Repellent and Antifeedant Activities of Citral-Derived Lactones against the Peach Potato Aphid
Abstract
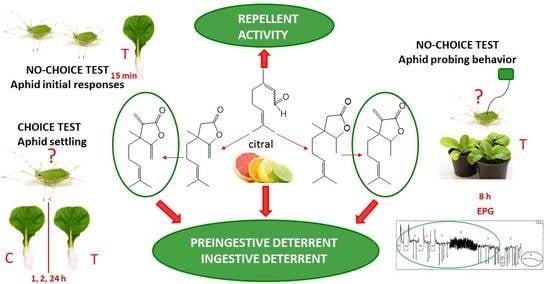
:1. Introduction
2. Results
2.1. Synthesis of Citral-Derived Lactones
2.2. Repellent and Feeding Deterrent Activities against Myzus Persicae
2.2.1. Aphid Settling (Choice-Test for Freely Moving Aphids)
2.2.2. Aphid Early Behavioral Responses (No-Choice Test for Freely Moving Aphids)
2.2.3. Aphid Probing and Feeding (No-Choice Test for Tethered Aphids)
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Lactones
4.1.1. Reagents
4.1.2. Purification and Analysis
4.1.3. Spectroscopic Analyses
4.2. Biological Studies
4.2.1. Cultures of Aphids and Plants
4.2.2. Preparation and Application of Compounds
4.2.3. Aphid Settling Deterrent Activity (Choice-Test for Freely Moving Aphids)
4.2.4. Aphid Repellent Activity (No-Choice Test for Freely Moving Aphids)
4.2.5. Probing and Feeding Deterrent Activity (No-Choice Test for Tethered Aphids)
4.2.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/news/story/en/item/1187738/icode/ (accessed on 9 September 2020).
- Wellings, P.W.; Ward, S.A.; Dixon, A.F.G.; Rabbinge, R. Crop loss assessment. In Aphids, Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherland, 1989; Volume C, pp. 49–64. [Google Scholar]
- Culliney, T. Crop losses to arthropods. In Integrated Pest Management; Pimentel, K., Peshin, R., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2014; pp. 201–224. [Google Scholar]
- Blackman, R.; Eastop, V.F. Taxonomic issues. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CAB International: Wallingford, UK, 2017; pp. 1–36. [Google Scholar]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Attempts to control aphid pests by integrated use of semiochemicals. In Brighton Crop Protection Conference—Pests and Diseases; British Crop Protection Council: Thornton Heath, UK, 1994; pp. 1239–1246. [Google Scholar]
- Isman, M. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Southwell, I.A.; Russell, M.; Smith, R.L.; Archer, D.W. Backhousia citriodora F. Muell. (Myrtaceae), a superior source of citral. J. Essent. Oil Res. 2000, 12, 735–741. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P. Review on the chemical composition of Litsea cubeba essential oils and the bioactivity of its major constituents citral and limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Punruckvong, A.; Bean, A.R.; Forster, P.I.; Lepschi, B.J.; Doran, J.C.; Rozefelds, A.C. Leaf essential oils of the genus Leptospermum (Myrtaceae) in eastern Australia. Part 7. Leptospermum petersonii, L. liversidgei and allies. Flavour Frag. J. 2000, 15, 342–351. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Cornell, J.A.; Preston, J.F.; Wei, C.-L. Antibacterial activity of carvacrol, citral and geraniol against Salmonella typhimurium in culture medium and on fish cubes. J. Food Sci. 1995, 60, 1364–1374. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Rauber, C.S.; Guterres, S.S.; Schapoval, E.E.S. LC determination of citral in Cymbopogon citrates volatile oil. J. Pharmaceut. Biomed. 2005, 37, 597–601. [Google Scholar] [CrossRef]
- Arias, R.J.; Schmeda-Hirschmann, G.; Falcao, A. Feeding deterrence and insecticidal effects of plant extracts on Lutzomyia longipalpis. Phytother. Res. 1992, 6, 64–67. [Google Scholar] [CrossRef]
- Salvatore, A.; Borkosky, S.; Willink, E.; Bardon, A. Toxic effects of lemon peel constituents on Ceratitis capitata. J. Chem. Ecol. 2004, 30, 323–333. [Google Scholar] [CrossRef]
- Choi, W.; Park, B.; Lee, Y.; Jang, D.Y.; Yoon, H.Y.; Lee, S. Fumigant toxicities of essential oils and monoterpenes against Lycoriella mali adults. Crop Prot. 2006, 25, 398–401. [Google Scholar] [CrossRef]
