GAGA Regulates Border Cell Migration in Drosophila
Abstract
:1. Introduction
2. Results
2.1. Molecular Characterization of Chromosomes Carrying Trl362 and Trl3609 Mutations
2.2. Decrease in Trl Expression Delays BC Migration
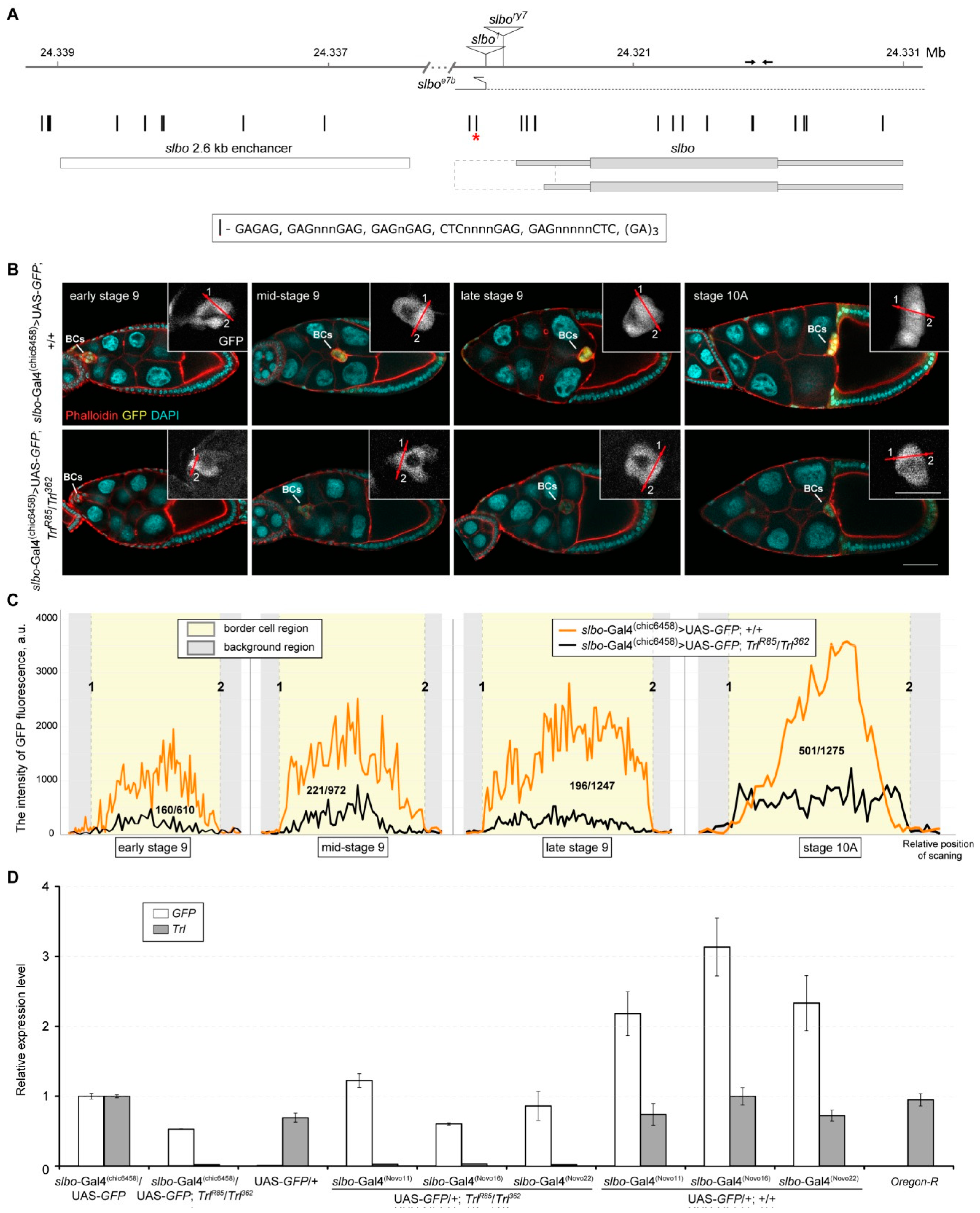
2.3. GAGA Regulates Transcriptional Activity of the slbo Gene during Migration of BCs
2.4. Genetic Interaction between Trl and slbo Genes
2.5. Transcriptional Activity of the slbo-Gal4 Drivers Depends on the GAGA Protein Level
2.6. Increase in the slbo Expression Level in Trl Mutants Enhances BC Migration Defects
2.7. Trl Expression Does Not Depend on Slbo
3. Discussion
4. Materials and Methods
4.1. Fly Stocks
4.2. Identification and Verification of P Element Transgene Insertion Sites
4.3. Generation of UAS-slbo Transgenic Flies
4.4. Immunofluorescent Staining
4.5. Quantitative Measurement of GFP Signal Intensity
4.6. Detection of BCs and Quantitative Analysis of Their Migration
4.7. Total RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rørth, P. Initiating and Guiding Migration: Lessons from Border Cells. Trends Cell Biol. 2002, 12, 325–331. [Google Scholar] [CrossRef]
- Montell, D.J. Border-Cell Migration: The Race Is on. Nat. Rev. Mol. Cell Biol. 2003, 4, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Rørth, P. Collective Cell Migration. Annu. Rev. Cell Dev. Biol. 2009, 25, 407–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montell, D.J.; Yoon, W.H.; Starz-Gaiano, M. Group Choreography: Mechanisms Orchestrating the Collective Movement of Border Cells. Nat. Rev. Mol. Cell Biol. 2012, 13, 631–645. [Google Scholar] [CrossRef] [Green Version]
- Spradling, A.C. Germline Cysts: Communes That Work. Cell 1993, 72, 649–651. [Google Scholar] [CrossRef]
- Ruohola, H.; Bremer, K.A.; Baker, D.; Swedlow, J.R.; Jan, L.Y.; Jan, Y.N. Role of Neurogenic Genes in Establishment of Follicle Cell Fate and Oocyte Polarity during Oogenesis in Drosophila. Cell 1991, 66, 433–449. [Google Scholar] [CrossRef]
- Margolis, J.; Spradling, A. Identification and Behavior of Epithelial Stem Cells in the Drosophila Ovary. Development 1995, 121, 3797–3807. [Google Scholar]
- Tworoger, M.; Larkin, M.K.; Bryant, Z.; Ruohola-Baker, H. Mosaic Analysis in the Drosophila Ovary Reveals a Common Hedgehog-Inducible Precursor Stage for Stalk and Polar Cells. Genetics 1999, 151, 739–748. [Google Scholar]
- Montell, D.J.; Rorth, P.; Spradling, A.C. Slow Border Cells, a Locus Required for a Developmentally Regulated Cell Migration during Oogenesis, Encodes Drosophila C/EBP. Cell 1992, 71, 51–62. [Google Scholar] [CrossRef]
- Saadin, A.; Starz-Gaiano, M. Circuitous Genetic Regulation Governs a Straightforward Cell Migration. Trends Genet. 2016, 32, 660–673. [Google Scholar] [CrossRef]
