Exosomes: Insights from Retinoblastoma and Other Eye Cancers
Abstract
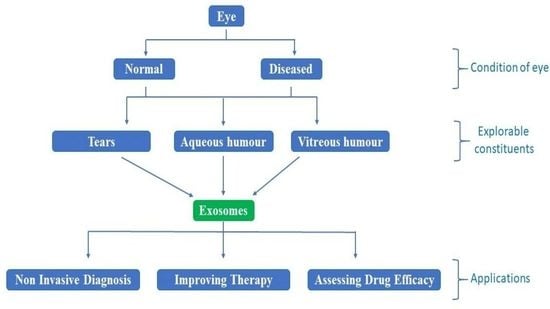
:1. Introduction: Exosomes
2. Exosomes of the Eye
2.1. Tear Fluid Exosomes
2.2. Retinal Exosomes
2.3. Corneal Exosomes
2.4. Exosomes of Aqueous Humor
2.5. Exosomes of Vitreous Humor
3. Eye Cancers
4. Retinoblastoma and Exosomes
5. miRNA and Their Targets in Retinoblastoma
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, M.; Sainte-Marie, J.; Philippot, J.R.; Bienvenue, A. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: Evidence precluding a role for “aminophospholipid translocase”. J. Cell Physiol. 1989, 140, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Exosomes: What do we love so much about them? Circ. Res. 2016, 119, 1280–1282. [Google Scholar] [CrossRef] [Green Version]
- Properzi, F.; Logozzi, M.; Fais, S. Exosomes: The future of biomarkers in medicine. Biomark. Med. 2013, 7, 769–778. [Google Scholar] [CrossRef]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [Green Version]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Hurley, J.H. ESCRTs are everywhere. EMBO J. 2015, 34, 2398–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell. 2011, 21, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef] [Green Version]
- Van den Boorn, J.G.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Nkosi, D.; Conlon, M.M.; York, S.B.; Liu, X.; Tremblay, D.C.; Meckes, D.G., Jr. CD63 regulates epstein-barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-κB signaling. J. Virol. 2017, 91, e02251-16. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef]
- Hoshino, D.; Kirkbride, K.C.; Costello, K.; Clark, E.S.; Sinha, S.; Grega-Larson, N.; Tyska, M.J.; Weaver, A.M. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013, 5, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Frängsmyr, L.; Baranov, V.; Nagaeva, O.; Stendahl, U.; Kjellberg, L.; Mincheva-Nilsson, L. Cytoplasmic microvesicular form of Fas ligand in human early placenta: Switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol. Hum. Reprod. 2005, 11, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Perez, V.L.; Caspi, R.R. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015, 36, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Stenqvist, A.C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 2013, 191, 5515–5523. [Google Scholar] [CrossRef] [Green Version]
- Kauma, S.W.; Huff, T.F.; Hayes, N.; Nilkaeo, A. Placental fas ligand expression is a mechanism for maternal immune tolerance to the fetus. J. Clin. Endocrinol. Metab. 1999, 84, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Klingeborn, M.; Dismuke, W.M.; Bowes Rickman, C.; Stamer, W.D. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res. 2017, 59, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.S.; Cho, J.H.; Kim, H.; Choi, E.J.; Rho, S.; Kim, J.; Kim, J.H.; Choi, D.S.; Kim, Y.K.; Hwang, D.; et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genom. 2009, 10, 556. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef] [Green Version]
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013, 32, 2747–2755. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Ragusa, M.; Barbagallo, C.; Statello, L.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Barbagallo, D.; et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol. Ther. 2015, 16, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, S.; Kawaguchi, H.; Mizumoto, S.; Kunihisa, T.; Baba, M.; Kitayama, Y.; Takeuchi, T.; Hoffman, R.M.; Tanino, H.; Sasaki, R. Oncogenic miRNAs identified in tear exosomes from metastatic breast cancer patients. Anticancer Res. 2020, 40, 3091–3096. [Google Scholar] [CrossRef]
- Cortes-Troncoso, J.; Jang, S.I.; Perez, P.; Hidalgo, J.; Ikeuchi, T.; Greenwell-Wild, T.; Warner, B.M.; Moutsopoulos, N.M.; Alevizos, I. T cell exosome-derived miR-142-3p impairs glandular cell function in Sjögren’s syndrome. JCI Insight 2020, 5, e133497. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Keino, H.; Horie, S.; Sugita, S. Immune privilege and eye-derived t-regulatory cells. J. Immunol. Res. 2018, 2018, 679197. [Google Scholar] [CrossRef]
- Biasutto, L.; Chiechi, A.; Couch, R.; Liotta, L.A.; Espina, V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013, 319, 2113–2123. [Google Scholar] [CrossRef] [Green Version]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J.M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef]