- Price, D.N.; Berry, M.S. Comparison of effects of octopamine and insecticidal essential oils on activity in the nerve cord, foregut, and dorsal unpaired median neurons of cockroaches. J. Insect Physiol. 2006, 52, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.C.R.; Henderson, G.; Chen, F.; Fei, H.; Laine, R.A. Evaluation of vetiver oil and seven insect-active essential oils against the Formosan subterranean termite. J. Chem. Ecol. 2001, 27, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Oyedele, A.O.; Gbolade, A.A.; Sosan, M.B.; Adewoyin, F.B.; Soyelu, O.L.; Orafidiya, O.O. Formulation of an effective mosquito-repellent topical product from Lemongrass oil. Phytomedicine 2002, 9, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Gabrys, B.; Dancewicz, K.; Halarewicz-Pacan, A.; Janusz, E. Effect of natural monoterpenes on behaviour of the peach potato aphid Myzus persicae (Sulz.). IOBC/WPRS Bull. 2005, 28, 29–34. [Google Scholar]
- Ley, S.V.; Toogood, P.L. Insect antifeedants. Chem. Br. 1990, 1, 31–35. [Google Scholar]
- Isman, M. Insect antifeedants. Pestic. Outlook 2002, 13, 152–157. [Google Scholar] [CrossRef]
- Szczepanik, M.; Szumny, A.; Grudniewska, A.; Wawrzeńczyk, C. Feeding deterrent activity of alpha-methylenelactones against the lesser mealworm (Alphitobius diaperinus Panzer). Pestycydy 2005, 4, 25–32. [Google Scholar]
- Korp, J.D.; Bernal, I.; Fischer, N.H.; Leonard, C.; Lee, I.Y.; LeVan, N. New guaianolides from Berlandiera pumila and B. texana, and the X-ray crystal structure of pumilin. J. Heterocycl. Chem. 1982, 19, 181–187. [Google Scholar] [CrossRef]
- Al-Massarani, S.M. Phytochemical and biological properties of sesquiterpene constituents from the marine red seaweed Laurencia: A review. Nat. Prod. Chem. Res. 2014, 2, 147. [Google Scholar]
- Miyazawa, M.; Shimabayashi, H.; Hayashi, S.; Hashimoto, S.; Nakamura, S.I.; Kosaka, H.; Kameoka, H. Synthesis and biological activity of α-methylene-γ-lactones as new aroma chemicals. J. Agric. Food Chem. 2000, 48, 5406–5410. [Google Scholar] [CrossRef]
- Asakawa, Y. Chemical constituents of the bryophytes. In Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, W.B., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer: Vienna, Austria, 1995; pp. 1–618. [Google Scholar]
- Gudjónsdóttir, G.A.; Ingólfsdóttir, K. Quantitative determination of protolichesterinic-and fumarprotocetraric acids in Cetraria islandica by high-performance liquid chromatography. J. Chromatogr. A 1997, 757, 303–306. [Google Scholar] [CrossRef]
- Quintana, J.; Estévez, F. Recent advances on cytotoxic sesquiterpene lactones. Curr. Pharm. Des. 2018, 24, 4355–4361. [Google Scholar] [CrossRef]
- Janecka, A.; Wyrębska, A.; Gach, K.; Fichna, J.; Janecki, T. Natural and synthetic α-methylenelactones and α-methylenelactams with anticancer potential. Drug Discov. Today 2012, 17, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, J.; Harmatha, J. Phytochemical feeding deterrents for stored product insect pests. Phytochem. Rev. 2012, 11, 543–566. [Google Scholar] [CrossRef]
- Padilla-Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Ciunik, Z.; Gabryś, B.; Wawrzeńczyk, C. Synthesis of piperitone-derived halogenated lactones and their effect on aphid probing, feeding, and settling behavior. RSC Adv. 2011, 1, 498–510. [Google Scholar] [CrossRef]
- Dams, I.; Białońska, A.; Ciunik, Z.; Wawrzeńczyk, C. Lactones. 21. Synthesis and odoriferous properties of lactones with the p-menthane system. J. Agric. Food Chem. 2004, 52, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Paruch, E.; Ciunik, Z.; Nawrot, J.; Wawrzeńczyk, C. Lactones. 9. Synthesis of terpenoid lactones active insect antifeedants. J. Agric. Food Chem. 2000, 48, 4973–4977. [Google Scholar] [CrossRef]
- Paruch, E.; Ciunik, Z.; Wawrzeńczyk, C. Lactones, 2. Synthesis of Enantiomeric pairs of lactones with the Pinane or the Fenchane system. Liebigs Ann. Chem. 1997, 11, 2341–2345. [Google Scholar] [CrossRef]