- Silver, D.L.; Montell, D.J. Paracrine Signaling through the JAK/STAT Pathway Activates Invasive Behavior of Ovarian Epithelial Cells in Drosophila. Cell 2001, 107, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Ghiglione, C.; Devergne, O.; Georgenthum, E.; Carballès, F.; Médioni, C.; Cerezo, D.; Noselli, S. The Drosophila Cytokine Receptor Domeless Controls Border Cell Migration and Epithelial Polarization during Oogenesis. Development 2002, 129, 5437–5447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beccari, S.; Teixeira, L.; Rørth, P. The JAK/STAT Pathway Is Required for Border Cell Migration during Drosophila Oogenesis. Mech. Dev. 2002, 111, 115–123. [Google Scholar] [CrossRef]
- Xi, R.; McGregor, J.R.; Harrison, D.A. A Gradient of JAK Pathway Activity Patterns the Anterior-Posterior Axis of the Follicular Epithelium. Dev. Cell 2003, 4, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sexton, T.R.; Venard, C.; Giedt, M.; Guo, Q.; Chen, Q.; Harrison, D.A. Pleiotropy of the Drosophila JAK Pathway Cytokine Unpaired 3 in Development and Aging. Dev. Biol. 2014, 395, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Arbouzova, N.I.; Zeidler, M.P. JAK/STAT Signalling in Drosophila: Insights into Conserved Regulatory and Cellular Functions. Development 2006, 133, 2605–2616. [Google Scholar] [CrossRef] [Green Version]
- Amoyel, M.; Anderson, A.M.; Bach, E.A. JAK/STAT Pathway Dysregulation in Tumors: A Drosophila Perspective. Semin. Cell Dev. Biol. 2014, 28, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Silver, D.L.; Geisbrecht, E.R.; Montell, D.J. Requirement for JAK/STAT Signaling Throughout Border Cell Migration in Drosophila. Development 2005, 132, 3483–3492. [Google Scholar] [CrossRef] [Green Version]
- Borghese, L.; Fletcher, G.; Mathieu, J.; Atzberger, A.; Eades, W.C.; Cagan, R.L.; Rørth, P. Systematic Analysis of the Transcriptional Switch Inducing Migration of Border Cells. Dev. Cell 2006, 10, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bo, J.; Bridges, T.; Dugan, K.D.; Pan, T.-C.; Chodosh, L.A.; Montell, D.J. Analysis of Cell Migration Using Whole-Genome Expression Profiling of Migratory Cells in the Drosophila Ovary. Dev. Cell 2006, 10, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Starz-Gaiano, M.; Melani, M.; Wang, X.; Meinhardt, H.; Montell, D.J. Feedback Inhibition of JAK/STAT Signaling by Apontic Is Required to Limit an Invasive Cell Population. Dev. Cell 2008, 14, 726–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Reyes, A.; St Johnston, D. Patterning of the Follicle Cell Epithelium along the Anterior-Posterior Axis during Drosophila Oogenesis. Development 1998, 125, 2837–2846. [Google Scholar] [PubMed]
- Levine, B.; Jean-Francois, M.; Bernardi, F.; Gargiulo, G.; Dobens, L. Notch Signaling Links Interactions Between the C/EBP Homolog Slow Border Cells and the GILZ Homolog Bunched During Cell Migration. Dev. Biol. 2007, 305, 217–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawan, F.; Arandjelovic, M.; Godt, D. The Maf Factor Traffic Jam Both Enables and Inhibits Collective Cell Migration in Drosophila Oogenesis. Development 2013, 140, 2808–2817. [Google Scholar] [CrossRef] [Green Version]