- Kang, G.Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.C.; Choi, S.; Yoon, S.; Kim, H.C.; Baek, J.H.; Park, H.S.; et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J. Proteome Res. 2014, 13, 581–595. [Google Scholar] [CrossRef]
- Lu, M.; Adamis, A.P. Molecular biology of choroidal neovascularization. Ophthalmol. Clin. N. Am. 2006, 19, 323–334. [Google Scholar]
- Wang, F.; Rendahl, K.G.; Manning, W.C.; Quiroz, D.; Coyne, M.; Miller, S.S. AAV-mediated expression of vascular endothelial growth factor induces choroidal neovascularization in rat. Investig. Ophthalmol. Vis. Sci. 2003, 44, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and autophagy: Coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [Green Version]
- Fader, C.M.; Sánchez, D.G.; Mestre, M.B.; Colombo, M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta 2009, 1793, 1901–1916. [Google Scholar] [CrossRef] [Green Version]
- Fader, C.M.; Colombo, M.I. Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy 2006, 2, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Kalani, A.; Tyagi, A.; Tyagi, N. Exosomes: Mediators of neurodegeneration, neuroprotection and therapeutics. Mol. Neurobiol. 2014, 49, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, K.B.; Fijalkowski, N.; Cano, M.; Handa, J.T. Oxidized low-density-lipoprotein-induced injury in retinal pigment epithelium alters expression of the membrane complement regulatory factors CD46 and CD59 through exosomal and apoptotic bleb release. Adv. Exp. Med. Biol. 2014, 801, 259–265. [Google Scholar]
- Ebrahimi, K.B.; Fijalkowski, N.; Cano, M.; Handa, J.T. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J. Pathol. 2013, 229, 729–742. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A.; Hussein, H.; Abd El-Baky, N. Molecular underpinnings of corneal angiogenesis: Advances over the past decade. Int. J. Ophthalmol. 2016, 9, 768–779. [Google Scholar]
- Clements, J.L.; Dana, R. Inflammatory corneal neovascularization: Etiopathogenesis. Semin. Ophthalmol. 2011, 26, 235–245. [Google Scholar] [CrossRef]
- Han, K.Y.; Dugas-Ford, J.; Seiki, M.; Chang, J.H.; Azar, D.T. Evidence for the involvement of MMP14 in MMP2 processing and recruitment in exosomes of corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5323–5329. [Google Scholar] [CrossRef] [Green Version]
- Han, K.Y.; Tran, J.A.; Chang, J.H.; Azar, D.T.; Zieske, J.D. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, J.; Paster, J.; Benichou, G. Allorecognition by T lymphocytes and allograft rejection. Front. Immunol. 2016, 7, 582. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Nolasco, B.; Wang, M.; Prunevieille, A.; Benichou, G. Emerging role of exosomes in allorecognition and allograft rejection. Curr. Opin. Organ. Transpl. 2018, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Rocchetta, F.; Longaretti, L.; Faravelli, S.; Todeschini, M.; Cassis, L.; Pezzuto, F.; Tomasoni, S.; Azzollini, N.; Mister, M.; et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci. Rep. 2017, 7, 11518. [Google Scholar] [CrossRef] [Green Version]
- Dismuke, W.M.; Challa, P.; Navarro, I.; Stamer, W.D.; Liu, Y. Human aqueous humor exosomes. Exp Eye Res. 2015, 132, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer Treat. Rev. 2012, 38, 549–553. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Andreoli, M.T.; Mieler, W.F.; Leiderman, Y.I. Epidemiological trends in uveal melanoma. Br. J. Ophthalmol. 2015, 99, 1550–1553. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Arepalli, S.; Atalay, H.T.; Manjandavida, F.P.; Pieretti, G.; Shields, J.A. Uveal melanoma in children and teenagers. Saudi. J. Ophthalmol. 2013, 27, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.D.; Schoenfield, L.A.; Bastian, B.C.; Aziz, H.A.; Marino, M.J.; Biscotti, C.V. Congenital uveal melanoma? Surv. Ophthalmol. 2016, 61, 59–64. [Google Scholar] [CrossRef]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E. EUROCARE working group. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.N.; Yu, G.P.; McCormick, S.A.; Schneider, S.; Finger, P.T. Population-based incidence of uveal melanoma in various races and ethnic groups. Am. J. Ophthalmol. 2005, 140, 612–617. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Cohen, M.N.; Shields, P.W.; Furuta, M.; Shields, J.A. Prognosis of uveal melanoma based on race in 8100 patients: The 2015 doyne lecture. Eye 2015, 29, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The national cancer data base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American college of surgeons commission on cancer and the American cancer society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Damato, B. Treatment of primary intraocular melanoma. Expert Rev. Anticancer Ther. 2006, 6, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Materin, M.; Gershenbaum, E.; Singh, A.D.; Smith, A. Iris melanoma: Risk factors for metastasis in 169 consecutive patients. Ophthalmology 2001, 108, 172–178. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Landreville, S.; Agapova, O.A.; Harbour, J.W. Emerging insights into the molecular pathogenesis of uveal melanoma. Future Oncol. 2008, 4, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Triozzi, P.L.; Eng, C.; Singh, A.D. Targeted therapy for uveal melanoma. Cancer Treat. Rev. 2008, 34, 247–258. [Google Scholar] [CrossRef]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Eldh, M.; Olofsson Bagge, R.; Lässer, C.; Svanvik, J.; Sjöstrand, M.; Mattsson, J.; Lindnér, P.; Choi, D.S.; Gho, Y.S.; Lötvall, J. MicroRNA in exosomes isolated directly from the liver circulation in patients with metastatic uveal melanoma. BMC Cancer 2014, 14, 962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bande Rodríguez, M.F.; Fernandez Marta, B.; Lago Baameiro, N.; Santiago-Varela, M.; Silva-Rodríguez, P.; Blanco-Teijeiro, M.J.; Pardo Perez, M.; Piñeiro Ces, A. Blood biomarkers of uveal melanoma: Current perspectives. Clin. Ophthalmol. 2014, 14, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Angi, M.; Kalirai, H.; Prendergast, S.; Simpson, D.; Hammond, D.E.; Madigan, M.C.; Beynon, R.J.; Coupland, S.E. In-depth proteomic profiling of the uveal melanoma secretome. Oncotarget 2016, 7, 49623–49635. [Google Scholar] [CrossRef] [Green Version]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [Green Version]
- Zieske, J.D.; Hutcheon, A.; Guo, X. Extracellular vesicles and cell-cell communication in the cornea. Anat. Rec. 2020, 303, 1727–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [Green Version]
- Hou, A.; Law, K.P.; Tin, M.Q.; Lim, Y.P.; Tong, L. In vitro secretomics study of pterygium-derived fibroblasts by iTRAQ-based quantitative proteomics strategy. Exp. Eye Res. 2016, 153, 14–22. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L. Pediatric ocular and periocular tumors. Pediatr. Ann. 2001, 30, 491–501. [Google Scholar] [CrossRef]
- McNamara, R.P.; Chugh, P.E.; Bailey, A.; Costantini, L.M.; Ma, Z.; Bigi, R.; Cheves, A.; Eason, A.B.; Landis, J.T.; Host, K.M.; et al. Extracellular vesicles from Kaposi Sarcoma-associated herpesvirus lymphoma induce long-term endothelial cell reprogramming. PLoS Pathog. 2019, 15, e1007536. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Feng, Z.; Yuan, G.; Emerson, C.C.; Stewart, P.L.; Ye, F.; Jin, G. Human immunodeficiency virus-associated exosomes promote kaposi’s sarcoma-associated herpesvirus infection via the epidermal growth factor receptor. J. Virol. 2020, 94, e01782-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakulinen, J.; Sankkila, L.; Sugiyama, N.; Lehti, K.; Keski-Oja, J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J. Cell Biochem. 2008, 105, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Sakwe, A.M.; Koumangoye, R.; Guillory, B.; Ochieng, J. Annexin A6 contributes to the invasiveness of breast carcinoma cells by influencing the organization and localization of functional focal adhesions. Exp. Cell Res. 2011, 317, 823–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepp, M.A.; Pal-Ghosh, S.; Tadvalkar, G.; Pajoohesh-Ganji, A. Syndecan-1 and its expanding list of contacts. Adv. Wound Care 2015, 4, 235–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.; Honavar, S.G. Retinoblastoma. Indian J. Pediatr. 2017, 84, 937–944. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Orbach, D.B.; VanderVeen, D. Retinoblastoma. Pediatr. Clin. N. Am. 2015, 62, 201–223. [Google Scholar] [CrossRef]
- Ortiz, M.V.; Dunkel, I.J. Retinoblastoma. J. Child. Neurol. 2016, 31, 227–236. [Google Scholar] [CrossRef]
