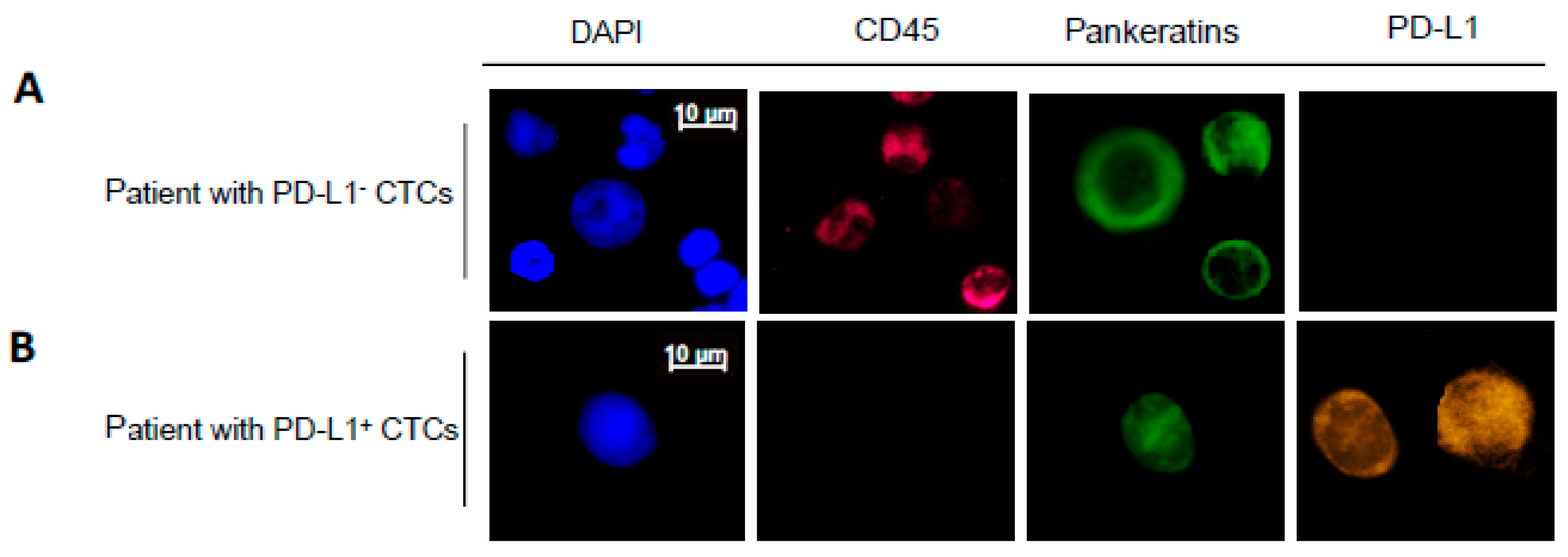
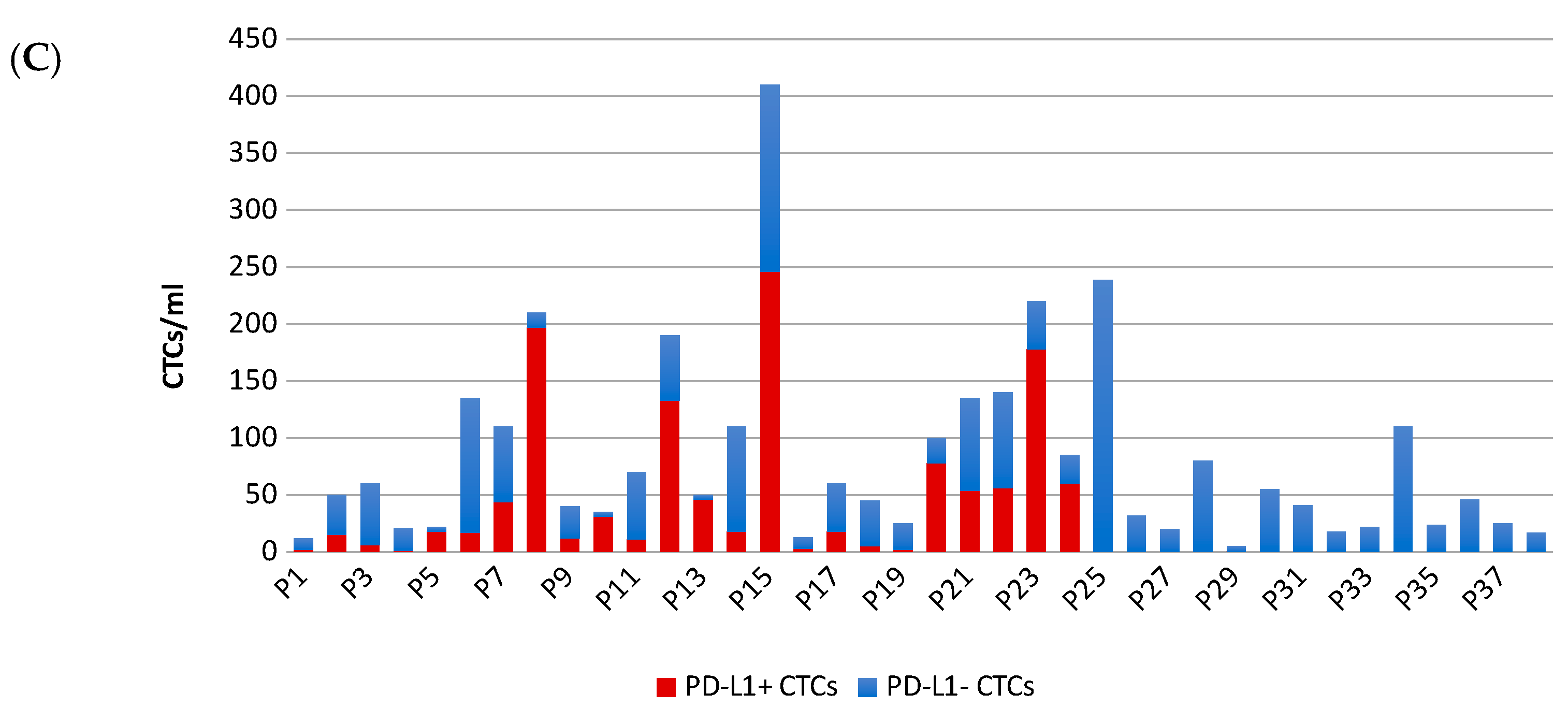
2.1. Patient Characteristics and PD-L1 Detection on CTCs
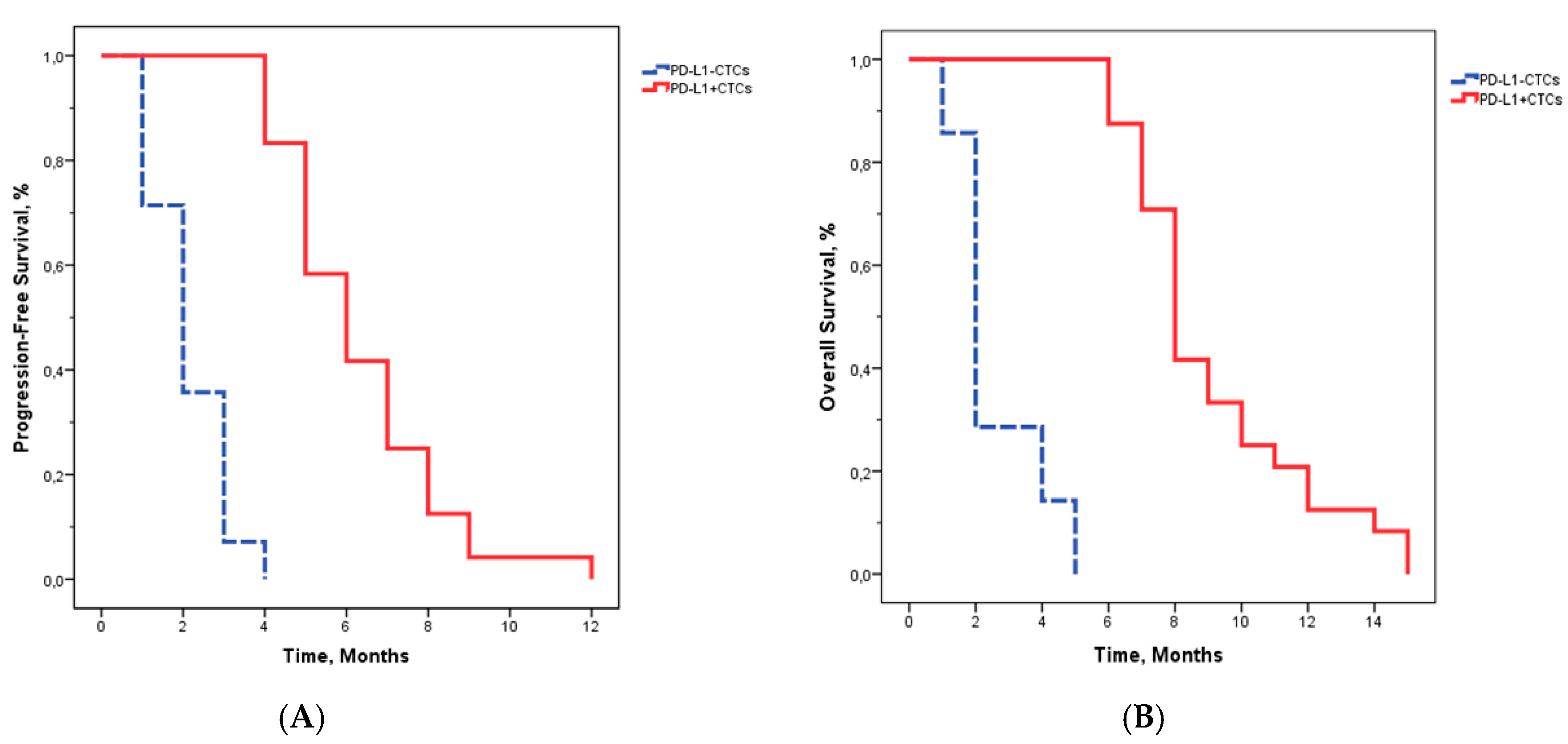
2.2. Patient Characteristics and PD-L1 Detection on CTCs
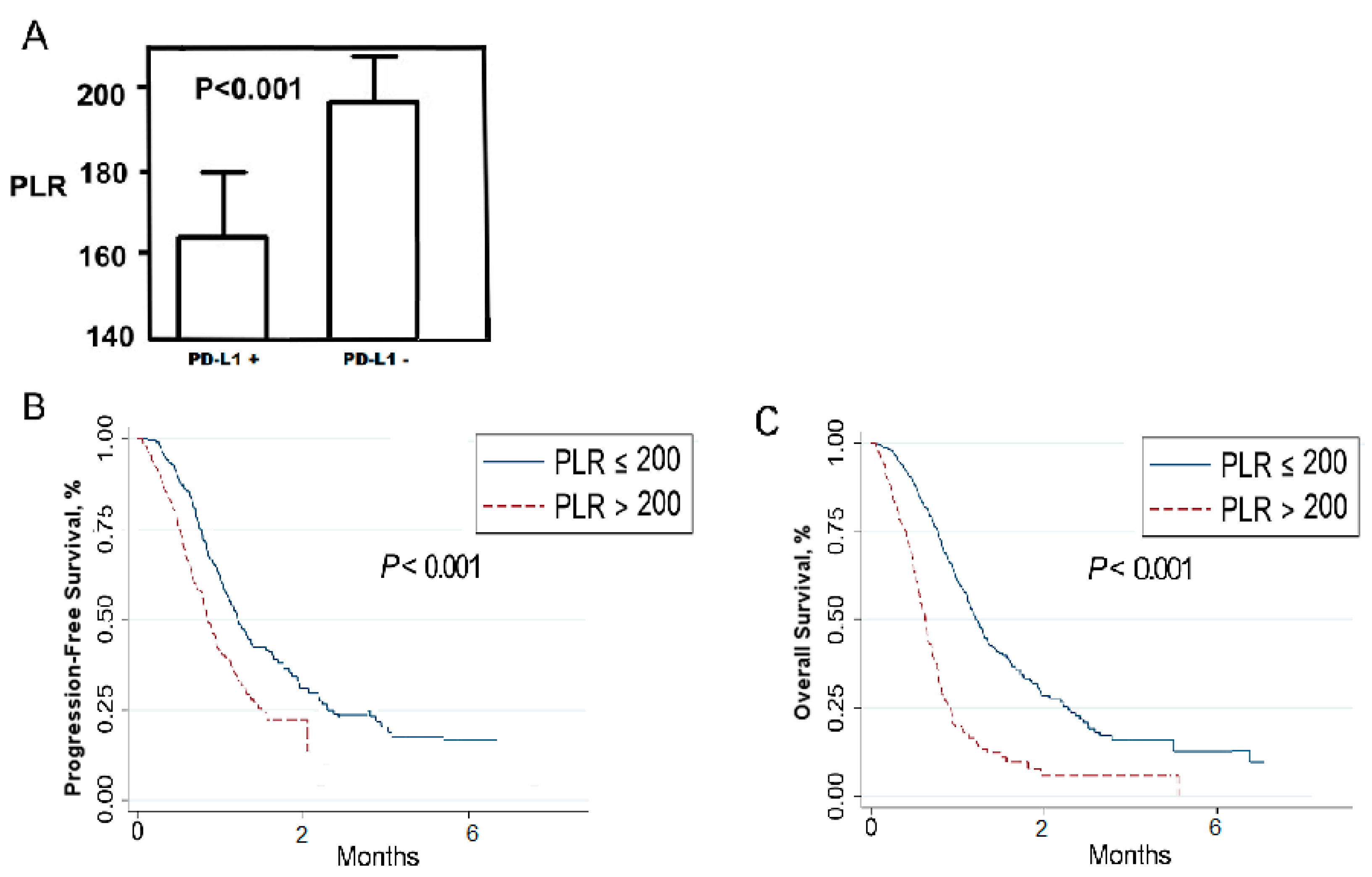
2.3. Relationship between Platelet-To-Lymphocyte Ratio (PLR) and Patients’ Outcomes
3. Discussion
4. Materials and Methods
4.1. Patients, Treatment and Blood Collection
4.2. Detection and Identification of CTCs
4.3. Treatment Response and Disease Progression Assessment
4.4. Measurements of Platelet to Lymphocyte Ratio (PLR)
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| mCRC | Metastatic Colorectal cancer |
| CTCs | Circulating Tumour Cells |
| PD-L1 | Programmed Death-Ligand 1 |
| PLR | Platelet-to-lymphocyte ratio |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| PFS | Progression-free survival |
| OS | Overall Survival |
| DAPI | 40,6-diamidino-2-phenylindole |
Appendix A
| Variable | No. of Patients (%) |
|---|---|
| Age (median = 65) | |
| <65 | 12 (30) |
| ≥65 | 28 (70) |
| Gender | |
| M | 25 (63) |
| F | 15 (37) |
| ECOG PS | |
| 0 | 29 (73) |
| 1–2 | 11 (27) |
| Primary tumour Location | |
