Functional Recovery of a GCDH Variant Associated to Severe Deflavinylation—Molecular Insights into Potential Beneficial Effects of Riboflavin Supplementation in Glutaric Aciduria-Type I Patients
Abstract
:1. Introduction
2. Results
2.1. Purified Human Recombinant GCDH-p.Val400Met Is Depleted of FAD Cofactor
2.2. Apo GCDH-p.Val400Met Presents a Less Compact Conformation
2.3. Deflavinylation Affects GCDH Quaternary Structure
2.4. Flavin Supplementation Rescues Apo GCDH-p.Val400Met Enzymatic Activity
2.5. Flavin Improves the Conformational Quality of Holo GCDH-p.Val400Met
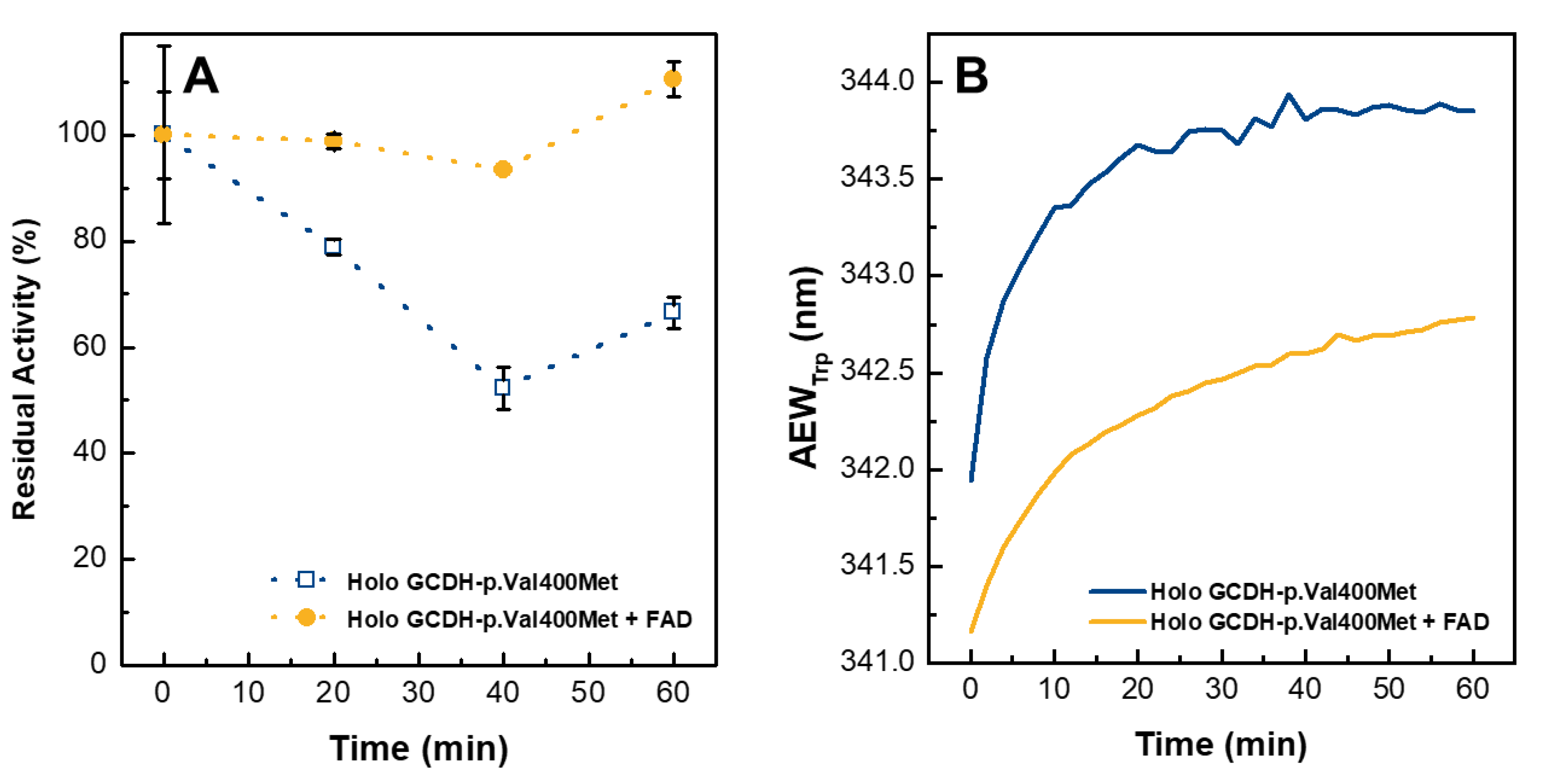
2.6. External FAD Preserves GCDH-p.Val400Met Enzymatic Activity during Thermal Stress
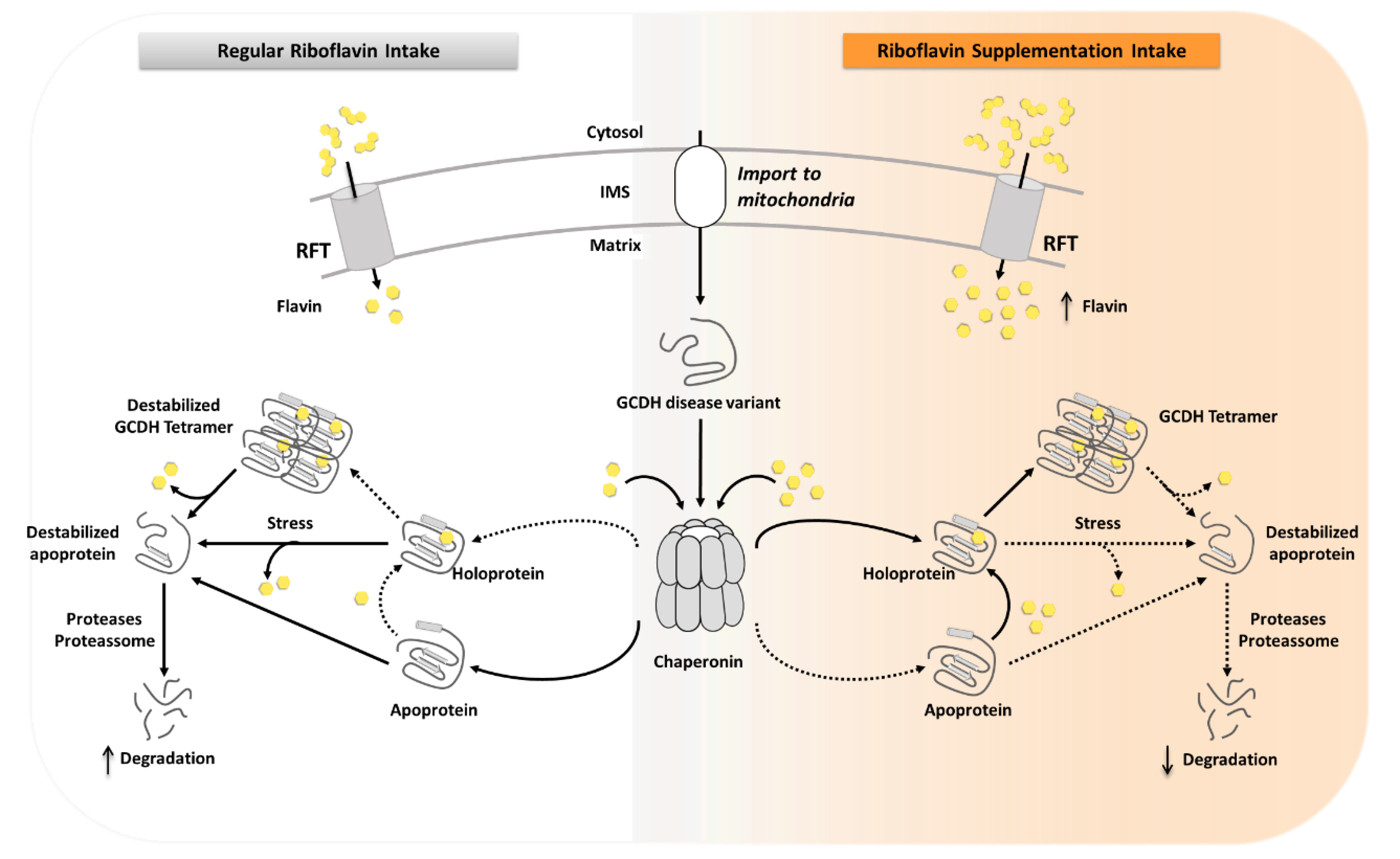
3. Discussion
4. Materials and Methods
4.1. Chemicals/Materials
4.2. Protein Purification and Biochemical Assays
4.3. Spectroscopic Methods
4.4. Fluorescence Quenching with Acrylamide
4.5. Size-Exclusion Chromatography
4.6. Thermal Stability
4.7. Limited Proteolysis with Trypsin
4.8. Thermal Stress Studies
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FMN | flavin mononucleotide |
| FAD | flavin adenine dinucleotide |
| ACDH | acyl-CoA dehydrogenase |
| GCDH | glutaryl-CoA dehydrogenase |
| GA-I | glutaric aciduria type I |
| GABA | γ-aminobutyric acid |
| CD | circular dichroism |
| AEW | average emission wavelength |
References
- Henriques, B.J.; Gomes, C.M. Riboflavin (vitamin B2) and mitochondrial energy. In Molecular Nutrition: Vitamins; Patel, V.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–244. [Google Scholar] [CrossRef]
- Henriques, B.J.; Olsen, R.K.; Bross, P.; Gomes, C.M. Emerging roles for riboflavin in functional rescue of mitochondrial beta-oxidation flavoenzymes. Curr. Med. Chem. 2010, 17, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Nisco, A.; Leone, P.; Barile, M. Development of Novel Experimental Models to Study Flavoproteome Alterations in Human Neuromuscular Diseases: The Effect of Rf Therapy. Int. J. Mol. Sci. 2020, 21, 5310. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, C.; DiMarco, J.P.; Tandler, B. Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J. Biol. Chem. 1979, 254, 4164–4170. [Google Scholar] [PubMed]
- Sakurai, T.; Miyazawa, S.; Furuta, S.; Hashimoto, T. Riboflavin deficiency and beta-oxidation systems in rat liver. Lipids 1982, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Veitch, K.; Draye, J.P.; Vamecq, J.; Causey, A.G.; Bartlett, K.; Sherratt, H.S.; Van Hoof, F. Altered acyl-CoA metabolism in riboflavin deficiency. Biochim. Biophys. Acta 1989, 1006, 335–343. [Google Scholar] [CrossRef]
