In Vivo Studies of Inoculated Plants and In Vitro Studies Utilizing Methanolic Extracts of Endophytic Streptomyces sp. Strain DBT34 Obtained from Mirabilis jalapa L. Exhibit ROS-Scavenging and Other Bioactive Properties
Abstract
:1. Introduction
2. Results
2.1. Catalase-Like and SOD-Like Activity
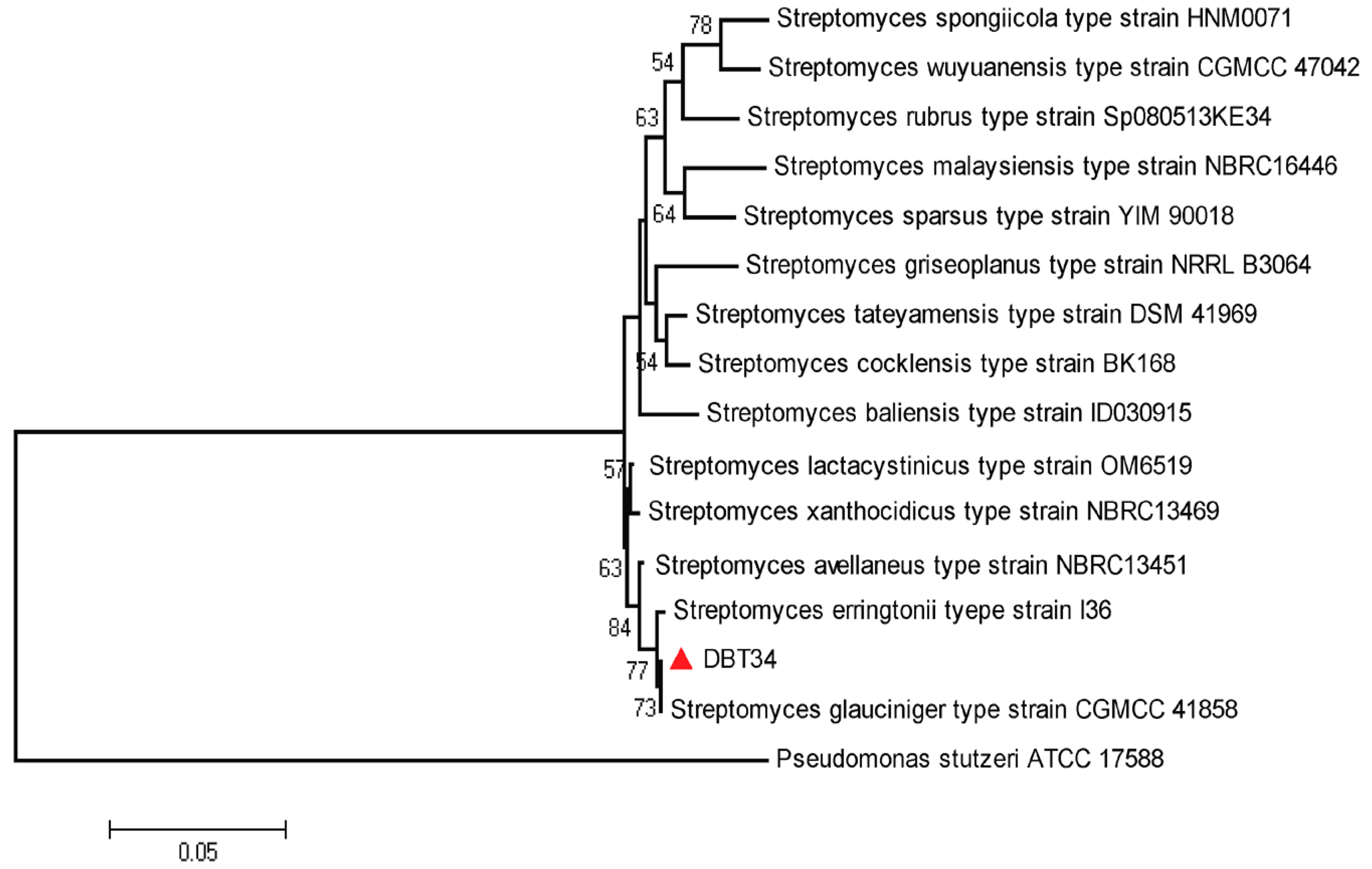
2.2. Identification and Phylogenetic Analysis
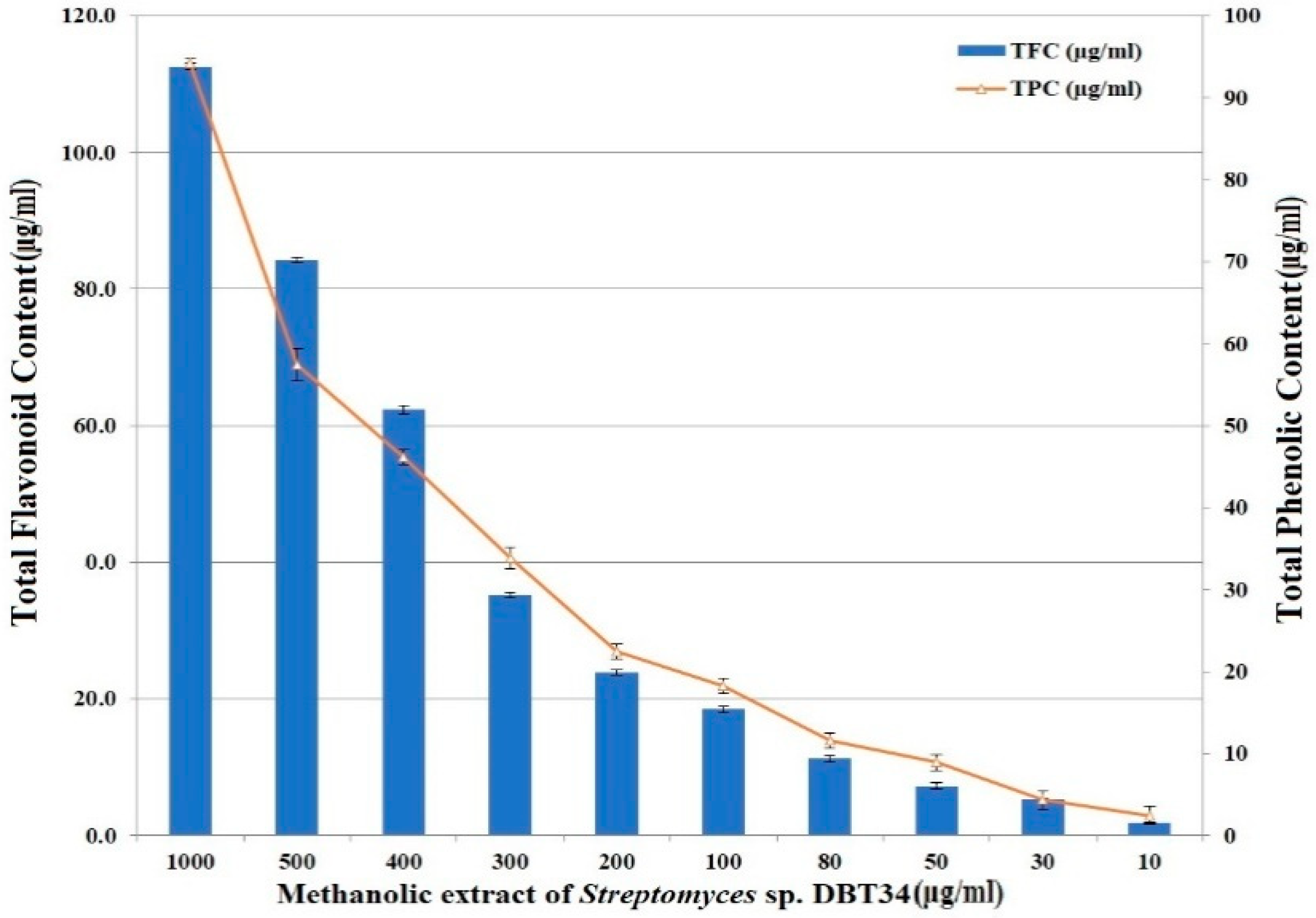
2.3. Total Phenolics and Flavonoids
2.4. Antioxidant Activity
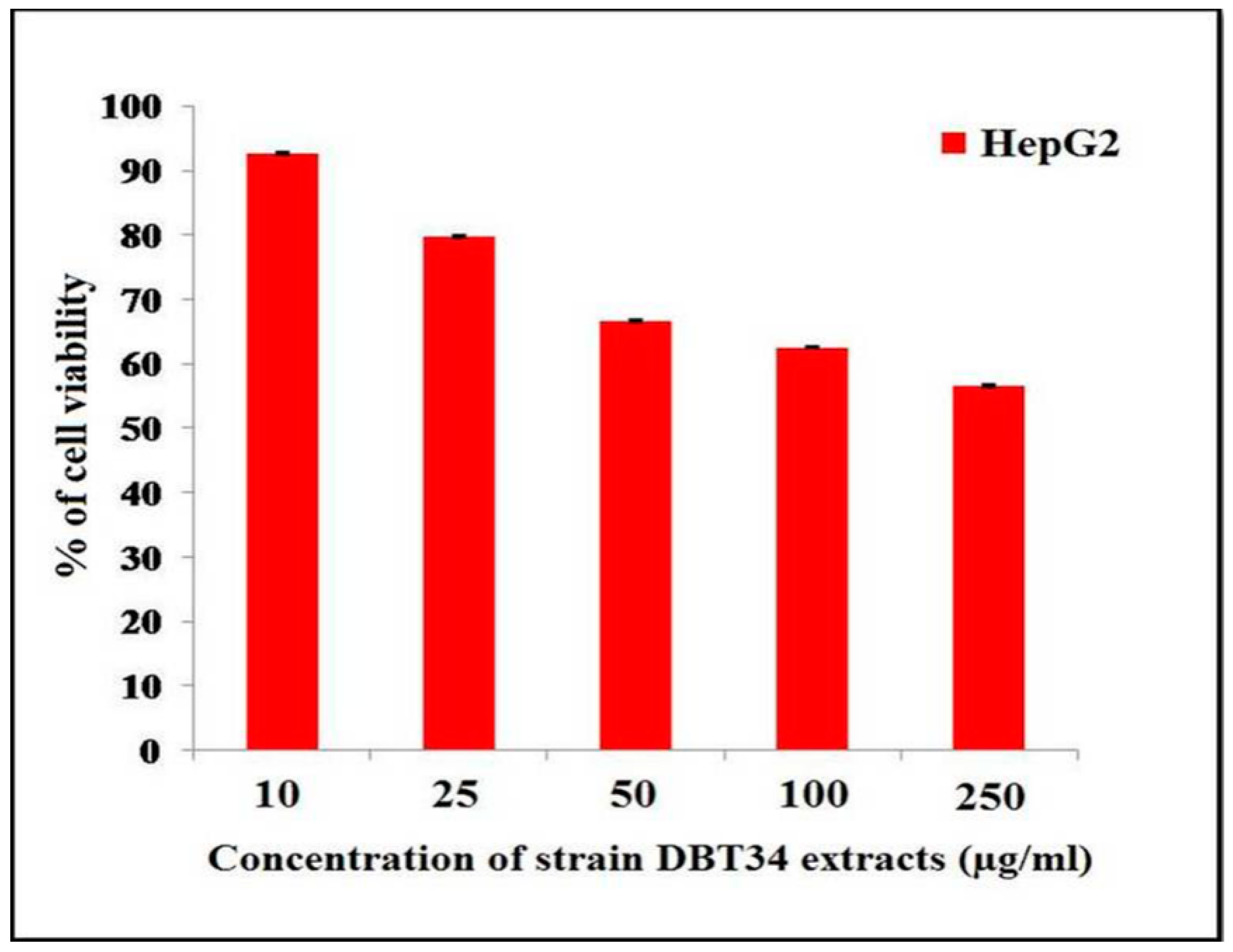
2.5. Cytotoxicity against Three Cancer Cell Lines
2.6. Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
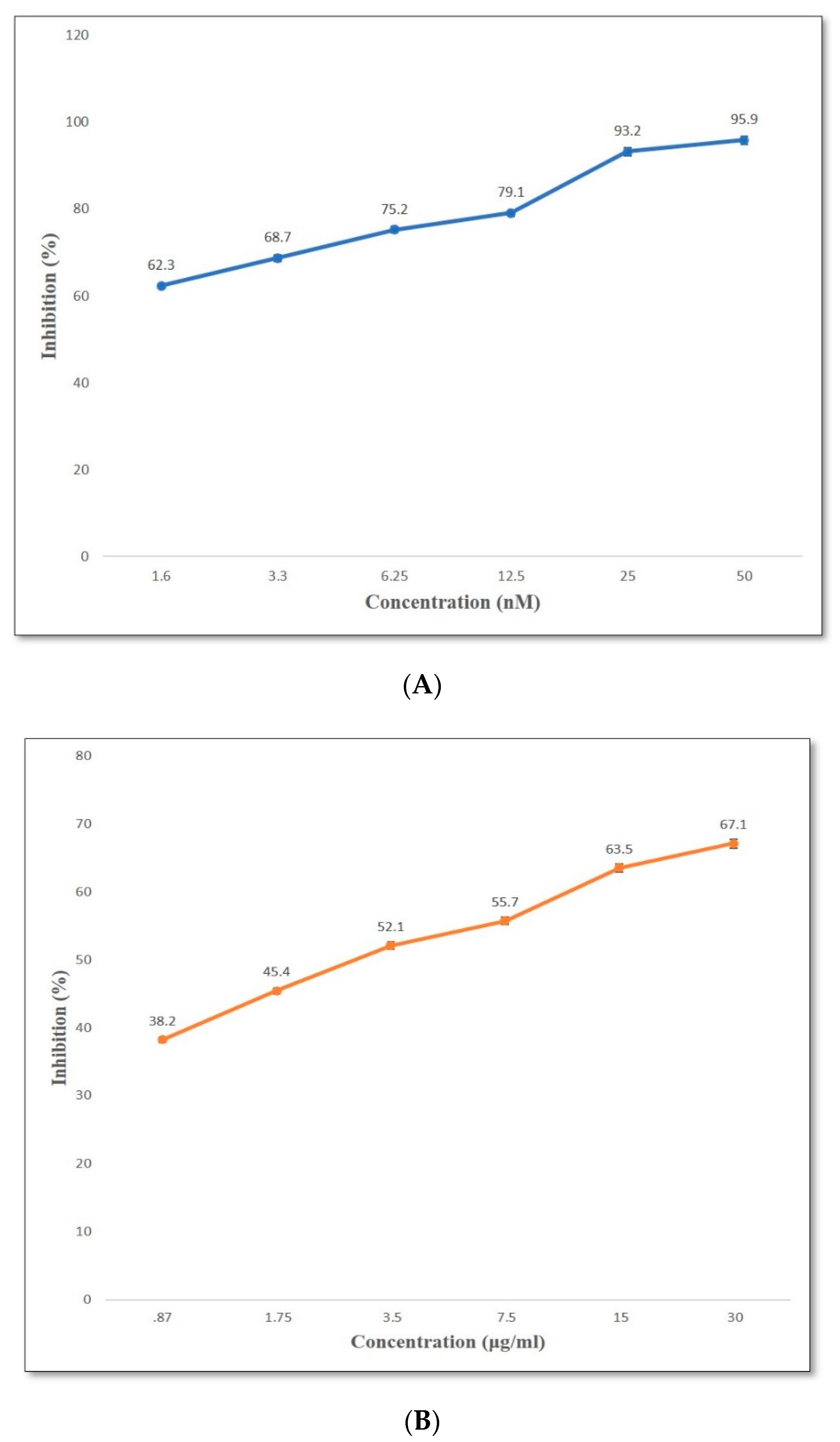
2.7. DPP-4 Inhibition In Vitro
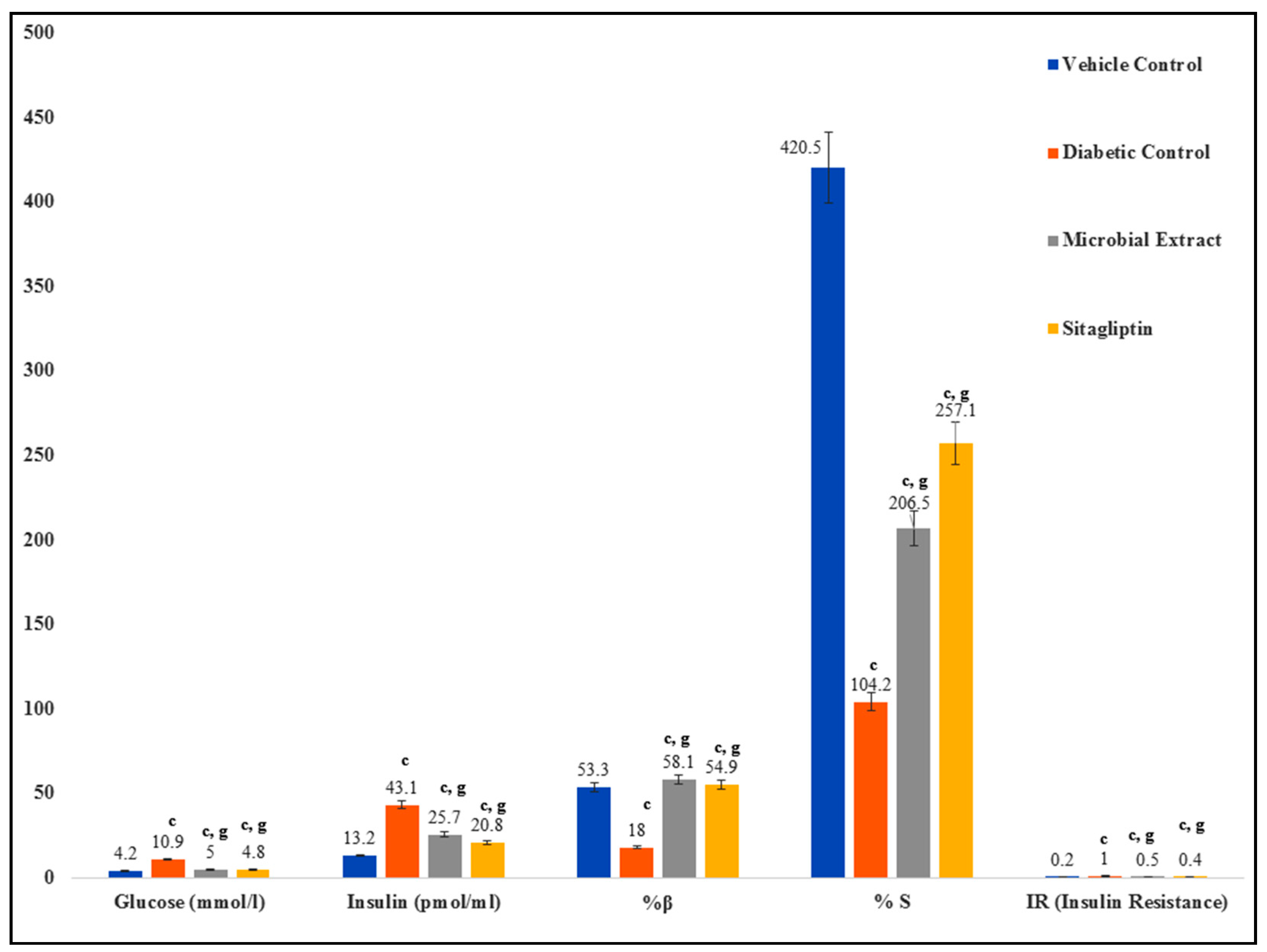
2.8. Glucose Levels, Insulin, and HOMA Indices
2.9. Alterations in the Lipid Profile of Treatment Groups
2.10. Changes in Serum Antioxidant Levels
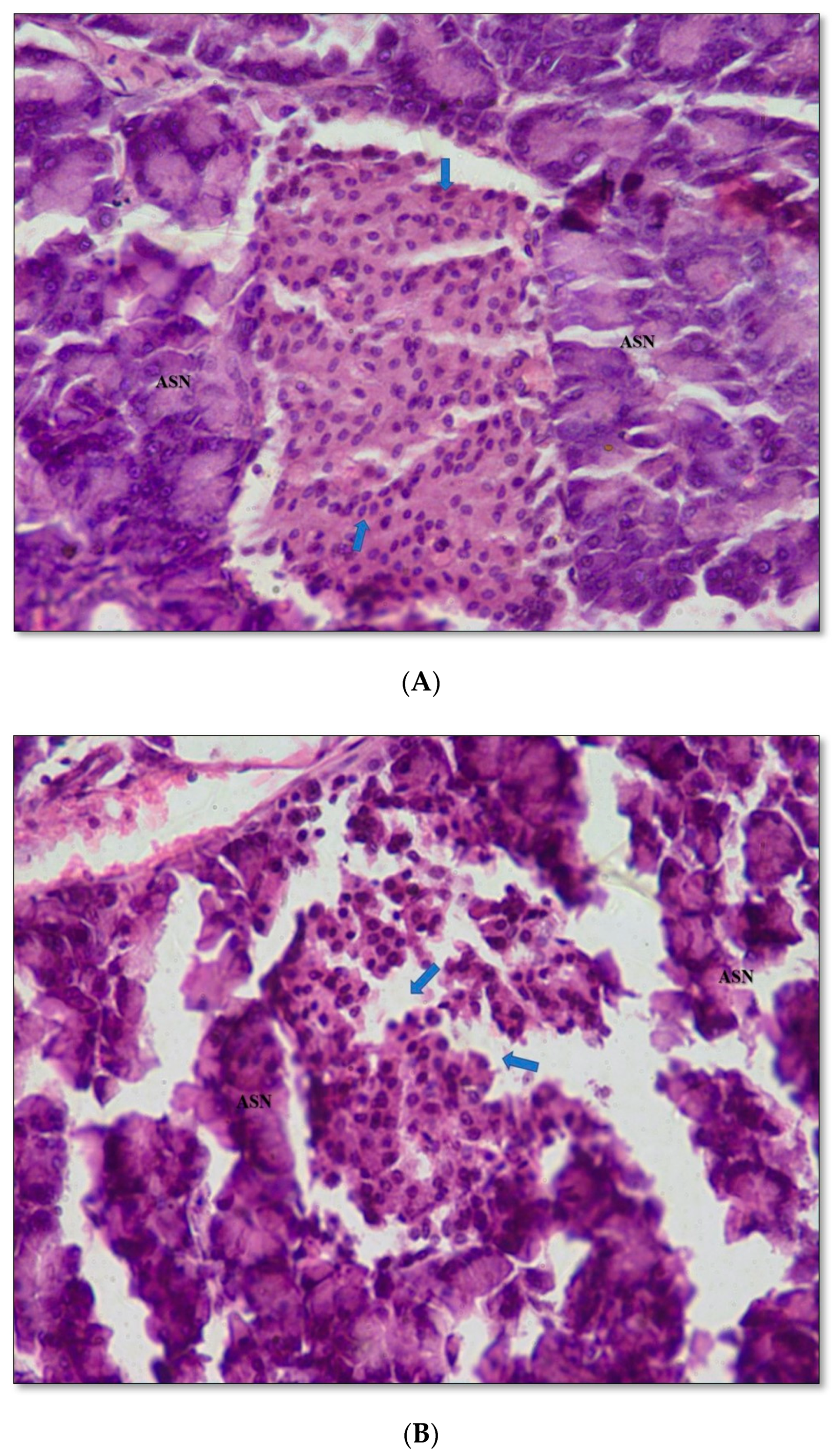
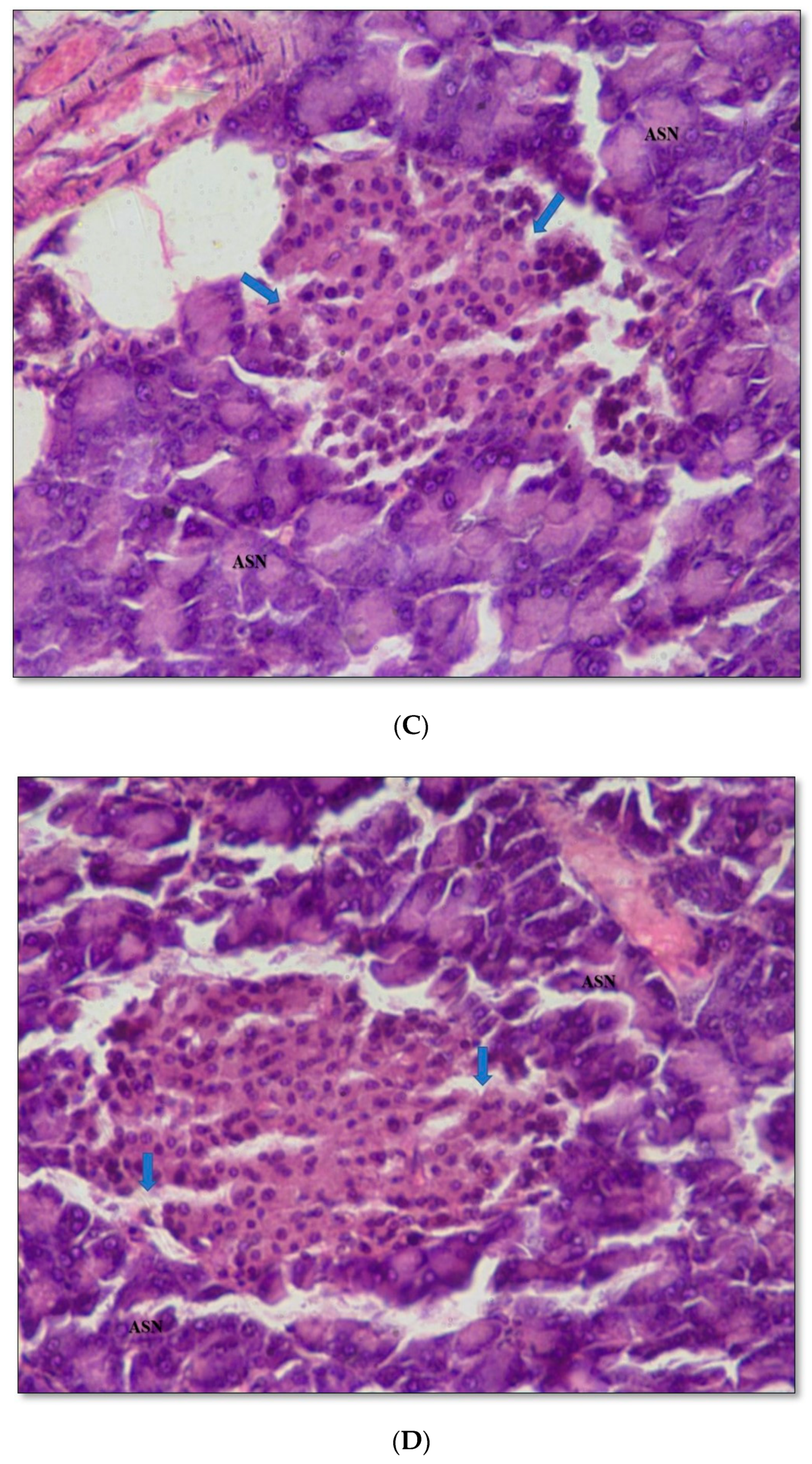
2.11. Pancreas Histopathology
2.12. Plant Growth Promotion in Chickpea Plants
Effect of Streptomyces sp. Strain DBT34 on Chickpea Plants In-Vivo and Antioxidant Activity in Derived Plant Extracts
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of Isolates of Actinobacteria
4.2. Extract Preparation
4.3. Reactive Oxygen Species (ROS)-Scavenging Assays
Catalase and Superoxide Dismutase-(SOD)-Like Activity
4.4. 16S rRNA Amplification and Phylogenetic Analysis
4.5. Phytochemical Analysis
Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
4.6. Determination of Antioxidant Potential
DPPH Radical Scavenging Assay and ABTS Radical Cation Decolorization Assay
4.7. Cytotoxicity
4.7.1. Cell Lines and Culture Medium
4.7.2. Cytotoxicity Assay
4.8. Determination of Volatile Organic Compounds (VOCs) Using Gas Chromatography-Mass Spectroscopy (GC/MS)
4.9. Antidiabetic Assay
4.9.1. Drugs and Chemicals
4.9.2. Animals
4.9.3. Induction of Type 2 Diabetes
4.9.4. In-Vitro DPP-4 Inhibition Assay
4.9.5. Biochemical Assessments of Serum
Glucose, Insulin, HbA1C and Lipid Profile
4.9.6. Homeostatic Model Assessment (HOMA)
4.9.7. Serum Antioxidant Assay
4.9.8. Histopathological Studies
4.10. Plant Growth Promotion Assay in Chickpea Plants (Cicer Arietinum)
Antioxidant Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| VOCs | Volatile compounds |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| GAE | Gallic Acid Equivalents |
| QE | Quercetin Equivalents |
| HepG2 | Human hepatocarcinoma |
| GSH | Glutathione |
References