| Colon | 39 (98) |
| Rectum | 1 (2) |
| KRAS status | |
| WT | 13 (35) |
| Mutated | 26 (65) |
| BRAF status | |
| WT | 39 (98) |
| V600E | 1 (2) |
| Metastatic sites | |
| Liver | 35 (88) |
| Lymph Nodes | 33 (83) |
| Lungs | 8 (20) |
| Others | 12 (30) |
| N° of metastatic sites | |
| 1 | 6 (15) |
| ≥2 | 34 (85) |
| PLR | |
| Mean (SD) | 212.5 (208.32) |
| <200 | 24 (60) |
| ≥200 | 16 (40) |
Appendix B
| Patients | Number of CTCs | % of PD-L1+CTCs |
|---|---|---|
| P1 | 12 | 20 |
| P2 | 50 | 30 |
| P3 | 60 | 10 |
| P4 | 21 | 5 |
| P5 | 22 | 80 |
| P6 | 135 | 50 |
| P7 | 110 | 40 |
| P8 | 210 | 94 |
| P9 | 40 | 81 |
| P10 | 35 | 88 |
| P11 | 70 | 16 |
| P12 | 190 | 70 |
| P13 | 50 | 91 |
| P14 | 110 | 16 |
| P15 | 410 | 60 |
| P16 | 13 | 21 |
| P17 | 60 | 30 |
| P18 | 45 | 12 |
| P19 | 25 | 6 |
| P20 | 100 | 78 |
| P21 | 135 | 40 |
| P22 | 140 | 40 |
| P23 | 220 | 81 |
| P24 | 85 | 70 |
| P25 | 70 | 0 |
| P26 | 70 | 0 |
| P27 | 10 | 0 |
| P28 | 10 | 0 |
| P29 | 20 | 0 |
| P30 | 12 | 0 |
| P31 | 41 | 0 |
| P32 | 18 | 0 |
| P33 | 22 | 0 |
| P34 | 110 | 0 |
| P35 | 24 | 0 |
| P36 | 46 | 0 |
| P37 | 25 | 0 |