- Xu, X.L.; Singh, H.P.; Wang, L.; Qi, D.L.; Poulos, B.K.; Abramson, D.H.; Jhanwar, S.C.; Cobrinik, D. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature 2014, 514, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, P.R.; Grossniklaus, H.E. The biology of retinoblastoma. Prog. Mol. Biol. Transl. Sci. 2015, 134, 503–516. [Google Scholar]
- Soliman, S.E.; Racher, H.; Zhang, C.; MacDonald, H.; Gallie, B.L. Genetics and molecular diagnostics in retinoblastoma-an update. Asia Pac. J. Ophthalmol. 2017, 6, 197–207. [Google Scholar]
- Munier, F.L. Classification and management of seeds in retinoblastoma. Ellsworth lecture Ghent August 24th 2013. Ophthalmic Genet. 2014, 35, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Colletti, M.; Petretto, A.; Galardi, A.; Di Paolo, V.; Tomao, L.; Lavarello, C.; Inglese, E.; Bruschi, M.; Lopez, A.A.; Pascucci, L.; et al. Proteomic analysis of neuroblastoma-derived exosomes: New insights into a metastatic signature. Proteomics 2017, 17, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Colletti, M.; Paolini, A.; Galardi, A.; Di Paolo, V.; Pascucci, L.; Russo, I.; De Angelis, B.; Peinado, H.; De Vito, R.; Milano, G.M.; et al. Expression profiles of exosomal miRNAs isolated from plasma of patients with desmoplastic small round cell tumor. Epigenomics 2019, 11, 489–500. [Google Scholar] [CrossRef]
- Danda, R.; Ganapathy, K.; Sathe, G.; Madugundu, A.K.; Ramachandran, S.; Krishnan, U.M.; Khetan, V.; Rishi, P.; Keshava Prasad, T.S.; Pandey, A.; et al. Proteomic profiling of retinoblastoma by high resolution mass spectrometry. Clin. Proteom. 2016, 13, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrie, C.H. MicroRNA expression in lymphoid malignancies: New hope for diagnosis and therapy? J. Cell Mol. Med. 2008, 12, 1432–1444. [Google Scholar] [CrossRef]
- Resnick, K.E.; Alder, H.; Hagan, J.P.; Richardson, D.L.; Croce, C.M.; Cohn, D.E. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 2009, 112, 55–59. [Google Scholar] [CrossRef]
- Lan, F.; Qing, Q.; Pan, Q.; Hu, M.; Yu, H.; Yue, X. Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell Oncol. 2018, 41, 25–33. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, Y.; Liang, C.; Cheng, M.; Jin, C.; Duan, Q.; Xu, D.; Yang, L.; Zhang, X.; Ren, B.; et al. Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer. J. Cell Biochem. 2018, 119, 4113–4119. [Google Scholar] [CrossRef]
- Jiao, C.; Jiao, X.; Zhu, A.; Ge, J.; Xu, X. Exosomal miR-34s panel as potential novel diagnostic and prognostic biomarker in patients with hepatoblastoma. J. Pediatr. Surg. 2017, 52, 618–624. [Google Scholar] [CrossRef]
- Yuwen, D.; Ma, Y.; Wang, D.; Gao, J.; Li, X.; Xue, W.; Fan, M.; Xu, Q.; Shen, Y.; Shu, Y. Prognostic role of circulating exosomal miR-425-3p for the response of NSCLC to platinum-based chemotherapy. Cancer Epidemiol. Biomark. Prev. 2019, 28, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Liu, X.; Pan, B.; Hu, X.; Zhu, Y.; Su, Y.; Guo, Z.; Zhang, G.; Xu, M.; Xu, X.; et al. Serum exosomal miR-122 as a potential diagnostic and prognostic biomarker of colorectal cancer with liver metastasis. J. Cancer 2020, 11, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.B.; Zhang, H.; Cai, T.T.; Liu, Y.N.; Ni, J.J.; He, J.; Peng, J.Y.; Chen, Q.Y.; Mo, H.Y.; Jun-Cui; et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016, 240, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Yang, J.; Lin, J.; Yao, N.; Zhu, Y.; Zheng, J.; Xu, J.; Cheng, J.Q.; Lin, J.Y.; Ma, X. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Childs Nerv. Syst. 2009, 25, 13–20. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Park, W.Y.; Kim, K.W.; Yu, Y.S.; Kim, J.H. Differential profiles of microRNAs in retinoblastoma cell lines of different proliferation and adherence patterns. J. Pediatr. Hematol. Oncol. 2011, 33, 529–533. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Zhu, D.; Zhou, J. microRNA profiling in the aqueous humor of highly myopic eyes using next generation sequencing. Exp. Eye Res. 2020, 195, 108034. [Google Scholar] [CrossRef]
- Ragusa, M.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Di Pietro, C.; Purrello, M.; Reibaldi, M. MicroRNAs in vitreus humor from patients with ocular diseases. Mol. Vis. 2013, 19, 430–440. [Google Scholar]
- Liu, N.; Sun, Q.; Chen, J.; Li, J.; Zeng, Y.; Zhai, S.; Li, P.; Wang, B.; Wang, X. MicroRNA-9 suppresses uveal melanoma cell migration and invasion through the NF-κB1 pathway. Oncol. Rep. 2012, 28, 961–968. [Google Scholar]