- Johnson, W.S.; Werthemann, L.; Bartlett, W.R.; Brocksom, T.J.; Li, T.; Faulkner, D.J.; Petersen, M.R. Simple stereoselective version of the Claisen rearrangement leading to trans-trisubstituted olefinic bonds. Synthesis of squalene. J. Am. Chem. Soc. 1970, 92, 741–743. [Google Scholar] [CrossRef]
- Srikrishna, A.; Nagaraju, S.; Kondiah, P. Application of microwave heating technique for rapid synthesis of -unsaturated esters. Tetrahedron 1995, 51, 1809–1816. [Google Scholar] [CrossRef]
- Mori, K.; Nakazono, Y. Pheromone synthesis. CV: Synthesis of lactone components of the pheromone of Anastrepha suspensa, suspensolide, and the enantiomers of anastrephin and epianastrephin. Liebigs Ann. Chem. 1988, 2, 167–174. [Google Scholar] [CrossRef]
- Fernández-Mateos, A.; de Pascual Teresa, J.; Rubio González, R. Synthesis of 8β-methyltestololactone. J. Chem. Soc. Perkin Trans. I 1990, 9, 2429–2435. [Google Scholar] [CrossRef]
- Parker, W.L.; Johnson, F. Total synthesis of dl-avenaciolide. J. Org. Chem. 1973, 38, 2489–2496. [Google Scholar] [CrossRef]
- Szumny, A.; Wawrzeńczyk, C. Lactones, part 28: A new approach for the synthesis of α-methylene lactones from alkenes. Synlett 2006, 10, 1523–1526. [Google Scholar] [CrossRef]
- Guntrum, E.; Kuhn, W.; Spönlein, W.; Jaeger, V. Synthesis of 2-Penten-4-olides, 3-Penten-4-olides, and 4,4-Dialkyl-, 1,3-cyclopentanediones by acid-and base-induced isomerization of 4-penten-4-olides (γ-Methylene-γ-butyrolactones). Synthesis 1986, 921–925. [Google Scholar] [CrossRef]
- Harrewijn, P. Resistance mechanisms of plant genotypes to various aphid species. In Aphid-Plant Genotype Interactions; Campbell, R.K., Eikenbary, R.D., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1990; pp. 117–130. [Google Scholar]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Developing sustainable pest control from chemical ecology. Agric. Ecosyst. Environ. 1997, 64, 149–156. [Google Scholar] [CrossRef]
- Isman, M. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [Green Version]
- Torto, B. Chemical signals as attractants, repellents and aggregation stimulants. In Chemical Ecology; Hardege, J.D., Ed.; Eoloss Publishers Co. Ltd.: Oxford, UK, 2009; pp. 186–199. [Google Scholar]
- Frazier, J.L.; Chyb, S. Use of feeding inhibitors in insect control. In Regulatory Mechanisms in Insect Feeding; Chapman, R.F., de Boer, G., Eds.; Chapman & Hall: New York, NY, USA, 1995; pp. 364–381. [Google Scholar]
- Tamiru, A.; Khan, Z.R. Volatile Semiochemical mediated plant defense in cereals: A novel strategy for crop protection. Agronomy 2017, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Leszczyński, B. Rola allelozwiązków w oddziaływaniach owady-rośliny. In Biochemiczne Oddziaływania Środowiskowe; Oleszek, W., Głowniak, K., Leszczyński, B., Eds.; Akademia Medyczna: Lublin, Poland, 2001; pp. 61–85. [Google Scholar]
- Gabryś, B.; Pawluk, M. Acceptability of different species of Brassicaceae as hosts for the cabbage aphid. Entomol. Exp. Appl. 1999, 91, 105–109. [Google Scholar] [CrossRef]
- Gabrys, B.; Tjallingii, W.F. The role of sinigrin in host plant recognition by aphids during initial plant penetration. Entomol. Exp. Appl. 2002, 104, 89–93. [Google Scholar] [CrossRef]
- Kordan, B.; Stec, K.; Słomiński, P.; Giertych, M.J.; Wróblewska-Kurdyk, A.; Gabryś, B. Susceptibility of forage legumes to infestation by the pea aphid Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae). Crop Pasture Sci. 2018, 69, 775–784. [Google Scholar] [CrossRef]
