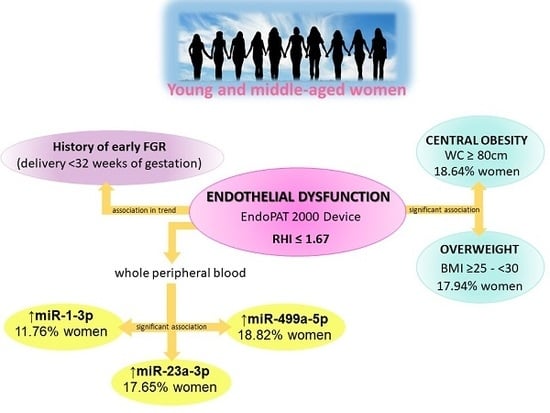
Evaluation of Vascular Endothelial Function in Young and Middle-Aged Women with Respect to a History of Pregnancy, Pregnancy-Related Complications, Classical Cardiovascular Risk Factors, and Epigenetics
Abstract
:1. Introduction
2. Results
2.1. Impact of A History of Pregnancy and Pregnancy-Related Complications on vascular Endothelial Function in Young and Middle-Aged Women
2.2. Impact of Classical Cardiovascular Risk Factors on Vascular Endothelial Function in Young and Middle-Aged Women
2.3. Association between Higher Expression Rates of miR-1–3p, miR-23a-3p, and miR-499a-5p in Whole Peripheral Blood and the Occurrence of Vascular Endothelial Dysfunction in Young and Middle-Aged Women
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Assessment of Vascular Endothelial Function
4.3. Blood Pressure Measurements
4.4. BMI and Waist Circumference Measurements
4.5. Biological Sampling
4.6. Gene Expression of Cardiovascular/Cerebrovascular diseAse Associated microRNAs in Whole Peripheral Blood
4.7. Statistical Analysis
5. Conclusions
6. Patent
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RHI | Reactive Hyperemia Index |
| BMI | Body Mass Index |
| FPR | False Positive Rate |
| GH | Gestational Hypertension |
| PE | Preeclampsia |
| FGR | Fetal Growth Restriction |
| w/o | without |
| w | with |
| HELLP | Hemolysis, Elevated Liver Enzymes, Low Platelet Count |
| WC | Waist Circumference |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| HDL | High-density Lipoprotein |
| LDL | Low-density Lipoprotein |
| Lp(a) | Lipoprotein A |
| CRP | C-reactive Protein) |
| FDA | Food and Drug Administration |
| SE | Standard Error |
| ANOVA | Analysis of Variance |
| ANCOVA | Analysis of Covariance |
| NTP | Normotensive Term Pregnancies |
| CI | Confidence Interval |
| OR | Odds Ratio |
| LDH | Lactate Dehydrogenase |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| AUC | Area under the Receive Operating Characteristic Curve |
| EFW | Estimated Fetal Weight |
| CS | Caesarean Section |
| PAT | Peripheral Arterial Tonometry |
| EDTA | Ethylenediaminetetraacetic Acid |
| LR + | Positive Likelihood Ratio |
| LR- | Negative Likelihood Ratio |
References
- Ray, J.G.; Vermeulen, M.J.; Schull, M.J.; Redelmeier, D.A. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet 2005, 366, 1797–1803. [Google Scholar] [CrossRef]
- Libby, G.; Murphy, D.J.; McEwan, N.F.; Greene, S.A.; Forsyth, J.S.; Chien, P.W.; Morris, A.D.; DARTS/MEMO Collaboration. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: An intergenerational study from the Walker cohort. Diabetologia 2007, 50, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykke, J.A.; Langhoff-Roos, J.; Sibai, B.M.; Funai, E.F.; Triche, E.W.; Paidas, M.J. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009, 53, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Männistö, T.; Mendla, P.; Vääräsmäki, M.; Järvelin, M.R.; Hartikainen, A.L.; Pouta, A.; Suvanto, E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013, 127, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Thilaganathan, B. Association of Higher Maternal Blood Pressure With Lower Infant Birthweight: Placental Cause or Cardiovascular Effect? Hypertension 2016, 67, 499–500. [Google Scholar] [CrossRef]
- Thilaganathan, B. Placental syndromes: Getting to the heart of the matter. Ultrasound Obstet. Gynecol. 2017, 49, 7–9. [Google Scholar] [CrossRef]
- Yang, J.J.; Lee, S.A.; Choi, J.Y.; Song, M.; Han, S.; Yoon, H.S.; Lee, Y.; Oh, J.; Lee, J.K.; Kang, D. Subsequent risk of metabolic syndrome in women with a history of preeclampsia: Data from the Health Examinees Study. J. Epidemiol. 2015, 25, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Udenze, I.C. Association of pre-eclampsia with metabolic syndrome and increased risk of cardiovascular disease in women: A systemic review. Niger. J. Clin. Pract. 2016, 19, 431–435. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [Green Version]
- Craici, I.M.; Wagner, S.J.; Hayman, S.R.; Garovic, V.D. Pre-eclamptic pregnancies: An opportunity to identify women at risk for future cardiovascular disease. Womens Health 2008, 4, 133–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Veerbeek, J.H.; Hermes, W.; Breimer, A.Y.; van Rijn, B.B.; Koenen, S.V.; Mol, B.W.; Franx, A.; de Groot, C.J.; Koster, M.P. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 2015, 65, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haukkamaa, L.; Moilanen, L.; Kattainen, A.; Luoto, R.; Kahonen, M.; Leinonen, M.; Jula, A.; Kesäniemi, Y.A.; Kaaja, R. Pre-eclampsia is a risk factor of carotid artery atherosclerosis. Cerebrovasc. Dis. 2009, 27, 599–607. [Google Scholar] [CrossRef]
- McDonald, S.D.; Ray, J.; Teo, K.; Jung, H.; Salehian, O.; Yusuf, S.; Lonn, E. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis 2013, 229, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Irgens, H.U.; Reisaeter, L.; Irgens, L.M.; Lie, R.T. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 2001, 323, 1213–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garovic, V.D.; Hayman, S.R. Hypertension in pregnancy: An emerging risk factor for cardiovascular disease. Nat. Clin. Pract. Nephrol. 2007, 3, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.L.; Cirillo, P.M.; John, B.A. Preeclampsia and cardiovascular disease death: Prospective evidence from the child health and development studies cohort. Hypertension 2010, 56, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borna, S.; Neamatipoor, E.; Radman, N. Risk of coronary artery disease in women with history of pregnancies complicated by preeclampsia and LBW. J. Matern. Fetal Neonatal Med. 2012, 25, 1114–1116. [Google Scholar] [CrossRef]
- Berks, D.; Hoedjes, M.; Raat, H.; Duvekot, J.J.; Steegers, E.A.; Habbema, J.D. Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: A literature-based study. BJOG 2013, 120, 924–931. [Google Scholar] [CrossRef]
- Carty, D.M.; Anderson, L.A.; Duncan, C.N.; Baird, D.P.; Rooney, L.K.; Dominiczak, A.F.; Delles, C. Peripheral arterial tone: Assessment of microcirculatory function in pregnancy. J. Hypertens. 2012, 30, 117–123. [Google Scholar] [CrossRef]