| P38 | 17 | 0 |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, Q.; Lou, Y.; Yang, J.; Nie, G.; Chen, Q.; Chen, Y.; Zhang, J.; Wang, J.; Wei, T.; et al. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene 2018, 37, 6105–6118. [Google Scholar] [CrossRef]
- Segal, N.H.; Saltz, L.B. Evolving treatment of advanced colon cancer. Annu. Rev. Med. 2009, 60, 207–219. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schutz, G.; Thierauch, K.-H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Konati, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib plus nivolumab in patients with advanced gastric (GC) or colorectal cancer (CRC): An open-label, dose-finding, and dose-expansion phase 1b trial (REGONIVO, EPOC1603) [ASCO abstract 2522]. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lampignano, R.; Yang, L.; Neumann, M.H.D.; Franken, A.; Fehm, T.; Niederacher, D.; Neubauer, H. A novel workflow to enrich and isolate patient-matched epcam(high) and epcam(low/negative) ctcs enables the comparative characterization of the pik3ca status in metastatic breast cancer. Int. J. Mol. Sci. 2017, 18, 1885. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.N., Jr.; Santoro, I.L.; Tadokoro, H.; de Lima Lopes, G.; Filardi, B.A.; Oliveira, P.; Mountzios, G.; de Mello, R.A. The role of pd-l1 expression as a predictive biomarker in advanced non-small-cell lung cancer: A network meta-analysis. Immunotherapy 2016, 8, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, B.S.P.; Hanks, D.; Venpanusa, B.; et al. PD-L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef]
- Sunshine, J.; Nguyen, P.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Esandrio, J.; Xu, H.; Ogurtsova, A.; Bleich, K.B.; Cornish, T.C.; et al. PD-L1 expression in melanoma: A quantitative immunohistochemical antibody comparison. Clin. Cancer Res. 2017, 23, 4938–4944. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Brabletz, T.; Kang, Y.; Nieto, M.A.; Weinberg, R.A.; Stanger, B.Z. Upholding a role for EMT in pancreatic cancer metastasis. Nature 2017, 547, E7–E8. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Kenny, L.; Warkiani, M.E.; Nelson, C.; Punyadeera, C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer 2017, 17, 333. [Google Scholar] [CrossRef]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabieres, C. Frequent expression of pd-l1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Tada, H.; Takahashi, H.; Kuwabara-Yokobori, Y.; Ishii, H.; Ida, S.; Shino, M. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral. Oncol. 2019, 89, 34–39. [Google Scholar] [CrossRef]