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Bini, Y.K.; Subila, K.P.; Aravind, R. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res. 2015, 173, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.E.; Gundel, P.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Dholakiya, R.N.; Kumar, R.; Mishra, A.; Mody, K.; Jha, B. Antibacterial and Antioxidant Activities of Novel Actinobacteria Strain Isolated from Gulf of Khambhat, Gujarat. Front. Microbiol. 2017, 8, 2420. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Genet. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Passari, A.K.; Mishra, V.K.; Saikia, R.; Gupta, V.K.; Singh, B.P. Isolation, abundance and phylogenetic affiliation of endophyticactinomycetes associated with medicinal plants and screening for their in vitro antimicrobial biosynthetic potential. Front. Microbiol. 2015, 6, 273. [Google Scholar] [CrossRef] [Green Version]
- Passari, A.K.; Mishra, V.K.; Gupta, V.K.; Yadav, M.K.; Saikia, R.; Singh, B.P. In Vitro and In Vivo Plant Growth Promoting Activities and DNA Fingerprinting of Antagonistic Endophytic Actinomycetes Associates with Medicinal Plants. PLoS ONE 2015, 10, e0139468. [Google Scholar] [CrossRef] [Green Version]
- Passari, A.K.; Chandra, P.; Mishra, V.K.; Leo, V.V.; Gupta, V.K.; Kumar, B.; Singh, B.P. Detection of biosynthetic gene and phytohormone production by endophytic actinobacteria associated with Solanumlycopersicum and their plant-growth-promoting effect. Res. Microbiol. 2016, 167, 692–705. [Google Scholar] [CrossRef]
- Passari, A.K.; Mishra, V.K.; Singh, G.; Singh, P.; Kumar, B.; Gupta, V.K.; Sarma, R.K.; Saikia, R.; Donovan, A.O.; Singh, B.P. Insights into the functionality of endophytic actinobacteria with a focus on their biosynthetic potential and secondary metabolites production. Sci. Rep. 2017, 7, 11809. [Google Scholar] [CrossRef]
- Salam, N.; Khieu, T.-N.; Liu, M.-J.; Vu, T.-T.; Chu-Ky, S.; Quach, N.-T.; Phi, Q.-T.; Rao, M.P.N.; Fontana, A.; Sarter, S.; et al. Endophytic Actinobacteria Associated with Dracaena cochinchinensisLour.: Isolation, Diversity, and Their Cytotoxic Activities. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Dubey, A.K. Diversity and Applications of Endophytic Actinobacteria of Plants in Special and Other Ecological Niches. Front. Microbiol. 2018, 9, 1767. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, R.; Sajid, I.; Hasnain, S. Larvicidal potential of Asteraceae family endophytic actinomycetes against Culex quinquefasciatus mosquito larvae. Nat. Prod. Res. 2014, 28, 2048–2052. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kalita, M.C.; Thakur, D. Broad Spectrum Antimicrobial Activity of Forest-Derived Soil Actinomycete, Nocardia sp. PB-52. Front. Microbiol. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozina, R. Pharmacological and biological activities of Mirabilis jalapa L. Int. J. Pharmacol. Res. 2016, 6, 160–168. [Google Scholar] [CrossRef]
- Dudai, N.; Fischer, R.; Segev, D.; Chaimovitsh, D.; Rosenzweig, N.; Shimoni, M. Antioxidative activity of khat (Catha edulisforsk.). Acta Hortic. 2008, 778, 85–92. [Google Scholar] [CrossRef]