- Takayama, K.; Kaneko, H.; Hwang, S.J.; Ye, F.; Higuchi, A.; Tsunekawa, T.; Matsuura, T.; Iwase, T.; Asami, T.; Ito, Y.; et al. Increased ocular levels of MicroRNA-148a in cases of retinal detachment promote epithelial-mesenchymal transition. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2699–2705. [Google Scholar] [CrossRef]
- Tsunekawa, T.; Kaneko, H.; Takayama, K.; Hwang, S.J.; Oishi, A.; Nagasaka, Y.; Ye, F.; Iwase, T.; Nonobe, N.; Ueno, S.; et al. Correlation between miR-148 expression in vitreous and severity of rhegmatogenous retinal detachment. Biomed. Res. Int. 2017, 2017, 3427319. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, H.; Mangani, A.S.; Phoebe Moses, G.L.; Mani, S.P.; Parameswaran, S.; Khetan, V.; Ganesan, S.; Krishnakumar, S. Serum exosomal miRNA as biomarkers for retinoblastoma. Exp. Eye Res. 2020, 108184. [Google Scholar] [CrossRef] [PubMed]
- Beta, M.; Venkatesan, N.; Vasudevan, M.; Vetrivel, U.; Khetan, V.; Krishnakumar, S. Identification and insilico analysis of retinoblastoma serum microRNA profile and gene targets towards prediction of novel serum biomarkers. Bioinform. Biol. Insights. 2013, 7, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Galardi, A.; Colletti, M.; Lavarello, C.; Di Paolo, V.; Mascio, P.; Russo, I.; Cozza, R.; Romanzo, A.; Valente, P.; De Vito, R.; et al. Proteomic profiling of retinoblastoma-derived exosomes reveals potential biomarkers of vitreous seeding. Cancers 2020, 12, 1555. [Google Scholar] [CrossRef]
- Peng, D.; Dong, J.; Zhao, Y.; Peng, X.; Tang, J.; Chen, X.; Wang, L.; Hu, D.N.; Reinach, P.S.; Qu, J.; et al. miR-142-3p suppresses uveal melanoma by targeting CDC25C, TGFβR1, GNAQ, WASL, and RAC1. Cancer Manag. Res. 2019, 11, 4729–4742. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Fu, Y.; Zhang, L.; Lu, X.; Li, Q. miR106b regulates retinoblastoma Y79 cells through Runx3. Oncol. Rep. 2017, 38, 3039–3043. [Google Scholar] [CrossRef]
- Tang, T.; Wong, H.K.; Gu, W.; Yu, M.Y.; To, K.F.; Wang, C.C.; Wong, Y.F.; Cheung, T.H.; Chung, T.K.; Choy, K.W. MicroRNA-182 plays an onco-miRNA role in cervical cancer. Gynecol. Oncol. 2013, 129, 199–208. [Google Scholar] [CrossRef]
- Kouri, F.M.; Hurley, L.A.; Daniel, W.L.; Day, E.S.; Hua, Y.; Hao, L.; Peng, C.Y.; Merkel, T.J.; Queisser, M.A.; Ritner, C.; et al. miR-182 integrates apoptosis, growth, and differentiation programs in glioblastoma. Genes Dev. 2015, 29, 732–745. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Amatya, V.J.; Kushitani, K.; Kai, Y.; Kambara, T.; Takeshima, Y. miR-182 and miR-183 promote cell proliferation and invasion by targeting FOXO1 in mesothelioma. Front. Oncol. 2018, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.G.; Shi, Y.; Hong, D.F.; Song, M.; Huang, D.; Wang, C.Y.; Zhao, G. MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway. Sci. Rep. 2015, 5, 8087. [Google Scholar] [CrossRef] [Green Version]
- Haflidadóttir, B.S.; Bergsteinsdóttir, K.; Praetorius, C.; Steingrímsson, E. miR-148 regulates Mitf in melanoma cells. PLoS ONE 2010, 5, e11574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Mao, H.L.; Zhao, X.R.; Li, Y.; Liu, P.S. MiR-29c-3p, a target miRNA of LINC01296, accelerates tumor malignancy: Therapeutic potential of a LINC01296/miR-29c-3p axis in ovarian cancer. J. Ovarian. Res. 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Lv, H.; Zhang, B.; Xu, F.; Zhu, H.; Chen, B.; Zhu, C.; Shen, J. miR-30b-5p acts as a tumor suppressor microRNA in esophageal squamous cell carcinoma. J. Thorac. Dis. 2019, 11, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Macedo, T.; Silva-Oliveira, R.J.; Silva, V.A.O.; Vidal, D.O.; Evangelista, A.F.; Marques, M.M.C. Overexpression of mir-183 and mir-494 promotes proliferation and migration in human breast cancer cell lines. Oncol. Lett. 2017, 14, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, L.; Li, Y.; Feng, G.H.; Teng, F.; Li, W.; Zhou, Q. MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol. Cancer 2018, 17, 1. [Google Scholar] [CrossRef]