- Satelli, A.; Batth, I.S.; Brownlee, Z.; Rojas, C.; Meng, Q.H.; Kopetz, S.; Li, S. Potential role of nuclear pd-l1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep. 2016, 6, 28910. [Google Scholar] [CrossRef]
- Anantharaman, A.; Friedlander, T.; Lu, D.; Krupa, R.; Premasekharan, G.; Hough, J.; Edwards, M.; Paz, R.; Lindquist, K.; Graf, R.; et al. Programmed death-ligand 1 (pd-l1) characterization of circulating tumor cells (ctcs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 2016, 16, 744. [Google Scholar] [CrossRef]
- Po, J.W.; Ma, Y.; Balakrishna, B.; Brungs, D.; Azimi, F.; de Souza, P.; Becker, T.M. Immunomagnetic isolation of circulating melanoma cells and detection of pd-l1 status. PLoS ONE 2019, 14, e0211866. [Google Scholar] [CrossRef]
- Dhar, M.; Wong, J.; Che, J.; Matsumoto, M.; Grogan, T.; Elasho, D.; Garon, E.B.; Goldman, J.W.; Sollier Christen, E.; Di Carlo, D.; et al. Evaluation of pd-l1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci. Rep. 2018, 8, 2592. [Google Scholar] [CrossRef] [PubMed]