- Autore, G.; Caruso, A.; Marzocco, S.; Nicolaus, B.; Palladino, C.; Pinto, A.; Popolo, A.; Sinicropi, M.S.; Tommonaro, G.; Saturnino, C. Acetamide Derivatives with Antioxidant Activity and Potential Anti-Inflammatory Activity. Molecules 2010, 15, 2028–2038. [Google Scholar] [CrossRef] [Green Version]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, M.G.; Fatma, A.R.; Helmy, H.; Hebaallah, M.A. Synthesis of some novel sulfonamides containing biologically active alkanoic acid, acetamide, thiazole, and pyrrole moieties of expected antitumor and radio sensietizing activities. J. Basic Appl. Chem. 2011, 1, 8–14. [Google Scholar]
- Fokialakis, N.; Alexi, X.; Aligiannis, N.; Siriani, D.; Meligova, A.K.; Pratsinis, H.; Mitakou, S.; Alexis, M. Ester and carbamate ester derivatives of Biochanin A: Synthesis and in vitro evaluation of estrogenic and antiproliferative activities. Bioorg. Med. Chem. 2012, 20, 2962–2970. [Google Scholar] [CrossRef]
- Ma, C.J.; Yun, B.-R.; Yang, H.J.; Weon, J.B.; Lee, J.; Eom, M.R. Neuroprotective properties of compounds extracted from Dianthus superbus L. against Glutamate-induced Cell death in HT22 Cells. Pharmacogn. Mag. 2016, 12, 109–113. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.-Y.; Yuan, J.; Li, X.; Ning, Y.-F.; Dai, C.-C. Endophytic Bacterium-Triggered Reactive Oxygen Species Directly Increase OxygenousSesquiterpenoid Content and Diversity in Atractylodeslancea. Appl. Environ. Microbiol. 2015, 82, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.T.-H.; Chan, K.-G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM212 as a Source of Antioxidants with Radical Scavenging and Metal Chelating Properties. Front. Pharmacol. 2017, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugapriya, K.; Senthil, M.T.; Udayabhanu, J.; Thayumanavan, T. Antioxidant investigation of dried methanolic extracts of GnaphaliumpolycaulonPers, an Indian folkloric ethnomedicinal plant of the Nilgiri, Tamil Nadu, India. Am. J. Phytomed. Clin. Ther. 2017, 5, 3. [Google Scholar] [CrossRef]
- De Carvalho, M.H.C. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2016, 8, 15996–16016. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Manhas, R.K.; Rani, R.; Arora, S. Actinobacteria from soil as potential free radical scavengers. Malays. J. Microbiol. 2017, 13, 1–5. [Google Scholar] [CrossRef]
- Lertcanawanichakul, M.; Pondet, K.; Kwantep, J. In vitro antimicrobial and antioxidant activities of bioactive compounds (secondary metabolites) extracted from Streptomyces lydicus A2. J. Appl. Pharm. Sci. 2015, 5, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukić, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Ser, H.-L.; Yin, W.-F.; Chan, K.G.; Lee, L.-H.; Goh, B.-H. Investigation of Antioxidative and Anticancer Potentials of Streptomyces sp. MUM256 Isolated from Malaysia Mangrove Soil. Front. Microbiol. 