- Zhan, M.N.; Yu, X.T.; Tang, J.; Zhou, C.X.; Wang, C.L.; Yin, Q.Q.; Gong, X.F.; He, M.; He, J.R.; Chen, G.Q.; et al. MicroRNA-494 inhibits breast cancer progression by directly targeting PAK1. Cell Death Dis. 2017, 8, e2529. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, T.; Tanaka, K.; Kawano, M.; Itonaga, I.; Tsumura, H. Tumor-suppressive microRNA-let-7a inhibits cell proliferation via targeting of E2F2 in osteosarcoma cells. Int. J. Oncol. 2015, 46, 1543–1550. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.; Gapihan, G.; Castro-Vega, L.J.; Acevedo, A.; Wang, L.; Li, Z.W.; El Bouchtaoui, M.; Di Benedetto, M.; Ratajczak, P.; Feugeas, J.P.; et al. Primary mediastinal large B-cell lymphoma: Transcriptional regulation by miR-92a through FOXP1 targeting. Oncotarget 2017, 8, 16243–16258. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, S.; Wu, Y.; Fang, X.; Wang, Y.; Song, Y.; Han, T. MicroRNA-216a inhibits the metastasis of gastric cancer cells by targeting the JAK2/STAT3-mediated EMT process. Oncotarget 2017, 8, 88870–88881. [Google Scholar] [CrossRef] [Green Version]
- Ben-Hamo, R.; Zilberberg, A.; Cohen, H.; Efroni, S. hsa-miR-9 controls the mobility behavior of glioblastoma cells via regulation of MAPK14 signaling elements. Oncotarget 2016, 7, 23170–23181. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.R.; Lee, S.T.; Kim, S.L.; Liu, Y.C.; Lee, M.R.; Shin, J.H.; Seo, S.Y.; Kim, S.H.; Kim, I.H.; Lee, S.O.; et al. MicroRNA-9 suppresses cell migration and invasion through downregulation of TM4SF1 in colorectal cancer. Int. J. Oncol. 2016, 48, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Lv, X.; Su, L.; Li, J.; Yu, Y.; Gu, Q.; Yan, M.; Zhu, Z.; Liu, B. MiR-148a functions as a tumor suppressor by targeting CCK-BR via inactivating STAT3 and Akt in human gastric cancer. PLoS ONE 2016, 11, e0158961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Liu, L. microRNA-148a inhibits hepatocellular carcinoma cell invasion by targeting sphingosine-1-phosphate receptor 1. Exp. Ther. Med. 2015, 9, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Floyd, D.H.; Zhang, Y.; Dey, B.K.; Kefas, B.; Breit, H.; Marks, K.; Dutta, A.; Herold-Mende, C.; Synowitz, M.; Glass, R.; et al. Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS ONE 2014, 9, e96239. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Tao, L.P.; Yao, S.C.; Huang, Q.K.; Chen, Z.F.; Sun, Y.J.; Jin, S.Q. MicroRNA-582-5p suppressed gastric cancer cell proliferation via targeting AKT3. Eur. Rev. Med. Pharm. Sci. 2017, 21, 5112–5120. [Google Scholar]
- Shen, H.; Lu, S.; Dong, L.; Xue, Y.; Yao, C.; Tong, C.; Wang, C.; Shu, X. hsa-miR-320d and hsa-miR-582, miRNA biomarkers of aortic dissection, regulate apoptosis of vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2018, 71, 275–282. [Google Scholar] [CrossRef]
- Yu, S.J.; Yang, L.; Hong, Q.; Kuang, X.Y.; Di, G.H.; Shao, Z.M. MicroRNA-200a confers chemoresistance by antagonizing TP53INP1 and YAP1 in human breast cancer. BMC Cancer 2018, 18, 74. [Google Scholar] [CrossRef]
- Su, J.; Zhang, A.; Shi, Z.; Ma, F.; Pu, P.; Wang, T.; Zhang, J.; Kang, C.; Zhang, Q. MicroRNA-200a suppresses the Wnt/β-catenin signaling pathway by interacting with β-catenin. Int. J. Oncol. 2012, 40, 1162–1170. [Google Scholar]
- Manavalan, T.T.; Teng, Y.; Litchfield, L.M.; Muluhngwi, P.; Al-Rayyan, N.; Klinge, C.M. Reduced expression of miR-200 family members contributes to antiestrogen resistance in LY2 human breast cancer cells. PLoS ONE 2013, 8, e62334. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; He, H.; Xie, Y.; Zhao, L.; Zhao, S.; Wan, X.; Yang, W.; Mo, Z. miR-125a-3p and miR-483-5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Sci. Rep. 2015, 5, 11909. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Yang, W.; Ma, J.; Zhang, H.; Li, Z.; Zhang, L.; Liu, J.; Han, Z.; Wang, H.; Hong, L. Role of miR-483 in digestive tract cancers: From basic research to clinical value. J. Cancer 2018, 9, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.A.; Zhang, Y.; Zheng, Z.; Li, K.; Wu, X.H.; Du, Q.G.; Ye, X.; Wang, L.; Zhu, L. MicroRNA-216b reduces growth, migration and invasion of pancreatic ductal adenocarcinoma cells by directly targeting ρ-associated coiled-coil containing protein kinase 1. Oncol. Lett. 2018, 15, 6745–6751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Liao, B.; Deng, Y.; Su, C.; Tuo, J.; Liu, J.; Yao, S.; Xu, L. MiR-216b inhibits cell proliferation by targeting FOXM1 in cervical cancer cells and is associated with better prognosis. BMC Cancer 2017, 17, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fort, R.S.; Mathó, C.; Oliveira-Rizzo, C.; Garat, B.; Sotelo-Silveira, J.R.; Duhagon, M.A. An integrated view of the role of miR-130b/301b miRNA cluster in prostate cancer. Exp. Hematol. Oncol. 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Huang, C.H.; Chou, W.G.; Juang, J.L. Abi enhances Abl-mediated CDC2 phosphorylation and inactivation. J. Biomed. Sci. 2004, 11, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Pendergast, A.M. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995, 9, 2569–2582. [Google Scholar] [CrossRef]