- Diggs, L.P.; Hsueh, E.C. Utility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark. Res. 2017, 5, 12. [Google Scholar] [CrossRef]
- Kallergi, G.; Vetsika, E.K.; Aggouraki, D.; Lagoudaki, E.; Koutsopoulos, A.; Koinis, F.; Katsarlinos, P.; Trypaki, M.; Messaritakis, I.; Stournaras, C.; et al. Evaluation of pd-l1/pd-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z.; et al. Dynamic change of pd-l1 expression on circulating tumor cells in advanced solid tumor patients undergoing pd-1 blockade therapy. Oncoimmunology 2018, 7. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; Del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring pd-l1 positive circulating tumor cells in non-small cell lung cancer patients treated with the pd-1 inhibitor nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Raimondi, C.; Carpino, G.; Nicolazzo, C.; Gradilone, A.; Gianni, W.; Gelibter, A.; Gaudio, E.; Cortesi, E.; Gazzaniga, P. Pd-l1 and epithelial-mesenchymal transition in circulating tumor cells from non-small cell lung cancer patients: A molecular shield to evade immune system? Oncoimmunology 2017, 6, e1315488. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.M.; Xia, W.; Hsu, Y.H.; Chan, L.C.; Yu, W.H.; Cha, J.H.; Chen, C.T.; Liao, H.W.; Kuo, C.W.; Khoo, K.H.; et al. Stt3-dependent pd-l1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