- Rodrigues, J.V.; Henriques, B.J.; Lucas, T.G.; Gomes, C.M. Cofactors and metabolites as protein folding helpers in metabolic diseases. Curr. Top. Med. Chem. 2012, 12, 2546–2559. [Google Scholar] [CrossRef]
- Nagao, M.; Tanaka, K. FAD-Dependent regulation of transcription, translation, post-translational processing, and post-processing stability of various mitochondrial acyl-CoA dehydrogenases and of electron transfer flavoprotein and the site of holoenzyme formation. J. Biol. Chem. 1992, 267, 17925–17932. [Google Scholar]
- Saijo, T.; Tanaka, K. Isoalloxazine ring of FAD is required for the formation of the core in the Hsp60-assisted folding of medium chain acyl-CoA dehydrogenase subunit into the assembly competent conformation in mitochondria. J. Biol. Chem. 1995, 270, 1899–1907. [Google Scholar] [CrossRef] [Green Version]
- Lucas, T.G.; Henriques, B.J.; Rodrigues, J.V.; Bross, P.; Gregersen, N.; Gomes, C.M. Cofactors and metabolites as potential stabilizers of mitochondrial acyl-CoA dehydrogenases. Biochim. Biophys. Acta 2011, 1812, 1658–1663. [Google Scholar] [CrossRef] [Green Version]
- Swigonova, Z.; Mohsen, A.W.; Vockley, J. Acyl-CoA dehydrogenases: Dynamic history of protein family evolution. J. Mol. Evol. 2009, 69, 176–193. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, T.M.; Rao, K.S.; Goodman, S.I.; Frerman, F.E. Proton abstraction reaction, steady-state kinetics, and oxidation-reduction potential of human glutaryl-CoA dehydrogenase. Biochemistry 2000, 39, 11488–11499. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Limon, A.; Alriquet, M.; Lang, W.H.; Calloni, G.; Wittig, I.; Vabulas, R.M. Recognition of enzymes lacking bound cofactor by protein quality control. Proc. Natl. Acad. Sci. USA 2016, 113, 12156–12161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedlund, G.L.; Longo, N.; Pasquali, M. Glutaric acidemia type 1. Am. J. Med. Genet. C Semin. Med. Genet. 2006, 142, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Boy, N.; Muhlhausen, C.; Maier, E.M.; Heringer, J.; Assmann, B.; Burgard, P.; Dixon, M.; Fleissner, S.; Greenberg, C.R.; Harting, I.; et al. Proposed recommendations for diagnosing and managing individuals with glutaric aciduria type I: Second revision. J. Inherit. Metab. Dis. 2017, 40, 75–101. [Google Scholar] [CrossRef]