- Rørth, P.; Szabo, K.; Texido, G. The Level of C/EBP Protein Is Critical for Cell Migration During Drosophila Oogenesis and Is Tightly Controlled by Regulated Degradation. Mol. Cell 2000, 6, 23–30. [Google Scholar] [CrossRef]
- Rørth, P.; Montell, D.J. Drosophila C/EBP: A Tissue-Specific DNA-Binding Protein Required for Embryonic Development. Genes Dev. 1992, 6, 2299–2311. [Google Scholar] [CrossRef]
- Levine, B.; Hackney, J.F.; Bergen, A.; Dobens III, L.; Truesdale, A.; Dobens, L. Opposing Interactions Between Drosophila Cut and the C/EBP Encoded by Slow Border Cells Direct Apical Constriction and Epithelial Invagination. Dev. Biol. 2010, 344, 196–209. [Google Scholar] [CrossRef] [Green Version]
- Melani, M.; Simpson, K.J.; Brugge, J.S.; Montell, D.J. Regulation of Cell Adhesion and Collective Cell Migration by Hindsight and Its Human Homolog RREB1. Curr. Biol. 2008, 18, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Rørth, P.; Szabo, K.; Bailey, A.; Laverty, T.; Rehm, J.; Rubin, G.M.; Weigmann, K.; Milán, M.; Benes, V.; Ansorge, W.; et al. Systematic Gain-of-Function Genetics in Drosophila. Development 1998, 125, 1049–1057. [Google Scholar]
- Ogienko, A.A.; Yarinich, L.A.; Fedorova, E.V.; Lebedev, M.O.; Andreyeva, E.N.; Pindyurin, A.V.; Baricheva, E.M. New Slbo-Gal4 Driver Lines for the Analysis of Border Cell Migration during Drosophila Oogenesis. Chromosoma 2018, 127, 475–487. [Google Scholar] [CrossRef]
- Ogienko, A.A.; Karagodin, D.A.; Fedorova, S.A.; Fedorova, E.V.; Lashina, V.V.; Baricheva, E.M. Analysis of a Novel Hypomorphic Mutation in Trithorax-Like Gene Affecting Drosophila Melanogaster Oogenesis. Russ. J. Dev. Biol. 2006, 37, 173–181. [Google Scholar] [CrossRef]
- Ogienko, A.A.; Karagodin, D.A.; Pavlova, N.V.; Fedorova, S.A.; Voloshina, M.V.; Baricheva, E.M. Molecular and Genetic Description of a New Hypomorphic Mutation of Trithorax-Like Gene and Analysis of Its Effect on Drosophila Melanogaster Oogenesis. Russ. J. Dev. Biol. 2008, 39, 108–115. [Google Scholar] [CrossRef]
- Fedorova, E.V.; Dorogova, N.V.; Bolobolova, E.U.; Fedorova, S.A.; Karagodin, D.A.; Ogienko, A.A.; Khruscheva, A.S.; Baricheva, E.M. GAGA Protein Is Required for Multiple Aspects of Drosophila Oogenesis and Female Fertility. Genesis 2018, 57, e23269. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.; Gausz, J.; Galloni, M.; Reuter, G.; Gyurkovics, H.; Karch, F. The Trithorax-Like Gene Encodes the Drosophila GAGA Factor. Nature 1994, 371, 806–808. [Google Scholar] [CrossRef]
- Granok, H.; Leibovitch, B.A.; Shaffer, C.D.; Elgin, S.C.R. Chromatin: Ga-Ga over GAGA Factor. Curr. Biol. 1995, 5, 238–241. [Google Scholar] [CrossRef] [Green Version]
- Berger, N.; Dubreucq, B. Evolution Goes GAGA: GAGA Binding Proteins across Kingdoms. Biochim. et Biophys. Acta (BBA)—Gene Regul. Mech. 2012, 1819, 863–868. [Google Scholar] [CrossRef]
- Tsukiyama, T.; Becker, P.B.; Wu, C. ATP-Dependent Nucleosome Disruption at a Heat-Shock Promoter Mediated by Binding of GAGA Transcription Factor. Nature 1994, 367, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, R.C.; Lis, J.T. DNA Distortion and Multimerization: Novel Functions of the Glutamine-Rich Domain of GAGA Factor. J. Mol. Biol. 1999, 285, 515–525. [Google Scholar] [CrossRef]