- Orabona, R.; Sciatti, E.; Vizzardi, E.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Endothelial dysfunction and vascular stiffness in women with previous pregnancy complicated by early or late pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 49, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Orabona, R.; Sciatti, E.; Vizzardi, E.; Bonadei, I.; Prefumo, F.; Valcamonico, A.; Metra, M.; Frusca, T. Maternal endothelial function and vascular stiffness after HELLP syndrome: A case-control study. Ultrasound Obstet. Gynecol. 2017, 50, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Namugowa, A.; Iputo, J.; Wandabwa, J.; Meeme, A.; Buga, G.A.B.; Abura, S.; Stofile, Y.Y. Arterial stiffness in women previously with preeclampsia from a semi-rural region of South Africa. Clin. Exp. Hypertens. 2019, 41, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kuvin, J.T.; Patel, A.R.; Sliney, K.A.; Pandian, N.G.; Sheffy, J.; Schnall, R.P.; Karas, R.H.; Udelson, J.E. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003, 146, 168–174. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R., Jr.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Maternal Cardiovascular Risk Assessment 3-to-11 Years Postpartum in Relation to Previous Occurrence of Pregnancy-Related Complications. J. Clin. Med. 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Llauradó, G.; Ceperuelo-Mallafré, V.; Vilardell, C.; Simó, R.; Albert, L.; Berlanga, E.; Vendrell, J.; González-Clemente, J.M. Impaired endothelial function is not associated with arterial stiffness in adults with type 1 diabetes. Diabetes Metab. 2013, 39, 355–362. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Postpartum profiling of microRNAs involved in pathogenesis of cardiovascular/cerebrovascular diseases in women exposed to pregnancy-related complications. Int. J. Cardiol. 2019, 291, 158–167. [Google Scholar] [CrossRef]
- Elmén, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjärn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M.; et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011, 585, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Small, E.M.; Sutherland, L.B.; Qi, X.; McAnally, J.; Plato, C.F.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009, 23, 2166–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhu, J.; Zhang, W.; Chen, Y.; Zhang, K.; Popescu, L.M.; Ma, X.; Lau, W.B.; Rong, R.; Yu, X.; et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 2011, 124, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.S.; Wang, H.Y.; Liao, Y.C.; Tsai, P.C.; Chen, K.C.; Cheng, H.Y.; Lin, R.T.; Juo, S.H. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc. Res. 2012, 95, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef]
- Ji, R.; Cheng, Y.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007, 100, 1579–1588. [Google Scholar] [CrossRef]
- Raitoharju, E.; Lyytikäinen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Doebele, C.; Bonauer, A.; Fischer, A.; Scholz, A.; Reiss, Y.; Urbich, C.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 2010, 115, 4944–4950. [Google Scholar] [CrossRef] [Green Version]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, S.; Kong, S.W.; Lu, J.; Bisping, E.; Zhang, H.; Allen, P.D.; Golub, T.R.; Pieske, B.; Pu, W.T. Altered microRNA expression in human heart disease. Physiol. Genom. 2007, 31, 367–373. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaumont, J.; López, B.; Hermida, N.; Schroen, B.; San José, G.; Heymans, S.; Valencia, F.; Gómez-Doblas, J.J.; De Teresa, E.; Díez, J.; et al. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-β1 up-regulation. Clin. Sci. (Lond.) 2014, 126, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Maitrias, P.; Metzinger-Le Meuth, V.; Nader, J.; Reix, T.; Caus, T.; Metzinger, L. The Involvement of miRNA in Carotid-Related Stroke. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1608–1617. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, M.; He, H.; Chen, J.; Zeng, H.; Li, J.; Duan, R. MicroRNA/mRNA profiling and regulatory network of intracranial aneurysm. BMC Med. Genom. 2013, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Bienertova-Vasku, J.; Novak, J.; Vasku, A. MicroRNAs in pulmonary arterial hypertension: Pathogenesis, diagnosis and treatment. J. Am. Soc. Hypertens. 2015, 9, 221–234. [Google Scholar] [CrossRef]
- Halkein, J.; Tabruyn, S.P.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.Q.; Scherr, M.; Castermans, K.; Malvaux, L.; Lambert, V.; Thiry, M.; et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investig. 2013, 123, 2143–2154. [Google Scholar] [CrossRef]
- Namugowa, A.V.; Meeme, A. PP017 Comparison of vascular function in preeclamptic and normotensive pregnant women in the rural eastern Cape province of South Africa. Pregnancy Hypertens. 2012, 2, 250–251. [Google Scholar] [CrossRef]
- Meeme, A.; Buga, G.A.; Mammen, M.; Namugowa, A. Endothelial dysfunction and arterial stiffness in pre-eclampsia demonstrated by the EndoPAT method. Cardiovasc. J. Afr. 2017, 28, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Mannaerts, D.; Faes, E.; Goovaerts, I.; Stoop, T.; Cornette, J.; Gyselaers, W.; Spaanderman, M.; Van Craenenbroeck, E.M.; Jacquemyn, Y. Flow-mediated dilation and peripheral arterial tonometry are disturbed in preeclampsia and reflect different aspects of endothelial function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R518–R525. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Cos, P.; Briedé, J.J.; Gyselaers, W.; Cornette, J.; Gorbanev, Y.; Bogaerts, A.; Spaanderman, M.; Van Craenenbroeck, E.; et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS ONE 2018, 13, e0202919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yinon, D.; Lowenstein, L.; Suraya, S.; Beloosesky, R.; Zmora, O.; Malhotra, A.; Pillar, G. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur. Respir. J. 2006, 27, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.F.; Lain, K.; Hansen, W.F.; Curry, T., Jr.; O’Brien, J.M. The use of digital peripheral artery tonometry to detect endothelial dysfunction in pregnant women who smoke. Am. J. Perinatol. 2014, 31, 113–118. [Google Scholar] [PubMed]
- Lobysheva, I.I.; Eeckhoudt, S.V.; Zotti, F.D.; Rifahi, A.; Pothen, L.; Beauloye, C.; Balligand, J.L. Clinical and biochemical data of endothelial function in Women Consuming Combined Contraceptives. Data Brief 2017, 13, 46–52. [Google Scholar] [CrossRef]
- Lowenstein, L.; Damti, A.; Pillar, G.; Shott, S.; Blumenfeld, Z. Evaluation of endothelial function in women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 134, 208–212. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Mammen, A.; Mooney, P.; Alsheikh-Ali, A.A.; Karas, R.H. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc. Med. 2007, 12, 13–16. [Google Scholar] [CrossRef]