- Scalais, E.; Bottu, J.; Wanders, R.J.; Ferdinandusse, S.; Waterham, H.R.; De Meirleir, L. Familial very long chain acyl-CoA dehydrogenase deficiency as a cause of neonatal sudden infant death: Improved survival by prompt diagnosis. Am. J. Med. Genet. A 2015, 167, 211–214. [Google Scholar] [CrossRef]
- Tonin, R.; Caciotti, A.; Funghini, S.; Pasquini, E.; Mooney, S.D.; Cai, B.; Proncopio, E.; Donati, M.A.; Baronio, F.; Bettocchi, I.; et al. Clinical relevance of short-chain acyl-CoA dehydrogenase (SCAD) deficiency: Exploring the role of new variants including the first SCAD-disease-causing allele carrying a synonymous mutation. BBA Clin. 2016, 5, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Brandt, N.J.; Gregersen, N.; Christensen, E.; Gron, I.H.; Rasmussen, K. Treatment of glutaryl-CoA dehydrogenase deficiency (glutaric aciduria). Experience with diet, riboflavin, and GABA analogue. J. Pediatr. 1979, 94, 669–673. [Google Scholar] [CrossRef]
- Dunger, D.B.; Snodgrass, G.J. Glutaric aciduria type I presenting with hypoglycaemia. J. Inherit. Metab. Dis. 1984, 7, 122–124. [Google Scholar] [CrossRef]
- Lipkin, P.H.; Roe, C.R.; Goodman, S.I.; Batshaw, M.L. A case of glutaric acidemia type I: Effect of riboflavin and carnitine. J. Pediatr. 1988, 112, 62–65. [Google Scholar] [CrossRef]
- Chalmers, R.A.; Bain, M.D.; Zschocke, J. Riboflavin-responsive glutaryl CoA dehydrogenase deficiency. Mol. Genet. Metab. 2006, 88, 29–37. [Google Scholar] [CrossRef]
- Kolker, S.; Christensen, E.; Leonard, J.V.; Greenberg, C.R.; Burlina, A.B.; Burlina, A.P.; Dixon, M.; Duran, M.; Goodman, S.I.; Koeller, D.M.; et al. Guideline for the diagnosis and management of glutaryl-CoA dehydrogenase deficiency (glutaric aciduria type I). J. Inherit. Metab. Dis. 2007, 30, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.V.; Lucas, T.G.; Bross, P.; Gomes, C.M.; Henriques, B.J. Potential complementation effects of two disease-associated mutations in tetrameric glutaryl-CoA dehydrogenase is due to inter subunit stability-activity counterbalance. Biochim. Biophys. Acta Proteins Proteom 2020, 1868, 140269. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Ghiron, C.A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry 1976, 15, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Westover, J.B.; Goodman, S.I.; Frerman, F.E. Pathogenic mutations in the carboxyl-terminal domain of glutaryl-CoA dehydrogenase: Effects on catalytic activity and the stability of the tetramer. Mol. Genet. Metab. 2003, 79, 245–256. [Google Scholar] [CrossRef]