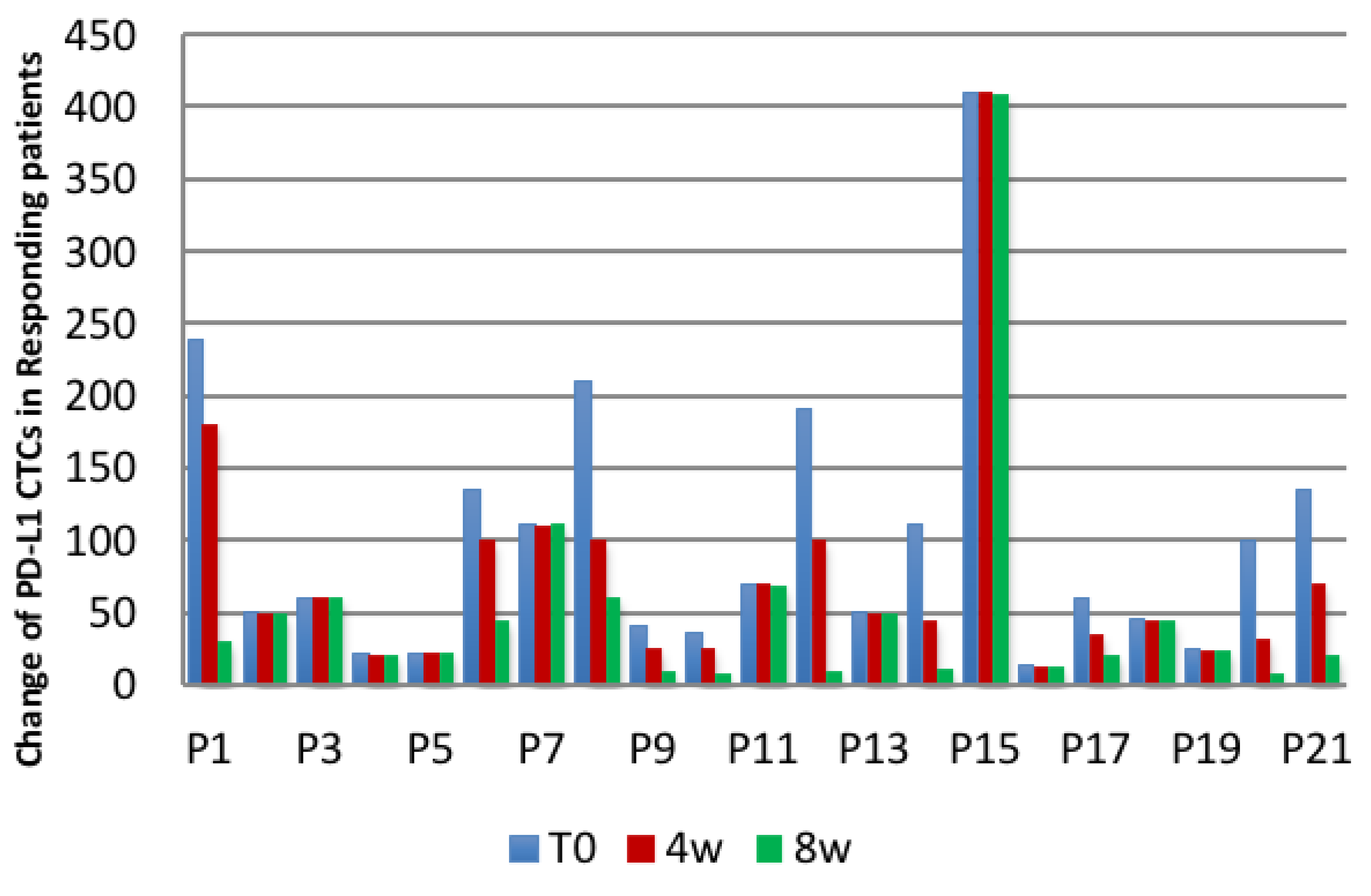
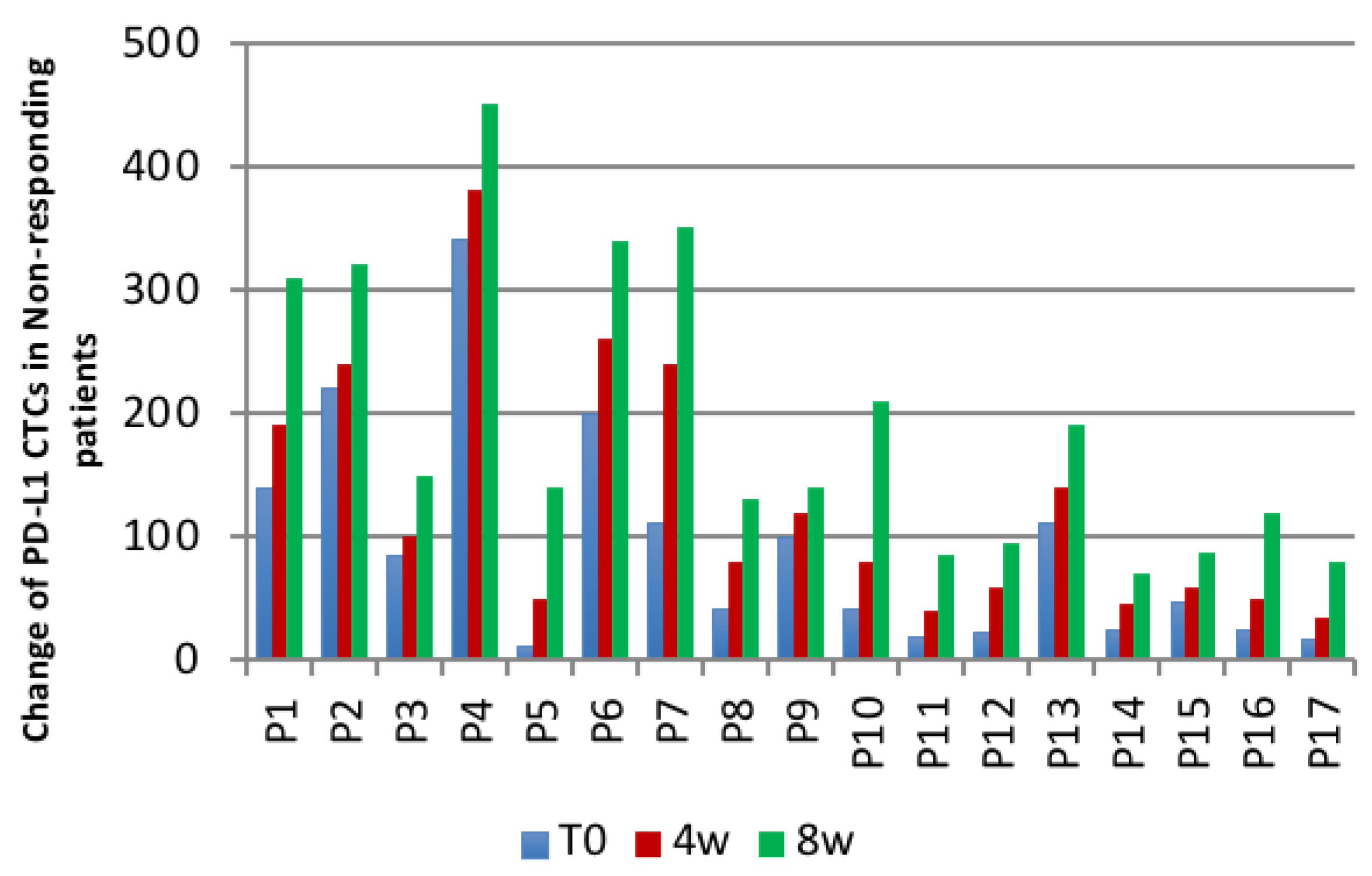
| Patients | Number of Patients with a | |
|---|---|---|
| Decrease/No Change of PD-L1 CTCs | Increase of PD-L1− CTCs | |
| Responding patients | 10 (decrease), 11 (no change) | 0 |
| Non-responding patients | 0 | 17 |
| Variable | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | ||
| All mCRC (n = 40) | |||||||
| PD-L1+ CTCs | Positive vs. Negative | 16.541 | 7.311–38.414 | <0.001 | 0.09 | 0.01–0.751 | 0.016 |
| ECOG PS | ≥1 vs. 0 | 1.444 | 0.511–3.121 | 0.481 | |||
| KRAS | Mutant vs. WT | 20.7 | 9.474–45.415 | <0.001 | 3.051 | 1.121–7.751 | 0.030 |
| BRAF | Mutant vs. WT | 0.399 | 0.210–0.851 | 0.004 | |||
| PLR | ≥200 vs. <200 | 0.21 | 0.041–0.815 | <0.001 | 0.345 | 0.131–0.845 | 0.018 |
| N sites metastases | ≥2 vs. 1 | 4.334 | 2.580–7.280 | <0.001 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimondi, L.; Raimondi, F.M.; Di Benedetto, L.; Cimino, G.; Spinelli, G.P. PD-L1 Expression on Circulating Tumour Cells May Be Predictive of Response to Regorafenib in Patients Diagnosed with Chemorefractory Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6907. https://doi.org/10.3390/ijms21186907