- Su, Z.; Kishida, S.; Tsubota, S.; Sakamoto, K.; Cao, D.; Kiyonari, S.; Ohira, M.; Kamijo, T.; Narita, A.; Xu, Y.; et al. Neurocan, an extracellular chondroitin sulfate proteoglycan, stimulates neuroblastoma cells to promote malignant phenotypes. Oncotarget 2017, 8, 106296–106310. [Google Scholar] [CrossRef]
- Gharbi-Ayachi, A.; Labbé, J.C.; Burgess, A.; Vigneron, S.; Strub, J.M.; Brioudes, E.; Van-Dorsselaer, A.; Castro, A.; Lorca, T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010, 330, 1673–1677. [Google Scholar] [CrossRef]
- Lu, M.; Ding, K.; Zhang, G.; Yin, M.; Yao, G.; Tian, H.; Lian, J.; Liu, L.; Liang, M.; Zhu, T.; et al. MicroRNA-320a sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by targeting ARPP-19 and ERRγ. Sci. Rep. 2015, 5, 8735. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Wu, W.; Zou, X.; Liu, F.; Wei, T.; Zhu, J. MiR-26a inhibits thyroid cancer cell proliferation by targeting ARPP19. Am. J. Cancer Res. 2018, 8, 1030–1039. [Google Scholar]
- Jeong, J.; VanHouten, J.N.; Dann, P.; Kim, W.; Sullivan, C.; Yu, H.; Liotta, L.; Espina, V.; Stern, D.F.; Friedman, P.A.; et al. PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E282–E290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, M.; Feng, S.; Chen, W.; Zhu, G.; Shen, D.; Ponnazhagan, S.; Deng, L.; Li, Y.P. Osteoclast proton pump regulator Atp6v1c1 enhances breast cancer growth by activating the mTORC1 pathway and bone metastasis by increasing V-ATPase activity. Oncotarget 2017, 8, 47675–47690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Liu, P.; Wei, L.; Wang, J.; Qi, J.; Feng, S.; Deng, L. Atp6v1c1 may regulate filament actin arrangement in breast cancer cells. PLoS ONE 2014, 9, e84833. [Google Scholar] [CrossRef]
- Feng, S.; Zhu, G.; McConnell, M.; Deng, L.; Zhao, Q.; Wu, M.; Zhou, Q.; Wang, J.; Qi, J.; Li, Y.P.; et al. Silencing of atp6v1c1 prevents breast cancer growth and bone metastasis. Int. J. Biol. Sci. 2013, 9, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z.M.; Song, Y.; Tai, X.H.; Ji, W.Y.; Gu, H. MicroRNA-126 modulates the tumor microenvironment by targeting calmodulin-regulated spectrin-associated protein 1 (Camsap1). Int. J. Oncol. 2014, 44, 1678–1684. [Google Scholar] [CrossRef] [Green Version]
- Toss, M.; Miligy, I.; Gorringe, K.; Mittal, K.; Aneja, R.; Ellis, I.; Green, A.; Rakha, E. Prognostic significance of cathepsin V (CTSV/CTSL2) in breast ductal carcinoma in situ. J. Clin. Pathol. 2020, 73, 76–82. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, R.; Ye, Q.; Zhuo, J.; Chen, K.; Lu, D.; Wei, X.; Xie, H.; Xu, X.; Zheng, S. High expression of human augmin complex submit 3 indicates poor prognosis and associates with tumor progression in hepatocellular carcinoma. J. Cancer. 2019, 10, 1434–1443. [Google Scholar] [CrossRef]
- Sun, A.G.; Meng, F.G.; Wang, M.G. CISD2 promotes the proliferation of glioma cells via suppressing beclin-1-mediated autophagy and is targeted by microRNA-449a. Mol. Med. Rep. 2017, 16, 7939–7948. [Google Scholar] [CrossRef] [Green Version]
- Ramdzan, Z.M.; Nepveu, A. CUX1, a haploinsufficient tumour suppressor gene overexpressed in advanced cancers. Nat. Rev. Cancer 2014, 14, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Itoh, F.; Hinoda, Y.; Ohe, M.; Ohe, Y.; Ban, T.; Endo, T.; Imai, K.; Yachi, A. Decreased expression of DCC mRNA in human colorectal cancers. Int. J. Cancer 1993, 53, 260–263. [Google Scholar] [CrossRef]
- Hedrick, L.; Cho, K.R.; Fearon, E.R.; Wu, T.C.; Kinzler, K.W.; Vogelstein, B. The DCC gene product in cellular differentiation and colorectal tumorigenesis. Genes Dev. 1994, 8, 1174–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.H.; Dai, W.G.; Xu, X.D.; Yu, Q.H.; Zhang, B.; Li, J.; Li, H.P. Downregulation of DPF3 promotes the proliferation and motility of breast cancer cells through activating JAK2/STAT3 signaling. Biochem. Biophys. Res. Commun. 2019, 514, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Luo, Y.; He, S. Knockdown of KDM1B inhibits cell proliferation and induces apoptosis of pancreatic cancer cells. Pathol. Res. Pract. 2019, 215, 1054–1060. [Google Scholar] [CrossRef]