- Truschel, E.; Jarczok, M.N.; Fischer, J.E.; Terris, D.D. High-throughput ambulatory assessment of digital reactive hyperemia: Concurrent validity with known cardiovascular risk factors and potential confounding. Prev. Med. 2009, 49, 468–472. [Google Scholar] [CrossRef]
- Ferré, R.; Aragonès, G.; Plana, N.; Merino, J.; Heras, M.; Buixadera, C.; Masana, L. High-density lipoprotein cholesterol and apolipoprotein A1 levels strongly influence the reactivity of small peripheral arteries. Atherosclerosis 2011, 216, 115–119. [Google Scholar] [CrossRef]
- Hamburg, N.M.; Palmisano, J.; Larson, M.G.; Sullivan, L.M.; Lehman, B.T.; Vasan, R.S.; Levy, D.; Mitchell, G.F.; Vita, J.A.; Benjamin, E.J. Relation of brachial and digital measures of vascular function in the community: The Framingham heart study. Hypertension 2011, 57, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Ravussin, E.; Johannsen, D.L.; Stull, A.J.; Cefalu, W.T.; Johnson, W.D. Endothelial Dysfunction: An Early Cardiovascular Risk Marker in Asymptomatic Obese Individuals with Prediabetes. Br. J. Med. Med. Res. 2012, 2, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Aversa, A.; Bruzziches, R.; Francomano, D.; Greco, E.A.; Violi, F.; Lenzi, A.; Donini, L.M. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J. Sex. Med. 2013, 10, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Cioni, G.; Boddi, M.; Fatini, C.; Romagnuolo, I.; Casini, A.; Gensini, G.F.; Abbate, R.; Sofi, F. Peripheral-arterial tonometry for assessing endothelial function in relation to dietary habits. J. Investig. Med. 2013, 61, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Hu, J.; Dong, Y.; Zhan, R.; Li, P.; Su, H.; Peng, Q.; Wu, T.; Lei, L.; Huang, X.; et al. Gender differences in the risk factors for endothelial dysfunction in Chinese hypertensive patients: Homocysteine is an independent risk factor in females. PLoS ONE 2015, 10, e0118686. [Google Scholar] [CrossRef]
- Michelsen, M.M.; Mygind, N.D.; Pena, A.; Aziz, A.; Frestad, D.; Høst, N.; Prescott, E. Steering Committee of the iPOWER Study. Peripheral Reactive Hyperemia Index and Coronary Microvascular Function in Women With no Obstructive CAD: The iPOWER Study. JACC Cardiovasc. Imaging 2016, 9, 411–417. [Google Scholar] [CrossRef]
- Kurozumi, A.; Okada, Y.; Arao, T.; Tanaka, Y. Excess Visceral Adipose Tissue Worsens the Vascular Endothelial Function in Patients with Type 2 Diabetes Mellitus. Intern. Med. 2016, 55, 3091–3095. [Google Scholar] [CrossRef] [Green Version]
- van der Heijden, D.J.; van Leeuwen, M.A.H.; Janssens, G.N.; Lenzen, M.J.; van de Ven, P.M.; Eringa, E.C.; van Royen, N. Body Mass Index Is Associated With Microvascular Endothelial Dysfunction in Patients With Treated Metabolic Risk Factors and Suspected Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6, e006082. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.L.; Seo, J.B.; Lee, J.Y.; Moon, M.K.; Chung, W.Y. Endothelial function estimated by digital reactive hyperemia in patients with atherosclerotic risk factors or coronary artery disease. Heart Vessels 2018, 33, 706–712. [Google Scholar] [CrossRef]
- Mori, H.; Okada, Y.; Kawaguchi, M.; Iwata, S.; Yoshikawa, M.; Sonoda, S.; Sugai, K.; Tanaka, K.; Hajime, M.; Narisawa, M.; et al. A Study of the Vascular Endothelial Function in Patients with Type 2 Diabetes Mellitus and Rheumatoid Arthritis. Intern. Med. 2019, 58, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Owei, I.; Umekwe, N.; Mohamed, H.; Ebenibo, S.; Wan, J.; Dagogo-Jack, S. Ethnic Disparities in Endothelial Function and Its Cardiometabolic Correlates: The Pathobiology of Prediabetes in A Biracial Cohort Study. Front. Endocrinol. 2018, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Czippelova, B.; Turianikova, Z.; Krohova, J.; Wiszt, R.; Lazarova, Z.; Pozorciakova, K.; Ciljakova, M.; Javorka, M. Arterial Stiffness and Endothelial Function in Young Obese Patients-Vascular Resistance Matters. J. Atheroscler. Thromb. 2019, 26, 1015–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taher, R.; Sara, J.D.; Heidari, B.; Toya, T.; Lerman, L.O.; Lerman, A. Metabolic syndrome is associated with peripheral endothelial dysfunction amongst men. Diabetes Metab. Syndr. Obes. 2019, 12, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, T.D.S.; Fernandes, J.F.R.; Araújo, L.D.S.; Nogueira, L.P.; Leal, P.M.; Antunes, V.P.; Rodrigues, M.L.G.; Valença, D.C.T.; Kaiser, S.E.; Klein, M.R.S.T. Serum Uric Acid Levels are Associated with Cardiometabolic Risk Factors in Healthy Young and Middle-Aged Adults. Arq. Bras. Cardiol. 2018, 111, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, J.; Lindholm, H.; Sinisalo, J.; Kuosma, E.; Halonen, J.; Hopsu, L.; Uitti, J. Association between lowered endothelial function measured by peripheral arterial tonometry and cardio-metabolic risk factors—A cross-sectional study of Finnish municipal workers at risk of diabetes and cardiovascular disease. BMC Cardiovasc. Disord. 2013, 13, 83. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.J.A.; Milne, B.J.; Ambler, A.; Theodore, R.; Ramrakha, S.; Caspi, A.; Moffitt, T.E.; Poulton, R. Childhood body mass index and endothelial dysfunction evaluated by peripheral arterial tonometry in early midlife. Int. J. Obes. 2017, 41, 1355–1360. [Google Scholar] [CrossRef] [Green Version]
- Randby, A.; Namtvedt, S.K.; Hrubos-Strøm, H.; Einvik, G.; Somers, V.K.; Omland, T. Sex-dependent impact of OSA on digital vascular function. Chest 2013, 144, 915–922. [Google Scholar] [CrossRef]
- Ertek, S.; Akgül, E.; Cicero, A.F.; Kütük, U.; Demirtaş, S.; Cehreli, S.; Erdoğan, G. 25-Hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Arch. Med. Sci. 2012, 8, 47–52. [Google Scholar] [CrossRef]
- Tang, D.; Bai, S.; Li, X.; Yao, M.; Gong, Y.; Hou, Y.; Li, J.; Yang, D. Improvement of microvascular endothelial dysfunction induced by exercise and diet is associated with microRNA-126 in obese adolescents. Microvasc. Res. 2019, 123, 86–91. [Google Scholar]