- Bhat, K.M.; Farkas, G.; Karch, F.; Gyurkovics, H.; Gausz, J.; Schedl, P. The GAGA Factor Is Required in the Early Drosophila Embryo Not Only for Transcriptional Regulation But Also for Nuclear Division. Development 1996, 122, 1113–1124. [Google Scholar]
- Hoskins, R.A.; Carlson, J.W.; Wan, K.H.; Park, S.; Mendez, I.; Galle, S.E.; Booth, B.W.; Pfeiffer, B.D.; George, R.A.; Svirskas, R.; et al. The Release 6 Reference Sequence of the Drosophila Melanogaster genome. Genome Res. 2015, 25, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Assaker, G.; Ramel, D.; Wculek, S.K.; González-Gaitán, M.; Emery, G. Spatial Restriction of Receptor Tyrosine Kinase Activity through a Polarized Endocytic Cycle Controls Border Cell Migration. Proc. Natl. Acad. Sci. USA 2010, 107, 22558–22563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, A.J.; Schedl, P. GAGA Factor Isoforms Have Distinct But Overlapping Functions In Vivo. Mol. Cell. Biol. 2001, 21, 8565–8574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omelina, E.S.; Baricheva, E.M.; Oshchepkov, D.Y.; Merkulova, T.I. Analysis and Recognition of the GAGA Transcription Factor Binding Sites in Drosophila Genes. Comput. Biol. Chem. 2011, 35, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R. Regulation of Cell Migration in Atherosclerosis. Curr. Atheroscler. Rep. 2000, 2, 445–452. [Google Scholar] [CrossRef]
- Mehlen, P.; Puisieux, A. Metastasis: A Question of Life or Death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef]
- Friedl, P.; Gilmour, D. Collective Cell Migration in Morphogenesis, Regeneration and Cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef]
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying Collective Cancer Cell Invasion. Nat. Cell Biol. 2012, 14, 777–783. [Google Scholar] [CrossRef]
- Chen, D.; Dale, R.K.; Lei, E.P. Shep Regulates Drosophila neuronal Remodeling by Controlling Transcription of Its Chromatin Targets. Development 2018, 145, dev154047. [Google Scholar] [CrossRef] [Green Version]
- Matzat, L.H.; Dale, R.K.; Moshkovich, N.; Lei, E.P. Tissue-Specific Regulation of Chromatin Insulator Function. PLoS Genet. 2012, 8, e1003069. [Google Scholar] [CrossRef] [Green Version]
- Bellen, H.J.; Kooyer, S.; D’Evelyn, D.; Pearlman, J. The Drosophila Couch Potato Protein Is Expressed in Nuclei of Peripheral Neuronal Precursors and Shows Homology to RNA-Binding Proteins. Genes Dev. 1992, 6, 2125–2136. [Google Scholar] [CrossRef] [Green Version]
- Bellen, H.J.; Vaessin, H.; Bier, E.; Kolodkin, A.; D’Evelyn, D.; Kooyer, S.; Jan, Y.N. The Drosophila Couch Potato Gene: An Essential Gene Required for Normal Adult Behavior. Genetics 1992, 131, 365–375. [Google Scholar] [PubMed]