Raimondi L, Raimondi FM, Di Benedetto L, Cimino G, Spinelli GP. PD-L1 Expression on Circulating Tumour Cells May Be Predictive of Response to Regorafenib in Patients Diagnosed with Chemorefractory Metastatic Colorectal Cancer. International Journal of Molecular Sciences. 2020; 21(18):6907. https://doi.org/10.3390/ijms21186907
Chicago/Turabian StyleRaimondi, Lucrezia, Filippo Maria Raimondi, Laura Di Benedetto, Giuseppe Cimino, and Gian Paolo Spinelli. 2020. "PD-L1 Expression on Circulating Tumour Cells May Be Predictive of Response to Regorafenib in Patients Diagnosed with Chemorefractory Metastatic Colorectal Cancer" International Journal of Molecular Sciences 21, no. 18: 6907. https://doi.org/10.3390/ijms21186907
APA StyleRaimondi, L., Raimondi, F. M., Di Benedetto, L., Cimino, G., & Spinelli, G. P. (2020). PD-L1 Expression on Circulating Tumour Cells May Be Predictive of Response to Regorafenib in Patients Diagnosed with Chemorefractory Metastatic Colorectal Cancer. International Journal of Molecular Sciences, 21(18), 6907. https://doi.org/10.3390/ijms21186907