2015, 6, 1316. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Khieu, T.-N.; Liu, M.-J.; Nimaichand, S.; Quach, N.-T.; Chu-Ky, S.; Phi, Q.-T.; Vu, T.-T.; Nguyen, T.-D.; Xiong, Z.; Prabhu, D.M.; et al. Characterization and evaluation of antimicrobial and cytotoxic effects of Streptomyces sp. HUST012 isolated from medicinal plant Dracaena cochinchinensisLour. Front. Microbiol. 2015, 6, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-R.; Lee, S.-K.; Choi, B.-K.; Cheng, J.; Lee, Y.-S.; Yang, S.H.; Suh, J.-W. Antioxidant activity and free radical scavenging activities of Streptomyces sp. strain MJM 10778. Asian Pac. J. Trop. Med. 2014, 7, 962–967. [Google Scholar] [CrossRef]
- Jog, R.; Pandya, M.; Nareshkumar, G.; Rajkumar, S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology 2014, 160, 778–788. [Google Scholar] [CrossRef] [Green Version]
- Choudoir, M.J.; Rossabi, S.; Gebert, M.; Helmig, D.; Fierer, N. A Phylogenetic and Functional Perspective on Volatile Organic Compound Production by Actinobacteria. MSystems 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial metabolites in nutrition, healthcare and agriculture. 3 Biotech 2017, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of Glucocorticoid-Induced Insulin Resistance. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.L.; Weiss, R.E. Steroid-induced diabetes: A clinical and molecular approach to understanding and treatment. Diabetes Metab. Res. Rev. 2014, 30, 96–102. [Google Scholar] [CrossRef]
- Gonçalves-Neto, L.M.; Ferreira, F.B.D.; Souza, L.; Dos Santos, C.; Boschero, A.C.; Facundo, V.A.; Santos, A.R.S.; Nunes, E.A.; Rafacho, A. Disruption of glucose tolerance caused by glucocorticoid excess in rats is partially prevented, but not attenuated, by arjunolic acid. Indian J. Exp. Biol. 2014, 52, 972–982. [Google Scholar]
- Martínez, B.B.; Pereira, A.C.C.; Muzetti, J.H.; Telles, F.D.P.; Mundim, F.G.L.; Teixeira, M.A. Experimental model of glucocorticoid-induced insulin resistance. Acta Cir. Bras. 2016, 31, 645–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Dong, R.R.; Zhong, K.L.; Ghosh, A.; Tang, S.S.; Long, Y.; Hu, M.; Miao, M.X.; Liao, J.M.; Sun, H.B.; et al. Antidiabetic drugs restore abnormal transport of amyloid-β across the blood–brain barrier and memory impairment in db/db mice. Neuropharmacology 2016, 101, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, N.; Patil, M.A.; Kumar, P.U.; Suryanarayana, P.; Reddy, G.B. Dietary ginger improves glucose dysregulation in a long-term high-fat high-fructose fed prediabetic rat model. Indian J. Exp. Biol. 2017, 55, 142–150. [Google Scholar] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, J.K.; Dubey, P.; Singh, S.; Bhat, H.R.; Kumawat, M.K.; Singh, U.P. Discovery of novel 1,3,5-triazine-thiazolidine-2,4-diones as dipeptidyl peptidase-4 (DPP-4) inhibitor targeting S1 pocket for the treatment of type 2 diabetes along with antibacterial activity. R. Soc. Chem. Adv. 2015, 5, 14095–14102. [Google Scholar] [CrossRef]