- Zhao, Y.; Samal, E.; Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005, 436, 214–220. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Rao, P.K.; Kumar, R.M.; Farkhondeh, M.; Baskerville, S.; Lodish, H.F. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc. Natl. Acad. Sci. USA 2006, 103, 8721–8726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Q.; Zhang, M.F.; Wen, H.Y.; Hu, C.L.; Liu, R.; Wei, H.Y.; Ai, C.M.; Wang, G.; Liao, X.X.; Li, X. Comparing the diagnostic values of circulating microRNAs and cardiac troponin T in patients with acute myocardial infarction. Clinics 2013, 68, 75–80. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zheng, J.; Sun, Y.; Wu, Z.; Liu, Z.; Huang, G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int. Heart J. 2009, 50, 377–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terentyev, D.; Belevych, A.E.; Terentyeva, R.; Martin, M.M.; Malana, G.E.; Kuhn, D.E.; Abdellatif, M.; Feldman, D.S.; Elton, T.S.; Györke, S. miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ. Res. 2009, 104, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Guo, X.; Long, C.L.; Li, C.; Zhang, Y.F.; Wang, J.; Wang, H. SUR2B/Kir6.1 channel openers correct endothelial dysfunction in chronic heart failure via the miR-1-3p/ET-1 pathway. Biomed. Pharmacother. 2019, 110, 431–439. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Cardiovascular and Cerebrovascular Disease Associated microRNAs Are Dysregulated in Placental Tissues Affected with Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction. PLoS ONE 2015, 10, e0138383. [Google Scholar] [CrossRef] [Green Version]
- Hromadnikova, I.; Kotlabova, K.; Ivankova, K.; Vedmetskaya, Y.; Krofta, L. Profiling of cardiovascular and cerebrovascular disease associated microRNA expression in umbilical cord blood in gestational hypertension, preeclampsia and fetal growth restriction. Int. J. Cardiol. 2017, 249, 402–409. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Gestational hypertension, preeclampsia and intrauterine growth restriction induce dysregulation of cardiovascular and cerebrovascular disease associated microRNAs in maternal whole peripheral blood. Thromb. Res. 2016, 137, 126–140. [Google Scholar] [CrossRef]
- Long, B.; Gan, T.Y.; Zhang, R.C.; Zhang, Y.H. miR-23a Regulates Cardiomyocyte Apoptosis by Targeting Manganese Superoxide Dismutase. Mol. Cells 2017, 40, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, W.; Wang, C. MiR-23a Regulates the Vasculogenesis of Coronary Artery Disease by Targeting Epidermal Growth Factor Receptor. Cardiovasc. Ther. 2016, 34, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, X.; Li, Y.; Lu, N.; Dai, Y.; Zhang, H.; Zhao, X.; Liu, Y. Resveratrol attenuates the inflammatory reaction induced by ischemia/reperfusion in the rat heart. Mol. Med. Rep. 2014, 9, 2528–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, K.N.; Umapathy, D.; Anandharaj, A.; Ravichandran, J.; Sasikumar, C.S.; Chandra, S.K.R.; Kesavan, R.; Kunka Mohanram, R. miR-23c regulates wound healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) among patients with diabetic foot ulcer. Microvasc. Res. 2020, 127, 103924. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L.; Sirc, J. Postnatal Expression Profile of microRNAs Associated with Cardiovascular and Cerebrovascular Diseases in Children at the Age of 3 to 11 Years in Relation to Previous Occurrence of Pregnancy-Related Complications. Int. J. Mol. Sci. 2019, 20, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.X.; Jiao, J.Q.; Li, Q.; Long, B.; Wang, K.; Liu, J.P.; Li, Y.R.; Li, P.F. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 2011, 17, 71–78. [Google Scholar] [CrossRef]
- Van Rooij, E.; Olson, E.N. MicroRNAs: Powerful new regulators of heart disease and provocative therapeutic targets. J. Clin. Investig. 2007, 117, 2369–2376. [Google Scholar] [CrossRef] [Green Version]
- Devaux, Y.; Vausort, M.; Goretti, E.; Nazarov, P.V.; Azuaje, F.; Gilson, G.; Corsten, M.F.; Schroen, B.; Lair, M.L.; Heymans, S.; et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin. Chem. 2012, 58, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Wang, Q.; You, W.; Chen, M.; Xia, J. MiRNAs as biomarkers of myocardial infarction: A meta-analysis. PLoS ONE 2014, 9, e88566. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Shen, B.; Li, J.; Lv, D.; Zhao, Y.; Wang, F.; Xu, J. Serum microRNA-499 and microRNA-208a as biomarkers of acute myocardial infarction. Int. J. Clin. Exp. Med. 2014, 7, 136–141. [Google Scholar]
- Toraih, E.A.; Hussein, M.H.; Al Ageeli, E.; Riad, E.; AbdAllah, N.B.; Helal, G.M.; Fawzy, M.S. Structure and functional impact of seed region variant in MIR-499 gene family in bronchial asthma. Respir. Res. 2017, 18, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Program, National High Blood Pressure Education. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000, 183, S1–S22. [Google Scholar] [CrossRef]
- Diagnosis and Management of Preeclampsia and Eclampsia. ACOG Practice Bulletin No. 33. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar]
- Espinoza, J.; Vidaeff, A.; Pettker, C.M.; Simhan, H. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar]
- Vyas, S.; Nicolaides, K.H.; Bower, S.; Campbell, S. Middle cerebral artery flow velocity waveforms in fetal hypoxaemia. Br. J. Obstet. Gynaecol. 1990, 97, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Cohn, H.E.; Sacks, E.J.; Heymann, M.A.; Rudolph, A.M. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am. J. Obstet. Gynecol. 1974, 120, 817–824. [Google Scholar] [CrossRef]
- Liz, S. Practice Guidelines: New AHA Recommendations for Blood Pressure Measurement. Am. Fam. Physician 2005, 72, 1391–1398. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]






| Endothelial Function (RHI) Mean (SE) | p Value | ||
|---|---|---|---|
| On blood pressure treatment [no (n = 253) vs. yes (n = 11)] | Unadjusted data | 2.046 (0.041) vs. 1.916 (0.195) | p = 0.516 |
| Adjusted data | 2.046 (0.041) vs. 1.921 (0.195) | p = 0.531 | |
| Trombophilic gene mutations [no (n = 246) vs. yes (n = 18)] | Unadjusted data | 2.042 (0.041) vs. 2.027 (0.153) | p = 0.924 |
| Adjusted data | 2.041 (0.041) vs. 2.036 (0.153) | p = 0.974 | |
| Current smoking of cigarettes [non-smokers + ex-smokers (n = 228) vs. smokers (n = 36)] | Unadjusted data | 2.028 (0.043) vs. 2.119 (0.108) | p = 0.435 |
| Adjusted data | 2.029 (0.043) vs. 2.113 (0.108) | p = 0.476 | |
| BMI [normal (n = 156) vs. abnormal (n = 108)] (<25 vs. ≥25) | Unadjusted data | 2.101 (0.052) vs. 1.953 (0.062) | p = 0.068 |
| Adjusted data | 2.099 (0.052) vs. 1.957 (0.062) | p = 0.082 | |
| BMI [normal (n = 156) vs. overweight (n = 68) vs. obese (n = 40)] (<25 vs. ≥ 25–29.9 vs. ≥30) | Unadjusted data | 2.101 (0.051) vs. 1.866 (0.078) vs. 2.102 (0.101) | Normal BMI vs. overweight p = 0.036 ↓ RHI in overweight women Normal BMI vs. obese p = 1.0 |
| Adjusted data | 2.099 (0.051) vs. 1.865 (0.078) vs. 2.117 (0.102) | Normal BMI vs. overweight p = 0.039 ↓ RHI in overweight women Normal BMI vs. obese p = 1.0 | |
| Waist circumference [normal (n = 146) vs. abnormal (n = 118)] (<80 cm vs. ≥ 80 cm) | Unadjusted data | 2.138 (0.053) vs. 1.920 (0.059) | p = 0.006 ↓ RHI in women with waist circumference ≥ 80 cm |