- Russo, A.; O’Bryan, J.P. Intersectin 1 is required for neuroblastoma tumorigenesis. Oncogene 2012, 31, 4828–4834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Liu, J.Y.; Shi, Y.; Tang, B.; He, T.; Liu, J.J.; Fan, J.Y.; Wu, B.; Xu, X.H.; Zhao, Y.L.; et al. MTMR2 promotes invasion and metastasis of gastric cancer via inactivating IFNγ/STAT1 signaling. J. Exp. Clin. Cancer Res. 2019, 38, 206. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Bartholomeusz, C.; Krishnamurthy, S.; Liu, P.; Saso, H.; Lafortune, T.A.; Hortobagyi, G.N.; Ueno, N.T. PEA-15 unphosphorylated at both serine 104 and serine 116 inhibits ovarian cancer cell tumorigenicity and progression through blocking β-catenin. Oncogenesis 2012, 1, e22. [Google Scholar] [CrossRef] [Green Version]

| Upregulated miRNA RB Tumor and RB Serum and Targets Identified in RB Tumor Exosomes | Downregulated miRNA RB Tumor and RB Serum and Targets Identified in RB Tumor Exosomes | miRNA Derived from RB Cell Lines Exosomes and Targets Identified in RB Tumor Exosomes | |||
|---|---|---|---|---|---|
| miRNA | Target Gene | miRNA | Target Gene | miRNA | Target Gene |
| hsa-miR-16-1 | KIAA1598 | hsa-let-7a | TJP3 | hsa-miR-148a | CAMSAP1, IKBIP, NUP50, PPP2R2A |
| hsa-miR-29b-1 | LPGAT1 | hsa-miR-92a | INTS1 | ||
| hsa-miR-34a | PTRHD1 | hsa-let-7f-2 | ERCC3, UNC13B | ||
| hsa-miR-96 | UNC13B | ||||
| hsa-miR-143 | ADD3, AK2 | hsa-miR-217 | ARPC2, EIF1 | hsa-miR-582 | CACNA2D2, MPP1, NEFL, PIN4, VDAC3 |
| hsa-miR-30d | DCK, POLD3 | hsa-let-7a-2 | CCNB1, GSTM1, TMEM55B | ||
| hsa-miR-16 | OS9, TPPP3 | hsa-miR-92a-1 | FAM120A, MPDZ, TNR, TPPP3 | hsa-miR-200a | CTSV, DAG1, GLRX, MTMR2, PRPS1, SLC27A4, UBXN4 |
| hsa-miR-142 | CCNB1, FAM49B, SDHB | ||||
| hsa-miR-106b | PPP2R2A, UBXN4, WDR36 | hsa-miR-216a | GLRX, POLD3, PPP2R2A, DLG5, PPIF, UNC119 | ||
| hsa-miR-182 | IKBIP, LMAN2, NUP50 | ||||
| hsa-miR-183 | MPP1, SEC61B, SNAP25 | ||||
| hsa-miR-148b | MPDZ, TRIM2, DKFZp781M17165, LONP1, PPP1R2 | hsa-miR-92a-2 | CACNA2D2, MPP1, OS9, P4HTM, PAK1, SIN3B | hsa-miR-483 | DCK, DKFZp781M17165, GSTM1, HAUS3, MYDGF, PPP2R1B, PTRHD1 |
| hsa-miR-29c | DAG1, NDUFS6, PDCD4, PPIF, NEFL, PRPS1 | hsa-let-7i | DNAJC5, ATP8A1, DAG1, PTRHD1, SLC25A13, TTC9C, UQCRFS1 | hsa-miR-216b | ABI2, ATP2B2, ATP8A1, CCDC85C, CISD2, CUX1, DNAJC5, FAM120A, ITSN1, LPGAT1, PPP1R2, RQCD1, TNR, TRMT6, TSPAN14, UBXN7, WIPF2 |
| hsa-miR-30b | CACNA2D2, ERCC3, GSTM1, SLC27A4, TMEM55B, MED4, ARPC2 | ||||
| hsa-miR-494 | ABI2, ATP2B2, ATP6V1C1, ATP8A1, CAMSAP1, CCDC85C, CISD2, CTSV, CUX1, DCC, DLG5, DNAJC5, DPF3, EIF1, FAM120A, GLRX, HAUS3, ITSN1, KDM1B, MTMR2, NCAN, PAK1, QSER1, SLC25A13, UBXN7, WDR11, WIPF2, ARPP19, PEA15, PIN4, PPP2R1B, RQCD1, SIN3B, SYNGR1, TNR, TRMT6, TSPAN14, UNC119, UQCRFS1 | hsa-miR-9 | ADD3, ARPP19, ATP6V1C1, CCDC85C, CISD2, CTSV, CUX1, DCC, DCK, FAM49B, ITSN1, KDM1B, LPGAT1, MED4, NCAN, PDCD4, PEA15, QSER1, RQCD1, TRIM2, UBXN4, WDR11, ABI2, ATP2B2, CAMSAP1, DKFZp781M17165, DPF3, HAUS3, IKBIP, MTMR2, NEFL, PPP1R2, PRPS1, SLC27A4, SNAP25, TSPAN14, UBXN7, WIPF2 | hsa-miR-301b | ADD3ARPP19ATP6V1C1CCNB1DCCDLG5DPF3FAM49BNCANPAK1PEA15QSER1SLC25A13SNAP25TMEM55BTRIM2UQCRFS1WDR11 |
| ABI2 | CAMSAP1 | CUX1 | ITSN1 | PEA15 | UBXN7 |
| ARPP19 | CCDC85C | DCC | KDM1B | QSER1 | WDR11 |
| ATP2B2 | CISD2 | DPF3 | MTMR2 | RQCD1 | WIPF2 |
| ATP6V1C1 | CTSV | HAUS3 | NCAN | TSPAN14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lande, K.; Gupta, J.; Ranjan, R.; Kiran, M.; Torres Solis, L.F.; Solís Herrera, A.; Aliev, G.; Karnati, R. Exosomes: Insights from Retinoblastoma and Other Eye Cancers. Int. J. Mol. Sci. 2020, 21, 7055. https://doi.org/10.3390/ijms21197055
Lande K, Gupta J, Ranjan R, Kiran M, Torres Solis LF, Solís Herrera A, Aliev G, Karnati R. Exosomes: Insights from Retinoblastoma and Other Eye Cancers. International Journal of Molecular Sciences. 2020; 21(19):7055. https://doi.org/10.3390/ijms21197055
Chicago/Turabian StyleLande, Kashmiri, Jitesh Gupta, Ravi Ranjan, Manjari Kiran, Luis Fernando Torres Solis, Arturo Solís Herrera, Gjumrakch Aliev, and Roy Karnati. 2020. "Exosomes: Insights from Retinoblastoma and Other Eye Cancers" International Journal of Molecular Sciences 21, no. 19: 7055. https://doi.org/10.3390/ijms21197055