- Luo, J.; Zuo, J.; Wu, J.; Wan, P.; Kang, D.; Xiang, C.; Zhu, H.; Chen, J. In Vivo RNAi Screen Identifies Candidate Signaling Genes Required for Collective Cell Migration in Drosophila Ovary. Sci. China Life Sci. 2015, 58, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischof, J.; Maeda, R.K.; Hediger, M.; Karch, F.; Basler, K. An Optimized Transgenesis System for Drosophila Using Germ-Line-Specific phiC31 Integrases. Proc. Natl. Acad. Sci. USA 2007, 104, 3312–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagodin, D.A.; Battulina, N.V.; Merkulova, T.I.; Baricheva, E.M. The Reasons of Trithorax-Like Expression Disturbance in Trl (3609) Allele of Drosophila Melanogaster. Dokl. Biochem. Biophys. 2016, 471, 443–446. [Google Scholar] [CrossRef]
- Pindyurin, A.V. Genome-Wide Cell Type-Specific Mapping of In Vivo Chromatin Protein Binding Using an FLP-Inducible DamID System in Drosophila. Methods Mol. Biol. 2017, 1654, 99–124. [Google Scholar]
- Ringrose, L. Transgenesis in Drosophila Melanogaster. Methods Mol. Biol. 2009, 561, 3–19. [Google Scholar]
- Xue, F.; Cooley, L. Kelch Encodes a Component of Intercellular Bridges in Drosophila Egg Chambers. Cell 1993, 72, 681–693. [Google Scholar] [CrossRef]
- Guild, G.M.; Connelly, P.S.; Shaw, M.K.; Tilney, L.G. Actin Filament Cables in Drosophila Nurse Cells Are Composed of Modules That Slide Passively Past One Another During Dumping. J. Cell Biol. 1997, 138, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Mao, F.; Lv, X.; Zhang, Z.; Fu, L.; Lu, Y.; Wu, W.; Zhou, Z.; Zhang, L.; Zhao, Y. Drosophila Vps36 Regulates Smo Trafficking in Hedgehog Signaling. J. Cell Sci. 2013, 126, 4230–4238. [Google Scholar] [CrossRef] [Green Version]




| Target Gene | Primer Sequence (5′->3′) | Reference | Amplicon Size, bp | Primer Efficiency, % |
|---|---|---|---|---|
| GFP | AGATCATATGAAACGGCATGACT | [30] | 124 | 100.5 |
| ACCTTCAAACTTGACTTCAGCAC | ||||
| slbo | GACAAGGGCACGGATGAGTA | This study | 198 | 100.0 |
| CTGCATGTAGATCTGCTTGTGT | ||||
| Trl | TTTCCCGCCCACAAGATAGT | This study | 118 | 97.0 |
| CCAGATCGTTCGCATTGACG | ||||
| RpL32 | CTAAGCTGTCGCACAAATGG | [59] | 148 | 99.6 |
| AGGAACTTCTTGAATCCGGTG | ||||
| Rap2l | TCTTGGAAATATTGGACACCGC | [30] | 197 | 102.0 |
| TTTGTTCGCGACTAGTAGGATG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogienko, A.A.; Yarinich, L.A.; Fedorova, E.V.; Dorogova, N.V.; Bayborodin, S.I.; Baricheva, E.M.; Pindyurin, A.V. GAGA Regulates Border Cell Migration in Drosophila. Int. J. Mol. Sci. 2020, 21, 7468. https://doi.org/10.3390/ijms21207468
Ogienko AA, Yarinich LA, Fedorova EV, Dorogova NV, Bayborodin SI, Baricheva EM, Pindyurin AV. GAGA Regulates Border Cell Migration in Drosophila. International Journal of Molecular Sciences. 2020; 21(20):7468. https://doi.org/10.3390/ijms21207468
Chicago/Turabian StyleOgienko, Anna A., Lyubov A. Yarinich, Elena V. Fedorova, Natalya V. Dorogova, Sergey I. Bayborodin, Elina M. Baricheva, and Alexey V. Pindyurin. 2020. "GAGA Regulates Border Cell Migration in Drosophila" International Journal of Molecular Sciences 21, no. 20: 7468. https://doi.org/10.3390/ijms21207468