| Adjusted data | 2.135 (0.053) vs. 1.924 (0.059) | p = 0.008 ↓ RHI in women with waist circumference ≥ 80 cm | |
| SBP [normal (n = 241) vs. abnormal (n = 23)] (<140 mmHg vs. ≥ 140 mmHg) | Unadjusted data | 2.033 (0.042) vs. 2.123 (0.135) | p = 0.526 |
| Adjusted data | 2.032 (0.042) vs. 2.134 (0.135) | p = 0.471 | |
| SBP [normal (n = 138) vs. prehypertension (n = 103) vs. hypertension (n = 23)] (<120 mmHg vs. ≥120–139 mmHg vs. ≥ 140 mmHg) | Unadjusted data | 2.030 (0.055) vs. 2.037 (0.064) vs. 2.123 (0.135) | Normal SBP vs. prehypertension p = 1.0 Normal SBP vs. hypertension p = 1.0 |
| Adjusted data | 2.029 (0.055) vs. 2.035 (0.064) vs. 2.134 (0.136) | Normal SBP vs. prehypertension p = 1.0 Normal SBP vs. hypertension p = 1.0 | |
| DBP [normal (n = 229) vs. abnormal (n = 35)] (< 90 mmHg vs. ≥ 90 mmHg) | Unadjusted data | 2.035 (0.043) vs. 2.077 (0.110) | p = 0.721 |
| Adjusted data | 2.034 (0.043) vs. 2.083 (0.110) | p = 0.678 | |
| DBP [normal (n = 172) vs. prehypertension (n = 57) vs. hypertension (n = 35)] (< 80 mmHg vs. ≥80–89 mmHg vs. ≥ 90 mmHg) | Unadjusted data | 2.019 (0.050) vs. 2.084 (0.086) vs. 2.077 (0.110) | Normal DBP vs. prehypertension p = 1.0 Normal DBP vs. hypertension p = 1.0 |
| Adjusted data | 2.020 (0.050) vs. 2.076 (0.086) vs. 2.083 (0.110) | Normal DBP vs. prehypertension p = 1.0 Normal DBP vs. hypertension p = 1.0 | |
| Serum total cholesterol [normal (n = 125) vs. abnormal (n = 139)] (≤ 5 mmol/L vs. > 5 mmol/L) | Unadjusted data | 2.004 (0.058) vs. 2.074 (0.055) | p = 0.386 |
| Adjusted data | 1.999 (0.058) vs. 2.078 (0.055) | p = 0.329 | |
| Serum HDL cholesterol [normal (n = 230) vs. abnormal (n = 34)] (≥ 1.2 mmol/L vs. <1.2 mmol/L) | Unadjusted data | 2.046 (0.043) vs. 2.002 (0.111) | p = 0.710 |
| Adjusted data | 2.046 (0.043) vs. 2.006 (0.111) | p = 0.741 | |
| Serum LDL cholesterol [normal (n = 100) vs. abnormal (n = 164)] (≤ 3 mmol/L vs. > 3 mmol/L) | Unadjusted data | 1.983 (0.065) vs. 2.074 (0.051) | p = 0.267 |
| Adjusted data | 1.976 (0.065) vs. 2.079 (0.051) | p = 0.217 | |
| Serum triglycerides [normal (n = 248) vs. abnormal (n = 16)] (≤ 1.7 mmol/L vs. > 1.7 mmol/L) | Unadjusted data | 2.039 (0.041) vs. 2.075 (0.162) | p = 0.827 |
| Adjusted data | 2.038 (0.041) vs. 2.083 (0.162) | p = 0.786 | |
| Serum Lp(a) [normal (n = 212) vs. abnormal (n = 52)] (≤ 72.0 nmol/L vs. > 72.0 nmol/L) | Unadjusted data | 2.032 (0.045) vs. 2.076 (0.090) | p = 0.665 |
| Adjusted data | 2.031 (0.045) vs. 2.082 (0.090) | p = 0.609 | |
| Serum CRP [normal (n = 223) vs. abnormal (n = 41)] (≤ 5 mg/L vs. > 5 mg/L) | Unadjusted data | 2.032 (0.043) vs. 2.089 (0.101) | p = 0.608 |
| Adjusted data | 2.032 (0.043) vs. 2.088 (0.101) | p = 0.613 | |
| Plasma homocysteine [normal (n = 229) vs. abnormal (n = 35)] (≤ 13.6 μmol/L vs. > 13.6 μmol/L) | Unadjusted data | 2.054 (0.043) vs. 1.954 (0.109) | p = 0.397 |
| Adjusted data | 2.054 (0.043) vs. 1.954 (0.109) | p = 0.394 | |
| Serum uric acid [normal (n = 233) vs. abnormal (n = 31)] (≤339 μmol/L vs. > 339 μmol/L) | Unadjusted data | 2.064 (0.042) vs. 1.870 (0.116) | p = 0.117 |
| Adjusted data | 2.063 (0.042) vs. 1.877 (0.116) | p = 0.134 | |
| Current hormonal contraceptive use [non-users + ex-users (n = 194) vs. users (n = 70)] | Unadjusted data | 2.038 (0.047) vs. 2.049 (0.077) | p = 0.901 |
| Adjusted data | 2.041 (0.047) vs. 2.041 (0.078) | p = 0.991 | |
| Total number of pregnancies per patient [1 (n = 61) vs. 2 (n = 115) vs. 3+ (n = 88)] | Unadjusted data | 2.050 (0.083) vs. 2.117 (0.061) vs. 1.935 (0.069) | 1 pregnancy vs. 2 pregnancies p = 1.0 1 pregnancy vs. 3+ pregnancies p = 0.857 |
| Adjusted data | 2.044 (0.083) 2.114 (0.061) vs. 1.943 (0.071) | 1 pregnancy vs. 2 pregnancies p = 1.0 1 pregnancy vs. 3+ pregnancies p = 1.0 | |
| Total parity per patient [1 (n = 77) vs. 2 (n = 150) vs. 3+ (n = 37)] | Unadjusted data | 2.038 (0.074) vs. 2.071 (0.053) vs. 1.927 (0.107) | 1 child vs. 2 children p = 1.0 1 child vs. 3+ children p = 1.0 |
| Adjusted data | 2.038 (0.074) vs. 2.066 (0.053) vs. 1.943 (0.109) | 1 child vs. 2 children p = 1.0 1 child vs. 3+ children p = 1.0 | |
| Infertility treatment [no (n = 226) vs. yes (n = 38)] | Unadjusted data | 2.021 (0.043) vs. 2.161 (0.105) | p = 0.216 |
| Adjusted data | 2.017 (0.043) vs. 2.183 (0.106) | p = 0.149 | |
| Prevalence of Vascular Endothelial Dysfunction | Group 1 (%) | Group 2 (%) | p Value | OR (95% CI) |
|---|---|---|---|---|
| On blood pressure treatment [no (n = 253) vs. yes (n = 11)] | 83 (32.81%) | 3 (27.27%) | 0.702 | 0.768 (0.199–2.971) |
| Trombophilic gene mutations [no (n = 246) vs. yes (n = 18)] | 80 (32.52%) | 6 (33.33%) | 0.943 | 1.038 (0.376–2.865) |
| Current smoking of cigarettes [non-smokers + ex-smokers (n = 228) vs. smokers (n = 36)] | 78 (34.21%) | 8 (22.22%) | 0.158 | 0.550 (0.239–1.263) |
| BMI [normal (n = 156) vs. abnormal (n = 108)] (<25 vs. ≥25) | 48 (30.77%) | 38 (35.19%) | 0.452 | 1.221 (0.725–2.057) |
| BMI | ||||
| [normal (n = 156) vs. overweight (n = 68)] (<25 vs. ≥25–29.9) | 48 (30.77%) | 29 (42.65%) | 0.087 | 1.673 (0.929–3.014) |
| [normal (n = 156) vs. obese (n = 40)] (<25 vs. ≥30) | 48 (30.77%) | 9 (22.50%) | 0.307 | 0.653 (0.289–1.478) |
| Waist circumference [normal (n = 146) vs. abnormal (n = 118)] (<80 cm vs. ≥ 80 cm) | 41 (28.08%) | 45 (38.14%) | 0.084 | 1.579 (0.941–2.650) |
| SBP [normal (n = 241) vs. abnormal (n = 23)] (<140 mmHg vs. ≥140 mmHg) | 82 (34.02%) | 4 (17.39%) | 0.114 | 0.408 (0.134–1.240) |
| SBP | ||||
| [normal (n = 138) vs. prehypertension (n = 103)] (<120 mmHg vs. ≥120–139 mmHg) | 49 (35.51%) | 33 (32.04%) | 0.574 | 0.856 (0.498–1.471) |
| [normal (n = 138) vs. hypertension (n = 23)] (<120 mmHg vs. ≥140 mmHg) | 49 (35.51%) | 4 (17.39%) | 0.096 | 0.382 (0.123–1.188) |
| DBP [normal (n = 229) vs. abnormal (n = 35)] (<90 mmHg vs. ≥90 mmHg) | 79 (34.50%) | 7 (20.0%) | 0.094 | 0.475 (0.199–1.135) |
| DBP | ||||
| [normal (n = 172) vs. prehypertension (n = 57)] (<80 mmHg vs. ≥80–89 mmHg) | 62 (36.05%) | 17 (29.82%) | 0.393 | 0.754 (0.395–1.440) |
| [normal (n = 172) vs. hypertension (n = 35)] (<80 mmHg vs. ≥90 mmHg) | 62 (36.05%) | 7 (20.00%) | 0.072 | 0.444 (0.183–1.075) |
| Serum total cholesterol [normal (n = 125) vs. abnormal (n = 139)] (≤5 mmol/L vs. >5 mmol/L) | 39 (31.30%) | 47 (33.81%) | 0.651 | 1.127 (0.672–1.888) |
| Serum HDL cholesterol [normal (n = 230) vs. abnormal (n = 34)] (≥1.2 mmol/L vs. <1.2 mmol/L) | 75 (32.61%) | 11 (32.35) | 0.976 | 0.988 (0.458–2.134) |