- Busquets, C.; Merinero, B.; Christensen, E.; Gelpi, J.L.; Campistol, J.; Pineda, M.; Fernandez-Alvarez, E.; Prats, J.M.; Sans, A.; Arteaga, R.; et al. Glutaryl-CoA dehydrogenase deficiency in Spain: Evidence of two groups of patients, genetically, and biochemically distinct. Pediatr. Res. 2000, 48, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianazza, E.; Vergani, L.; Wait, R.; Brizio, C.; Brambilla, D.; Begum, S.; Giancaspero, T.A.; Conserva, F.; Eberini, I.; Bufano, D.; et al. Coordinated and reversible reduction of enzymes involved in terminal oxidative metabolism in skeletal muscle mitochondria from a riboflavin-responsive, multiple acyl-CoA dehydrogenase deficiency patient. Electrophoresis 2006, 27, 1182–1198. [Google Scholar] [CrossRef]
- Henriques, B.J.; Rodrigues, J.V.; Olsen, R.K.; Bross, P.; Gomes, C.M. Role of flavinylation in a mild variant of multiple acyl-CoA dehydrogenation deficiency: A molecular rationale for the effects of riboflavin supplementation. J. Biol. Chem. 2009, 284, 4222–4229. [Google Scholar] [CrossRef] [Green Version]
- Muntau, A.C.; Leandro, J.; Staudigl, M.; Mayer, F.; Gersting, S.W. Innovative strategies to treat protein misfolding in inborn errors of metabolism: Pharmacological chaperones and proteostasis regulators. J. Inherit. Metab. Dis. 2014, 37, 505–523. [Google Scholar] [CrossRef]
- Keyser, B.; Muhlhausen, C.; Dickmanns, A.; Christensen, E.; Muschol, N.; Ullrich, K.; Braulke, T. Disease-Causing missense mutations affect enzymatic activity, stability and oligomerization of glutaryl-CoA dehydrogenase (GCDH). Hum. Mol. Genet. 2008, 17, 3854–3863. [Google Scholar] [CrossRef]
- Schmiesing, J.; Lohmoller, B.; Schweizer, M.; Tidow, H.; Gersting, S.W.; Muntau, A.C.; Braulke, T.; Muhlhausen, C. Disease-Causing mutations affecting surface residues of mitochondrial glutaryl-CoA dehydrogenase impair stability, heteromeric complex formation and mitochondria architecture. Hum. Mol. Genet. 2017, 26, 538–551. [Google Scholar] [CrossRef] [Green Version]
- Leandro, P.; Gomes, C.M. Protein misfolding in conformational disorders: Rescue of folding defects and chemical chaperoning. Mini Rev. Med. Chem. 2008, 8, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency-Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef]