- Patel, B.D.; Bhadada, S.V.; Ghate, M. Design, synthesis and anti-diabetic activity of triazolotriazine derivatives as dipeptidyl peptidase-4 (DPP-4) inhibitors. Bioorg. Chem. 2017, 72, 345–358. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. Natural compounds with DPP-4 inhibitory effects: Implications for the treatment of diabetes. J. Cell. Biochem. 2019, 120, 10909–10913. [Google Scholar] [CrossRef]
- Hoda, Q.; Ahmad, S.; Akhtar, M.; Najmi, A.K.; Pillai, K.; Ahmad, S.J. Antihyperglycaemic and antihyperlipidaemic effect of poly-constituents, in aqueous and chloroform extracts, of Withaniacoagulans Dunal in experimental type 2 diabetes mellitus in rats. Hum. Exp. Toxicol. 2010, 29, 653–658. [Google Scholar] [CrossRef]
- Al-Qudah, M.M.A.; Haddad, M.A.; El-Qudah, J.M.F. The effects of aqueous ginger extract on pancreas histology and on blood glucose in normal and alloxan monohydrate-induced diabetic rats. Biomed. Res. 2016, 27, 350–356. [Google Scholar]
- Liu, R.; Cheng, J.; Wu, H. Discovery of Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: A Review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [Green Version]
- Quintero-Soto, M.F.; Saracho-Peña, A.G.; Chavez-Ontiveros, J.; Garzón-Tiznado, J.A.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Phenolic profiles and their contribution to the antioxidant activity of selected chickpea genotypes from Mexico and ICRISAT collections. Plant Foods Hum. Nutr. 2018, 73, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Passari, A.K.; Upadhyaya, K.; Singh, G.; Abdel-Azeem, A.; Thankappan, S.; Uthandi, S.; Hashem, A.; Abd_Allah, E.F.; Malik, J.A.; As, A.; et al. Enhancement of disease resistance, growth potential, and photosynthesis in tomato (Solanumlycopersicum) by inoculation with an endophyticactinobacterium, Streptomyces thermocarboxydus strain BPSAC147. PLoS ONE 2019, 14, e0219014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wu, Z.; He, Y.; Huang, Y.; Li, X.; Ye, B.-C. Plant growth promotion and alleviation of salinity stress in Capsicum annuum L. by Bacillus isolated from saline soil in Xinjiang. Ecotoxicol. Environ. Saf. 2018, 164, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Solanki, M.K.; Yu, Z.-X.; Yang, L.-T.; An, Q.-L.; Dong, D.-F.; Li, Y.-R. Draft Genome Analysis Offers Insights into the Mechanism by Which Streptomyces chartreusis WZS021 Increases Drought Tolerance in Sugarcane. Front. Microbiol. 2019, 9, 3262. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A. Three new cyclotetrapeptides isolated from Streptomyces sp. 447. Nat. Prod. Res. 2016, 31, 1014–1021. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.