| Serum LDL cholesterol [normal (n = 100) vs. abnormal (n = 164)] (≤3 mmol/L vs. >3 mmol/L) | 32 (32.0%) | 54 (32.91%) | 0.808 | 1.068 (0.628–1.817) |
| Serum triglycerides [normal (n = 248) vs. abnormal (n = 16)] (≤1.7 mmol/L vs. >1.7 mmol/L) | 81 (32.66%) | 5 (31.25%) | 0.907 | 0.937 (0.315–2.787) |
| Serum Lp(a) [normal (n = 212) vs. abnormal (n = 52)] (≤72.0 nmol/L vs. >72.0 nmol/L) | 71 (33.49%) | 15 (28.85%) | 0.522 | 0.805 (0.414–1.564) |
| Serum CRP [normal (n = 223) vs. abnormal (n = 41)] (≤5 mg/L vs. >5 mg/L) | 75 (33.63%) | 11 (26.83%) | 0.974 | 0.991 (0.586–1.678) |
| Plasma homocysteine [normal (n = 229) vs. abnormal (n = 35)] (≤13.6 μmol/L vs. >13.6 μmol/L) | 72 (31.44%) | 14 (40.0%) | 0.316 | 1.454 (0.700–3.021) |
| Serum uric acid [normal (n = 233) vs. abnormal (n = 31)] (≤339 μmol/L vs. >339 μmol/L) | 72 (30.90%) | 14 (45.16%) | 0.115 | 1.842 (0.861–3.938) |
| Current hormonal contraceptive use [non-users + ex-users (n = 194) vs. users (n = 70)] | 64 (32.99%) | 22 (31.43%) | 0.811 | 0.931 (0.518–1.674) |
| Total number of pregnancies per patient | ||||
| [1 (n = 61) vs. 2 (n = 115)] | 17 (27.87%) | 32 (27.83%) | 0.995 | 0.998 (0.499–1.995) |
| [1 (n = 61) vs. 3+ (n = 88)] | 17 (27.87%) | 37 (42.05%) | 0.078 | 1.878 (0.931–3.788) |
| Total parity per patient | ||||
| [1 (n = 77) vs. 2 (n = 150)] | 24 (31.17%) | 49 (32.67%) | 0.819 | 1.071 (0.593–1.934) |
| [1 (n = 77) vs. 3+ (n = 37)] | 24 (31.17%) | 13 (35.14%) | 0.672 | 1.196 (0.522–2.742) |
| Infertility treatment [no (n = 226) vs. yes (n = 38)] | 75 (33.19%) | 11 (28.95%) | 0.606 | 0.820 (0.386–1.743) |
| RHI (Non-Normal Distribution) | Data Distribution | Pearson Correlation Coefficient, p Value | Spearman’s Coefficient of Rank Correlation (rho), p Value |
|---|---|---|---|
| age | Non-normal distribution | - | ρ = −0.046, p = 0.457 |
| BMI | Non-normal distribution | - | ρ = −0.063, p = 0.307 |
| Waist circumference | Non-normal distribution | - | ρ = −0.081, p = 0.188 |
| SBP | Non-normal distribution | - | ρ = 0.079, p = 0.199 |
| DBP | Non-normal distribution | - | ρ = 0.044, p = 0.478 |
| Heart rate at rest | Non-normal distribution | - | ρ = 0.051, p = 0.407 |
| Serum total cholesterol | Non-normal distribution | - | ρ = 0.015, p = 0.810 |
| Serum HDL cholesterol | Non-normal distribution | - | ρ = −0.030, p = 0.631 |
| Serum LDL cholesterol | Non-normal distribution | - | ρ = −0.002, p = 0.973 |
| Serum triglycerides | Non-normal distribution | - | ρ = −0.008, p = 0.903 |
| Serum Lp(a) | Non-normal distribution | - | ρ = −0.002, p = 0.981 |
| Serum CRP | Non-normal distribution | - | ρ = 0.057, p = 0.357 |
| Plasma homocysteine | Non-normal distribution | - | ρ = −0.020, p = 0.753 |
| Serum uric acid | Normal distribution | r = −0.111, p = 0.072 | ρ = −0.111, p = 0.072 |
| Time elapsed since the delivery | Non-normal distribution | - | ρ = 0.014, p = 0.816 |
| Total number of pregnancies per patient | Non-normal distribution | - | ρ = −0.114, p = 0.064 |
| Total parity per patient | Non-normal distribution | - | ρ = −0.037, p = 0.548 |
| NTP (n = 74) | GH (n = 48) | PE (n = 114) | FGR (n = 28) | Diagnostic Groups (Normal vs. Diseased) | p Value (ANOVA, ANCOVA) | ||
| RHI | Unadjusted data | 2.066 (0.075) | 1.907 (0.093) | 2.094 (0.060) | 1.989 (0.122) | NTP vs. GH | p = 1.0 |
| NTP vs. PE | p = 1.0 | ||||||
| NTP vs. FGR | p = 1.0 | ||||||
| Adjusted data | 2.068 (0.075) A | 1.910 (0.093) A | 2.091 (0.060) A | 1.987 (0.122) A | NTP vs. GH | p = 1.0 | |
| NTP vs. PE | p = 1.0 | ||||||
| NTP vs. FGR | p = 1.0 | ||||||
| NTP (n = 74) | PE w/o SF (n = 28) | PE w SF (n = 86) | DiagnosticGroups (Normal vs. Diseased) | p Value (ANOVA, ANCOVA) | |||
| RHI | Unadjusted data | 2.066 (0.075) | 2.200 (0.121) | 2.059 (0.069) | NTP vs. PE w/o SF | p = 1.0 | |
| NTP vs. PE w SF | p = 1.0 | ||||||
| Adjusted data | 2.066 (0.075) A | 2.201 (0.122) A | 2.059 (0.069) A | NTP vs. PE w/o SF | p = 1.0 | ||
| NTP vs. PE w SF | p = 1.0 | ||||||
| NTP (n = 74) | Early PE (n = 40) | Late PE (n = 74) | DiagnosticGroups (Normal vs. Diseased) | p Value (ANOVA, ANCOVA) | |||
| RHI | Unadjusted data | 2.066 (0.075) | 2.074 (0.102) | 2.104 (0.075) | NTP vs. early PE | p = 1.0 | |
| NTP vs. late PE | p = 1.0 | ||||||
| Adjusted data | 2.066 (0.075) A | 2.074 (0.102) A | 2.105 (0.075) A | NTP vs. early PE | p = 1.0 | ||
| NTP vs. late PE | p = 1.0 | ||||||
| NTP (n = 74) | PE w/o HELLP (n = 98) | PE w HELLP (n = 16) | DiagnosticGroups (Normal vs. Diseased) | p Value (ANOVA, ANCOVA) | |||
| RHI | Unadjusted data | 2.066 (0.075) | 2.067 (0.065) | 2.256 (0.160) | NTP vs. PE w/o HELLP | p = 1.0 | |
| NTP vs. PE w HELLP | p = 0.848 | ||||||
| Adjusted data | 2.066 (0.075) A | 2.067 (0.065) A | 2.256 (0.161) A | NTP vs. PE w/o HELLP | p = 1.0 | ||
| NTP vs. PE w HELLP | p = 0.852 | ||||||
| NTP (n = 74) | PE w/o FGR (n = 98) | PE w FGR (n = 16) | DiagnosticGroups (Normal vs. Diseased) | p Value (ANOVA, ANCOVA) | |||
| RHI | Unadjusted data | 2.066 (0.075) | 2.104 (0.065) | 2.029 (0.161) | NTP vs. PE w/o FGR | p = 1.0 | |
| NTP vs. PE w FGR | p = 1.0 | ||||||
| Adjusted data | 2.066 (0.075) A | 2.104 (0.065) A | 2.028 (0.162) A | NTP vs. PE w/o FGR | p = 1.0 | ||
| NTP vs. PE w FGR | p = 1.0 | ||||||
| NTP (n = 74) | Early FGR (n = 9) | Late FGR (n = 19) | DiagnosticGroups (Normal vs. Diseased) | p Value (ANOVA, ANCOVA) | |||
| RHI | Unadjusted data | 2.066 (0.085) | 2.038 (0.243) | 1.965 (0.168) | NTP vs. early FGR | p = 1.0 | |
| NTP vs. late FGR | p = 1.0 | ||||||
| Adjusted data | 2.065 (0.085) A | 2.037 (0.245) A | 1.969 (0.169) A | NTP vs. early FGR | p = 1.0 | ||
| NTP vs. late FGR | p = 1.0 | ||||||
| Prevalence of Vascular Endothelial Dysfunction | Case, n (%) | NTP, n (%) | p Value | OR (95% CI) |
|---|---|---|---|---|
| Pregnancy-related complications irrespective of the severity of the disease | ||||
| PE | 29 (25.44%) | 24 (32.43%) | 0.299 | 0.711 (0.373–1.353) |
| GH | 18 (37.50%) | 24 (32.43%) | 0.565 | 1.250 (0.584–2.674) |
| FGR | 15 (53.57%) | 24 (32.43%) | 0.053 | 2.404 (0.989–5.842) |
| Women with a history of FGR with respect to the disease severity | ||||
| Early FGR | 6 (66.67%) | 24 (32.43%) | 0.057 | 4.167 (0.959–18.102) |
| Late FGR | 9 (47.37%) | 24 (32.43%) | 0.229 | 1.875 (0.674–5.219) |
| Women with a history of PE with respect to the disease severity | ||||
| PE w/o SF | 6 (21.43%) | 24 (32.43%) | 0.280 | 0.568 (0.204–1.585) |
| PE w SF | 23 (26.74%) | 24 (32.43%) | 0.432 | 0.761 (0.385–1.504) |
| Early PE | 13 (32.50%) | 24 (32.43%) | 0.994 | 1.003 (0.441–2.281) |