- Henriques, B.J.; Lucas, T.G.; Gomes, C.M. Therapeutic Approaches Using Riboflavin in Mitochondrial Energy Metabolism Disorders. Curr. Drug Targets 2016, 17, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Barile, M.; Giancaspero, T.A.; Leone, P.; Galluccio, M.; Indiveri, C. Riboflavin transport and metabolism in humans. J. Inherit. Metab. Dis. 2016, 39, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Perez-Duenas, B.; De La Osa, A.; Capdevila, A.; Navarro-Sastre, A.; Leist, A.; Ribes, A.; Garcia-Cazorla, A.; Serrano, M.; Pineda, M.; Campistol, J. Brain injury in glutaric aciduria type I: The value of functional techniques in magnetic resonance imaging. Eur. J. Paediatr. Neurol. 2009, 13, 534–540. [Google Scholar] [CrossRef]
- Thorpe, C.; Matthews, R.G.; Williams, C.H., Jr. Acyl-coenzyme A dehydrogenase from pig kidney. Purification and properties. Biochemistry 1979, 18, 331–337. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Springer: Boston, MA, USA, 1999; p. 698. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Niklasson, M.; Andresen, C.; Helander, S.; Roth, M.G.; Zimdahl Kahlin, A.; Lindqvist Appell, M.; Martensson, L.G.; Lundstrom, P. Robust and convenient analysis of protein thermal and chemical stability. Protein Sci. A Publ. Protein Soc. 2015, 24, 2055–2062. [Google Scholar] [CrossRef] [Green Version]





| GCDH | Ka (M−1) | fa |
|---|---|---|
| Wild-Type | 5.88 ± 0.42 | 0.84 ± 0.04 |
| Holo p.Val400Met | 7.43 ± 0.08 | 0.80 ± 0.01 |
| Apo p.Val400Met | 8.99 ± 0.33 | 0.89 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, J.V.; Gomes, C.M.; Henriques, B.J. Functional Recovery of a GCDH Variant Associated to Severe Deflavinylation—Molecular Insights into Potential Beneficial Effects of Riboflavin Supplementation in Glutaric Aciduria-Type I Patients. Int. J. Mol. Sci. 2020, 21, 7063. https://doi.org/10.3390/ijms21197063
Ribeiro JV, Gomes CM, Henriques BJ. Functional Recovery of a GCDH Variant Associated to Severe Deflavinylation—Molecular Insights into Potential Beneficial Effects of Riboflavin Supplementation in Glutaric Aciduria-Type I Patients. International Journal of Molecular Sciences. 2020; 21(19):7063. https://doi.org/10.3390/ijms21197063
Chicago/Turabian StyleRibeiro, Joana V., Cláudio M. Gomes, and Bárbara J. Henriques. 2020. "Functional Recovery of a GCDH Variant Associated to Severe Deflavinylation—Molecular Insights into Potential Beneficial Effects of Riboflavin Supplementation in Glutaric Aciduria-Type I Patients" International Journal of Molecular Sciences 21, no. 19: 7063. https://doi.org/10.3390/ijms21197063