-S.; Cho, Y.-J.; Lee, K.; Yoon, S.-H.; Kim, M.; Na, H.; Park, S.-C.; Jeon, Y.S.; Lee, J.-H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and Antiproliferative Activities of Raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chiang, L.-C.; Chen, C.-C.; Liu, L.-T.; Wang, K.-C.; Lin, C.-C. Antileukemic Activity of Bidenspilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am. J. Chin. Med. 2001, 29, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thompson, M.D.; Gallagher, W.J.; Iaizzo, P.A.; Lanier, W.L. The Effect of Chronic Dexamethasone-induced Hyperglycemia and Its Acute Treatment with Insulin on Brain Glucose and Glycogen Concentrations in Rats. Anesthesiology 2000, 93, 1279–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoduwa, S.I.R.; Umar, I.A.; James, D.B.; Inuwa, H.M. Appropriate Insulin Level in Selecting Fortified Diet-Fed, Streptozotocin-Treated Rat Model of Type 2 Diabetes for Anti-Diabetic Studies. PLoS ONE 2017, 12, e0170971. [Google Scholar] [CrossRef]
- Jatwa, R.; Parmar, H.S.; Panda, S.; Kar, A. Amelioration of Corticosteroid-Induced Type 2 Diabetes Mellitus by Rosiglitazone Is Possibly Mediated through Stimulation of Thyroid Function and Inhibition of Tissue Lipid Peroxidation in Mice. Basic Clin. Pharmacol. Toxicol. 2007, 101, 177–180. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; De Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid. Based Complement. Altern. Med. 2013, 2013, 479505. [Google Scholar] [CrossRef] [Green Version]
- Ekayanti, M.; Sauriasari, R.; Elya, B. Dipeptidyl peptidase IV Inhibitory Activity of Fraction from White Tea Ethanolic Extract (Camellia sinensis (L.) Kuntze) ex vivo. Pharmacogn. J. 2017, 10, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Yalow, R.S.; Berson, S.A. Assay of Plasma Insulin in Human Subjects by Immunological Methods. Nature 1959, 184, 1648–1649. [Google Scholar] [CrossRef]
- Jeppsson, J.-O.; Kobold, U.; Barr, J.; Finke, A.; Hoelzel, W.; Hoshino, T.; Miedema, K.; Mosca, A.; Mauri, P.; Paroni, R.; et al. Approved IFCC Reference Method for the Measurement of HbA1c in Human Blood. Clin. Chem. Lab. Med. 2002, 40, 78–89. [Google Scholar] [CrossRef]
- Ojiako, O.A.; Chikezie, P.C.; Ogbuji, A.C. Blood glucose level and lipid profile of alloxan-induced hyperglycemic rats treated with single and combinatorial herbal formulations. J. Tradit. Complement. Med. 2015, 6, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic Determination of Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Nohtomi, K.; Koba, S.; Muroi, A.; Ito, Y. A simple and precise method for measuring HDL-cholesterol subfractions by a single precipitation followed by homogenous HDL-cholesterol assay. J. Lipid Res. 2008, 49, 1130–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotzsch, S.G.; McNamara, J.R. Triglyceride measurements: A review of methods and interferences. Clin. Chem. 1990, 36, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.K. Calculated Low Density Lipoprotein-Cholesterol: Friedewald’s Formula versus Other Modified Formulas. Int. J. Life Sci. Med. Res. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Haffner, S.M.; Miettinen, H.; Stern, M.P. The homeostasis model in the San Antonio Heart Study. Diabetes Care 1997, 20, 1087–1092. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Mantha, S.V.; Prasad, M.; Kalra, J.; Prasad, K. Antioxidant enzymes in hypercholesterolemia and effects of vitamin E in rabbits. Atherosclerosis 1993, 101, 135–144. [Google Scholar] [CrossRef]
- Kalkan, I.H.; Suher, M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak. J. Med. Sci. 2013, 29, 938–942. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2009, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Jatwa, R.; Kar, A. Amelioration of metformin-induced hypothyroidism by Withaniasomnifera and Bauhinia purpurea extracts in Type 2 diabetic mice. Phytother. Res. 2009, 23, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Sözmen, E.Y.; Sözmen, B.; Delen, Y.; Onat, T. Catalase/Superoxide Dismutase (SOD) and Catalase/Paraoxonase (PON) Ratios May Implicate Poor Glycemic Control. Arch. Med. Res. 2001, 32, 283–287. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Baskin, D.G. A Historical Perspective on the Identification of Cell Types in Pancreatic Islets of Langerhans by Staining and Histochemical Techniques. J. Histochem. Cytochem. 2015, 63, 543–558. [Google Scholar] [CrossRef] [Green Version]
- Elayat, A.A.; El-Naggar, M.M.; Tahir, M. An immunocytochemical and morphometric study of the rat pancreatic islets. J. Anat. 1995, 186, 629–637. [Google Scholar]
- Lal, K.; Purohit, A.; Ram, H. Insulin mimetic and pancreas-protective effect of Tecomella undulata leaves extract in diabetic rats. World J. Pharm. Pharm. Sci. 2017, 6, 924–938. [Google Scholar] [CrossRef]




| ROS/Strain | DBT33 | DBT34 | DBT35 | DBT36 | DBT37 | DBT39 |
|---|---|---|---|---|---|---|
| Catalase-like activity (Unit) | 64.31 | 158.21 | 49.27 | 19.49 | 65.5 | 39.4 |
| SOD-like activity (% of inhibition) | 53.51 | 55.15 | 59.84 | 61.89 | 56.47 | 61.75 |
| Sl.No | Compound Name | Formula | MW | RT | Height | Area % | Norm % | Structure | Activities & References |
|---|---|---|---|---|---|---|---|---|---|
| DBT34 | |||||||||
| 1 | ETHYL 6-[P-Chlorophenacylamino]-4-[[Diphenylmethyl]Amino]-5-Nitro-2 | C29H26O5N5Cl | 559 | 21.390 | 1,814,810 | 6.514 | 37.38 |  | - |
| 2 | Cathinone | C9H11ON | 149 | 21.635 | 1,398,351 | 5.655 | 32.45 | 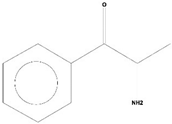 | Antioxidant activity [17] |
| 3 | 4-Chlorobenzoic Acid, 4-Hexadecyl Ester | C23H37O2Cl | 380 | 22.295 | 2,297,526 | 17.427 | 100.00 | 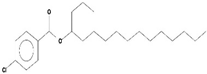 | - |