| Late PE | 16 (21.62%) | 24 (32.43%) | 0.141 | 0.575 (0.275–1.201) |
| PE w/o HELLP | 27 (27.55%) | 24 (32.43%) | 0.488 | 0.792 (0.410–1.530) |
| PE w HELLP | 2 (12.50%) | 24 (32.43%) | 0.128 | 0.298 (0.063–1.416) |
| PE w/o FGR | 24 (24.49%) | 24 (32.43%) | 0.251 | 0.676 (0.346–1.320) |
| PE w FGR | 5 (31.25%) | 24 (32.43%) | 0.927 | 0.947 (0.296–3.032) |
| microRNA | Normal Endothelial Function RHI > 1.67 (n = 178) Median (25th Percentile–75th Percentile) | Endothelial Dysfunction RHI ≤ 1.67 (n = 86) Median (25th Percentile–75th Percentile) | p Value |
|---|---|---|---|
| miR-1-3p | 4.170 (1.970–12.300) × 10−2 | 6.840 (3.780–13.000) × 10−2 | p = 0.008 |
| miR-16-5p | 0.868 (0.625–1.249) | 0.933 (0.708–1.147) | p = 0.548 |
| miR-17-5p | 1.044 (0.674–1.664) | 1.174 (0.820–1.669) | p = 0.421 |
| miR-20a-5p | 1.085 (0.550–1.633) | 0.995 (0.549–1.521) | p = 0.672 |
| miR-20b-5p | 1.051 (0.595–1.535) | 1.111 (0.564–1.592) | p = 0.677 |
| miR-21-5p | 0.180 (0.101–0.293) | 0.200 (0.133–0.295) | p = 0.215 |
| miR-23a-3p | 0.109 (0.051–0.200) | 0.123 (0.073–0.239) | p = 0.030 |
| miR-24-3p | 0.204 (0.139–0.293) | 0.213 (0.148–0.298) | p = 0.726 |
| miR-26a-5p | 0.346 (0.188–0.542) | 0.406 (0.248–0.536) | p = 0.163 |
| miR-29a-3p | 0.187 (0.112–0.335) | 0.211 (0.119–0.388) | p = 0.460 |
| miR-92a-3p | 1.759 (1.208–2.560) | 1.704 (1.144–2.589) | p = 0.759 |
| miR-100-5p | 0.975 (0.554–1.880) × 10−3 | 1.000 (0.514–1.720) × 10−3 | p = 0.950 |
| miR-103a-3p | 0.759 (0.397–1.294) | 0.944 (0.493–1.365) | p = 0.095 |
| miR-125b-5p | 0.257 (0.121–0.447) × 10−2 | 0.254 (0.140–0.449) × 10−2 | p = 0.808 |
| miR-126-3p | 0.178 (0.097–0.279) | 0.191 (0.115–0.279) | p = 0.442 |
| miR-130b-3p | 0.345 (0.177–0.677) | 0.379 (0.251–0.698) | p = 0.301 |
| miR-133a-3p | 7.440 (2.840–15.500) × 10−2 | 7.890 (3.870–18.600) × 10−2 | p = 0.221 |
| miR-143-3p | 1.320 (0.614–2.920) × 10−2 | 1.430 (0.792–2.990) × 10−2 | p = 0.485 |
| miR-145-5p | 7.450 (4.710–11.100) × 10−2 | 7.860 (4.630–12.000) × 10−2 | p = 0.758 |
| miR-146a-5p | 0.844 (0.482–1.267) | 0.845 (0.490–1.240) | p = 0.997 |
| miR-155-5p | 0.962 (0.680–1.448) | 1.129 (0.731–1.599) | p = 0.353 |
| miR-181a-5p | 0.153 (0.086–0.245) | 0.161 (0.093–0.254) | p = 0.708 |
| miR-195-5p | 3.240 (1.150–8.860) × 10−2 | 3.770 (1.360–9.730) × 10−2 | p = 0.600 |
| miR-199a-5p | 2.420 (1.320–5.180) × 10−2 | 2.880 (1.510–5.890) × 10−2 | p = 0.223 |
| miR-210-3p | 8.840 (5.680–14.300) × 10−2 | 9.150 (5.640–14.800) × 10−2 | p = 0.953 |
| miR-221-3p | 0.353 (0.190–0.601) | 0.340 (0.212–0.550) | p = 0.675 |
| miR-342-3p | 2.194 (1.409–3.206) | 2.296 (1.752–3.532) | p = 0.341 |
| miR-499a-5p | 9.400 (3.160–17.000) × 10−2 | 14.600 (3.710–34.700) × 10−2 | p = 0.010 |
| miR-574-3p | 0.108 (0.067–0.175) | 0.110 (0.081–0.175) | p = 0.375 |
| microRNA | Data Distribution RHI (Non-Normal Distribution) | Spearman’s Coefficient of Rank Correlation (rho), p Value |
|---|---|---|
| miR-1-3p | Non-normal distribution | ρ = −0.156, p = 0.012 weak negative correlation (↓ RHI ≈ ↑ miR-1-3p) |
| miR-16-5p | Non-normal distribution | ρ = −0.157, p = 0.361 |
| miR-17-5p | Non-normal distribution | ρ = −0.083, p = 0.179 |
| miR-20a-5p | Non-normal distribution | ρ = −0.034, p = 0.581 |
| miR-20b-5p | Non-normal distribution | ρ = −0.060, p = 0.331 |
| miR-21-5p | Non-normal distribution | ρ = −0.102, p = 0.102 |
| miR-23a-3p | Non-normal distribution | ρ = −0.154, p = 0.013 weak negative correlation (↓ RHI ≈ ↑ miR-23a-3p) |
| miR-24-3p | Non-normal distribution | ρ = −0.055, p = 0.381 |
| miR-26a-5p | Non-normal distribution | ρ = −0.101, p = 0.103 |
| miR-29a-3p | Non-normal distribution | ρ = −0.060, p = 0.332 |
| miR-92a-3p | Non-normal distribution | ρ = 0.025, p = 0.692 |
| miR-100-5p | Non-normal distribution | ρ = −0.002, p = 0.981 |
| miR-103a-3p | Non-normal distribution | ρ = −0.086, p = 0.165 |
| miR-125b-5p | Non-normal distribution | ρ = −0.001, p = 0.987 |
| miR-126-3p | Non-normal distribution | ρ = −0.047, p = 0.453 |
| miR-130b-3p | Non-normal distribution | ρ = −0.066, p = 0.291 |
| miR-133a-3p | Non-normal distribution | ρ = −0.054, p = 0.385 |
| miR-143-3p | Non-normal distribution | ρ = −0.104, p = 0.092 |
| miR-145-5p | Non-normal distribution | ρ = −0.052, p = 0.402 |
| miR-146a-5p | Non-normal distribution | ρ = −0.022, p = 0.727 |
| miR-155-5p | Non-normal distribution | ρ = −0.006, p = 0.918 |
| miR-181a-5p | Non-normal distribution | ρ = −0.051, p = 0.411 |
| miR-195-5p | Non-normal distribution | ρ = −0.073, p = 0.239 |
| miR-199a-5p | Non-normal distribution | ρ = −0.114, p = 0.065 |
| miR-210-3p | Non-normal distribution | ρ = −0.042, p = 0.505 |
| miR-221-3p | Non-normal distribution | ρ = −0.041, p = 0.512 |
| miR-342-3p | Non-normal distribution | ρ = −0.035, p = 0.572 |
| miR-499a-5p | Non-normal distribution | ρ = −0.193, p = 0.002 weak negative correlation (↓ RHI ≈ ↑ miR-499a-5p) |
| miR-574-3p | Non-normal distribution | ρ = −0.102, p = 0.100 |
| Normal Pregnancies (n = 74) | PE (n = 114) | FGR (n = 28) | GH (n = 48) | p-Value 1 | p-Value 2 | p-Value 3 | |
|---|---|---|---|---|---|---|---|
| At follow-up | |||||||
| Age (years) Age (range) | 38.49 ± 0.40 31–50 | 38.05 ± 0.41 28–52 | 38.11 ± 0.65 32–45 | 38.67 ± 0.68 31–58 | 1.000 | 1.000 | 1.000 |
| Time elapsed since delivery (years) | 5.74 ± 0.21 | 5.52 ± 0.21 | 5.25 ± 0.35 | 4.96 ± 0.31 | 1.000 | 1.000 | 0.259 |
| Family medical history Angina or heart attack in a first degree relative before the age of 60 years | 1 (1.35%) | 2 (1.75%) | 0 (0%) | 1 (2.08%) | - | - | - |
| Dispensarisation at Dpt. of Cardiology (valve problems and heart defects) | 0 (0%) | 1 (0.88%) Sinus tachycardia | 1 (3.45%) Leaky heart valve | 2 (4.17%) Mitral valve prolapse | - | - | - |
| On blood pressure treatment | 1 (1.35%) | 7 (6.14%) | 0 (0%) | 3 (6.25%) | - | - | - |
| Lipid-lowering medication | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - | - | - |
| DM type I | 0 (0%) | 1 (0.88%) | 0 (0%) | 1 (2.08%) | - | - | - |
| DM type II | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - | - | - |
| Rheumatoid arthritis | 0 (0%) | 0 (0%) | 1 (3.45%) | 2 (4.17%) | - | - | - |
| Chronic venous insufficiency | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.08%) | - | - | - |
| Thrombosis | 1 (1.35%) | 2 (1.75%) | 1 (3.45%) | 0 (0%) | - | - | - |
| Trombophilic gene mutations | 0 (0%) | 10 (8.77%) | 4 (14.29%) | 4 (8.33%) | - | - | - |
| Presence of risk factors for chronic kidney disease | 0 (%) | 1 (0.88%) Haematuria | 0 (0%) | 2 (4.17%) Abnormal kidney structure (n = 1) Glomerulonephritis in childhood (n = 1) | - | - | - |
| Chronic kidney disease | 0 (%) | 1 (0.88%) Nephrotic syndrome | 0 (0%) | 0 (0%) | - | - | - |
| Smoking of cigarettes | |||||||
| Non-Smoker | 46 (62.16%) | 70 (61.40%) | 22 (78.57%) | 31 (64.58%) | - | - | - |
| Ex-smoker | 17 (22.97%) | 28 (24.56%) | 2 (7.14%) | 12 (25.0%) | - | - | - |
| Smoker | 11 (14.86%) | 16 (14.04%) | 4 (14.29%) | 5 (10.42%) | - | - | - |
| BMI | |||||||
| Normal (<25) | 56 (75.68%) | 62 (54.39%) | 19 (67.86%) | 19 (39.58%) | - | - | - |
| Overweight (≥25-<30) | 15 (20.27%) | 32 (28.07%) | 3 (10.71%) | 18 (37.50%) | - | - | - |
| Obese (≥30) | 3 (4.05%) | 20 (17.54%) | 6 (21.43%) | 11 (22.92%) | - | - | - |
| Waist circumference | |||||||
| Normal (< 80cm) | 54 (72.97%) | 59 (51.75%) | 18 (64.29%) | 15 (31.25%) | - | - | - |
| Central Obesity (≥80 cm) | 20 (27.03%) | 55 (48.25%) | 10 (35.71%) | 33 (68.75%) | - | - | - |
| SBP | |||||||
| Normal (<120 mmHg) | 56 (75.68%) | 48 (42.11%) | 17 (60.71%) | 17 (35.42%) | - | - | - |
| Prehypertension (≥120–<140 mmHg) | 18 (24.32%) | 53 (46.49%) | 11 (39.29%) | 21 (43.75%) | - | - | - |
| Hypertension (≥140 mmHg) | 0 (0%) | 13 (11.40%) | 0 (0%) | 10 (20.83%) | - | - | - |
| DBP | |||||||
| Normal (<80 mmHg) | 66 (89.19%) | 65 (57.01%) | 18 (64.29%) | 23 (47.92%) | - | - | - |
| Prehypertension (≥80–<90 mmHg) | 6 (8.11%) | 30 (26.32%) | 7 (25.0%) | 14 (29.16%) | - | - | - |
| Hypertension (≥90 mmHg) | 2 (2.70%) | 19 (16.67%) | 3 (10.71%) | 11 (22.92%) | - | - | - |
| Heart rate at rest | |||||||
| Bradycardia (< 60 bpm) | 7 (9.46%) | 6 (5.26%) | 3 (10.71%) | 4 (8.33%) | - | - | - |
| Normal (60–100 bpm) | 66 (89.19%) | 108 (94.74%) | 25 (89.29%) | 42 (87.50%) | - | - | - |
| Tachycardia (>100 bpm) | 1 (1.35%) | 0 (0%) | 0 (0%) | 2 (4.17%) | - | - | - |
| Serum total cholesterol | |||||||
| Normal (2.9–5.0 mmol/L) | 35 (47.30%) | 53 (46.49%) | 15 (53.57%) | 22 (45.83%) | - | - | - |
| High (>5.0 mmol/L) | 39 (52.70%) | 61 (53.51%) | 13 (46.43%) | 26 (54.17%) | - | - | - |
| Serum HDL cholesterol | |||||||
| Normal (1.2–2.7 mmol/L) | 69 (93.24%) | 100 (87.72%) | 24 (85.71%) | 37 (77.08%) | - | - | - |
| Low (<1.2 mmol/L) | 5 (6.76%) | 14 (12.28%) | 4 (14.29%) | 11 (22.92%) | - | - | - |
| Serum LDL cholesterol | |||||||
| Normal (1.2–3.0 mmol/L) | 35 (47.30%) | 38 (33.33%) | 12 (42.86%) | 15 (31.25%) | - | - | - |
| High (>3.0 mmol/L) | 39 (52.70%) | 76 (66.67%) | 16 (57.14%) | 33 (68.75%) | - | - | - |
| Serum triglycerides | |||||||
| Normal (0.45–1.7 mmol/L) | 73 (98.65%) | 105 (92.11%) | 25 (89.29%) | 45 (93.75%) | - | - | - |
| High (>1.7 mmol/L) | 1 (1.35%) | 9 (7.89%) | 3 (10.71%) | 3 (6.25%) | - | - | - |
| Serum Lp(a) | |||||||
| Normal (0–72.0 nmol/L) | 63 (85.14%) | 85 (74.56%) | 22 (78.57%) | 42 (87.50%) | - | - | - |
| High (>72.0 nmol/L) | 11 (14.86%) | 29 (25.44%) | 6 (21.43%) | 6 (12.50%) | - | - | - |
| Serum CRP | |||||||
| Normal (0–5.0 mg/L) | 69 (93.24%) | 93 (81.58%) | 20 (71.43%) | 41 (85.42%) | - | - | - |
| High (>5.0 mg/L) | 5 (6.76%) | 21 (18.42%) | 8 (28.57%) | 7 (14.58%) | - | - | - |
| Plasma homocysteine | |||||||
| Normal (4.4–13.6 µmol/L) | 64 (86.49%) | 98 (85.96%) | 23 (82.14%) | 44 (91.67%) | - | - | - |
| High (>13.6 µmol/L) | 10 (13.51%) | 16 (14.04%) | 5 (17.86%) | 4 (8.33%) | - | - | - |
| Serum uric acid | |||||||
| Normal (143–339 µmol/L) | 71 (95.95%) | 99 (86.84%) | 22 (78.57%) | 41 (85.42%) | - | - | - |
| High (>339 µmol/L) | 3 (4.05%) | 15 (13.16%) | 6 (21.43%) | 7 (14.58%) | - | - | - |
| Hormonal contraceptive use | |||||||
| No | 26 (35.14%) | 29 (25.44%) | 7 (25.0%) | 15 (31.25%) | - | - | - |
| In the past | 25 (33.78%) | 54 (47.37%) | 12 (42.86%) | 26 (54.17%) | - | - | - |
| Yes | 23 (31.08%) | 31 (27.19%) | 9 (32.14%) | 7 (14.58%) | - | - | - |
| Total number of pregnancies per patient | |||||||
| 1 | 7 (9.46%) | 37 (32.46%) | 8 (28.57%) | 9 (18.75%) | - | - | - |
| 2 | 35 (47.30%) | 46 (40.35%) | 13 (46.43%) | 21 (43.75%) | - | - | - |
| 3+ | 32 (43.24%) | 31 (27.19%) | 7 (25.0%) | 18 (37.50%) | - | - | - |
| Total parity per patient | |||||||
| 1 | 11 (14.86%) | 39 (34.21%) | 11 (39.29%) | 16 (33.33%) | - | - | - |
| 2 | 50 (67.57%) | 59 (51.75%) | 15 (53.57%) | 26 (54.17%) | - | - | - |
| 3+ | 13 (17.57%) | 16 (14.04%) | 2 (7.14%) | 6 (12.50%) | - | - | - |
| Infertility treatment | |||||||
| Yes | 3 (4.05%) | 23 (20.18%) | 6 (21.43%) | 6 (12.50%) | - | - | - |
| No | 71 (95.95%) | 91 (79.82%) | 22 (78.57%) | 42 (87.50%) | - | - | - |
| During gestation | |||||||
| Maternal age at delivery (years) | 32.78 ± 0.38 | 32.26 ± 0.41 | 32.86 ± 0.58 | 33.65 ± 0.61 | 1.000 | 1.000 | 1.000 |
| GA at delivery (weeks) | 39.85 ± 0.10 | 35.91 ± 0.33 | 35.23 ± 0.67 | 38.64 ± 0.21 | <0.001 | <0.001 | 0.106 |
| Fetal birth weight (g) | 3390.14 ± 41.55 | 2403.45 ± 80.99 | 1831.43 ± 125.48 | 3226.46 ± 69.50 | <0.001 | <0.001 | 1.000 |
| Mode of delivery | |||||||
| Vaginal | 69 (93.24%) | 19 (16.67%) | 6 (21.43%) | 21 (43.75%) | <0.001 | <0.001 | <0.001 |
| CS | 5 (6.76%) | 95 (83.33%) | 23 (78.57%) | 27 (56.25%) | |||
| Fetal sex | |||||||
| Boy | 37 (50.00%) | 49 (42.98%) | 15 (53.57%) | 23 (47.92%) | 0.345 | 0.747 | 0.822 |
| Girl | 37 (50.00%) | 65 (57.02%) | 13 (46.43%) | 25 (52.08%) | |||
| Blood pressure (mmHg) | |||||||
| Systolic | 120.70 ± 1.13 | 157.32 ± 1.49 | 127.21 ± 3.06 | 148.73 ± 2.17 | <0.001 | 0.232 | <0.001 |
| Diastolic | 75.85 ± 0.76 | 98.71 ± 1.01 | 78.07 ± 2.18 | 94.96 ± 1.45 | <0.001 | 1.000 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Evaluation of Vascular Endothelial Function in Young and Middle-Aged Women with Respect to a History of Pregnancy, Pregnancy-Related Complications, Classical Cardiovascular Risk Factors, and Epigenetics. Int. J. Mol. Sci. 2020, 21, 430. https://doi.org/10.3390/ijms21020430
Hromadnikova I, Kotlabova K, Dvorakova L, Krofta L. Evaluation of Vascular Endothelial Function in Young and Middle-Aged Women with Respect to a History of Pregnancy, Pregnancy-Related Complications, Classical Cardiovascular Risk Factors, and Epigenetics. International Journal of Molecular Sciences. 2020; 21(2):430. https://doi.org/10.3390/ijms21020430
Chicago/Turabian StyleHromadnikova, Ilona, Katerina Kotlabova, Lenka Dvorakova, and Ladislav Krofta. 2020. "Evaluation of Vascular Endothelial Function in Young and Middle-Aged Women with Respect to a History of Pregnancy, Pregnancy-Related Complications, Classical Cardiovascular Risk Factors, and Epigenetics" International Journal of Molecular Sciences 21, no. 2: 430. https://doi.org/10.3390/ijms21020430