| 4 | 2,3-Dihydroxystearic Acid | C18H36O4 | 316 | 22.445 | 1,336,178 | 9.799 | 56.23 | 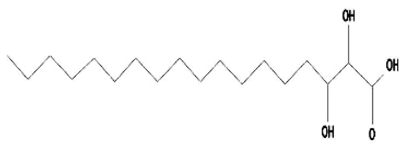 | - |
| 5 | Acetamide, 2-Amino | C2H6ON2 | 74 | 22.530 | 1,669,673 | 7.104 | 40.76 | 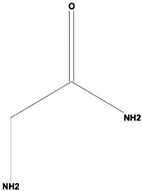 | Antioxidant activity [16] & anticancer activity [18] |
| 6 | 1,4-Cyclohexadiene, 1,3,6 Tris(Trimethylsilyl) | C15H32Si3 | 296 | 23.171 | 1,601,816 | 11.259 | 64.61 |  | - |
| 7 | Benzeneethanamine, N-[(Pentafluorophenyl)Methylene]-Beta,4-Bis [(trimethylsilyl)oxy] | C21H26O2NF5Si2 | 475 | 28.408 | 1,640,921 | 10.878 | 62.42 | 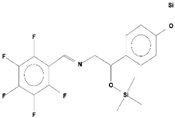 | - |
| 8 | Benzeneacetic Acid, Alpha-Oxo Trimethylsilyl Ester | C11H14O3Si | 222 | 28.488 | 2,008,596 | 5.629 | 32.30 | 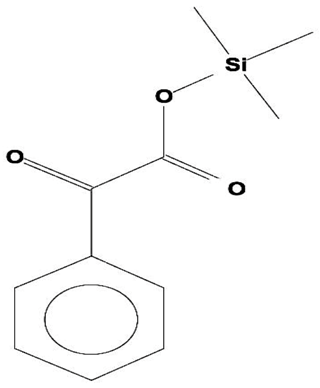 | |
| 9 | Benzeneacetic Acid, 4-Methoxy-Alpha-[(Trimethylsilyl)Oxy]- Methyl | C13H20O4Si | 268 | 28.688 | 1,468,754 | 5.683 | 32.61 |  | Anticancer activity [19]; Neuroprotective activity [20] |
| 10 | 1,2-Benzenedimethanethiol, S-Trimethylsilyl | C11H18S2Si | 242 | 28.793 | 1,568,043 | 6.188 | 35.51 | 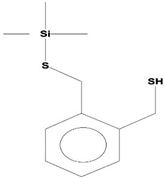 | |
| 11 | Trimethyl[4-(1,1,3,3,-Tetramethylbutyl)Phenoxy]Silane | C17H30OSi | 278 | 29.698 | 1,952,357 | 7.912 | 45.40 | 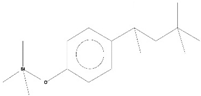 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passari, A.K.; Leo, V.V.; Singh, G.; Samanta, L.; Ram, H.; Siddaiah, C.N.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; Fathi Abd_Allah, E.; et al. In Vivo Studies of Inoculated Plants and In Vitro Studies Utilizing Methanolic Extracts of Endophytic Streptomyces sp. Strain DBT34 Obtained from Mirabilis jalapa L. Exhibit ROS-Scavenging and Other Bioactive Properties. Int. J. Mol. Sci. 2020, 21, 7364. https://doi.org/10.3390/ijms21197364
Passari AK, Leo VV, Singh G, Samanta L, Ram H, Siddaiah CN, Hashem A, Al-Arjani A-BF, Alqarawi AA, Fathi Abd_Allah E, et al. In Vivo Studies of Inoculated Plants and In Vitro Studies Utilizing Methanolic Extracts of Endophytic Streptomyces sp. Strain DBT34 Obtained from Mirabilis jalapa L. Exhibit ROS-Scavenging and Other Bioactive Properties. International Journal of Molecular Sciences. 2020; 21(19):7364. https://doi.org/10.3390/ijms21197364
Chicago/Turabian StylePassari, Ajit Kumar, Vincent Vineeth Leo, Garima Singh, Loknath Samanta, Heera Ram, Chandra Nayak Siddaiah, Abeer Hashem, Al-Bandari Fahad Al-Arjani, Abdulaziz A. Alqarawi, Elsayed Fathi Abd_Allah, and et al. 2020. "In Vivo Studies of Inoculated Plants and In Vitro Studies Utilizing Methanolic Extracts of Endophytic Streptomyces sp. Strain DBT34 Obtained from Mirabilis jalapa L. Exhibit ROS-Scavenging and Other Bioactive Properties" International Journal of Molecular Sciences 21, no. 19: 7364. https://doi.org/10.3390/ijms21197364