- Paprocka, M.; Gliszczyńska, A.; Dancewicz, K.; Gabryś, B. Novel hydroxy- and epoxy-cis-jasmone and dihydrojasmone derivatives affect the foraging activity of the peach potato aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Molecules 2018, 23, 2362. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Kurdyk, A.; Gniłka, R.; Dancewicz, K.; Grudniewska, A.; Wawrzeńczyk, C.; Gabryś, B. β-Thujone and its derivatives modify the probing behavior of the peach potato aphid. Molecules 2019, 24, 1847. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska-Kurdyk, A.; Dancewicz, K.; Gliszczyńska, A.; Gabryś, B. New insight into the behaviour modifying activity of two natural sesquiterpenoids farnesol and nerolidol towards Myzus persicae (Sulzer) (Homoptera: Aphididae). Bull. Entomol. Res. 2020, 110, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Tjallingii, W.F. Regulation of phloem sap feeding by aphids. In Regulatory Mechanisms in Insect Feeding; Chapman, R.F., de Boer, G., Eds.; Chapman & Hall: New York, NY, USA, 1995; pp. 190–209. [Google Scholar]
- Polonsky, J.; Bhatnagar, S.C.; Griffiths, D.C.; Pickett, J.A.; Woodcock, C.M. Activity of qassinoids as antifeedants against aphids. J. Chem. Ecol. 1989, 15, 933–998. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J.; Pickett, J.A. Laboratory evaluation of antifeedant compounds for inhibiting settling by cereal aphids. Entomol. Exp. Appl. 1997, 84, 189–193. [Google Scholar] [CrossRef]
- Halarewicz-Pacan, A.; Gabryś, B.; Dancewicz, K.; Wawrzeńczyk, C. Enantiospecific effect of limonene and limonene-derived bicyclic lactones on settling and probing behaviour of the peach-potato aphid Myzus persicae (Sulz.). J. Plant Prot. Res. 2003, 43, 133–142. [Google Scholar]
- Hardie, J.; Holyoak, M.; Taylor, N.J.; Griffiths, D.C. The combination of electronic monitoring and video-assisted observations of plant penetration by aphids and behavioural effects of polygodial. Entomol. Exp. Appl. 1992, 62, 233–239. [Google Scholar] [CrossRef]
- Pettersson, J.; Tjallingii, W.F.; Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 173–195. [Google Scholar]
- Tjallingii, W.F.; Hogen Esch, T.H. Fine-structure of aphid stylet routes in plant-tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J.; Pickett, J. Effects of the antifeedant polygodial on plant penetration by aphids, assessed by video and electrical recording. Entomol. Exp. Appl. 1993, 68, 193–200. [Google Scholar] [CrossRef]
- Gabryś, B.; Dancewicz, K.; Gliszczyńska, A.; Kordan, B.; Wawrzeńczyk, C. Systemic deterrence of aphid probing and feeding by β-damascone analogues. J. Pest. Sci. 2015, 88, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stompor, M.; Dancewicz, K.; Gabryś, B.; Anioł, M. Insect antifeedant potential of xanthohumol, isoxanthohumol, and their derivatives. J. Agric. Food Chem. 2015, 63, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Philippi, J.; Schliephake, E.; Jurgens, H.U.; Jansen, G.; Ordon, F. Feeding behavior of aphids on narrow-leafed lupin (Lupinus angustifolius) genotypes varying in the content of quinolizidine alkaloids. Entomol. Exp. Appl. 2005, 156, 37–51. [Google Scholar] [CrossRef]




| Compounds | Number of Aphids | |||||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 1 h | 2 h | 24 h | ||
| (1) citral | treated | 2.1 (±0.3) | 2.9 (±0.4) | 3.1 (±0.4) | 2.3 (±0.3) | 1.4 (±0.4) |
| control | 10.8 (±1.0) | 11.0 (±1.1) | 9.8 (±1.0) | 10.9 (±0.3) | 8.0 (±1.3) | |
| p | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0002 | |
| (4) β,γ-dimethyllactone | treated | 3.9 (±0.8) | 4.5 (±0.6) | 5.0 (±0.6) | 5.0 (±0.8) | 3.9 (±0.7) |
| control | 11.0 (±1.0) | 11.9 (±1.2) | 11.3 (±1.1) | 11.9 (±1.0) | 8.0 (±1.1) | |
| p | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0064 | |
| (5) α-methylenelactone | treated | 1.8 (±0.5) | 1.5 (±0.4) | 1.0 (±0.4) | 1.6 (±0.6) | 0.6 (±0.3) |
| control | 11.0 (±1.0) | 11.8 (±1.1) | 11.3 (±1.1) | 9.5 (±1.2.) | 5.0 (±1.7) | |
| p | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0214 | |
| (6) γ-methylenelactone | treated | 6.6 (±1.0) | 5.8 (±1.1) | 6.6 (±1.1) | 6.4 (±0.8) | 3.3 (±0.6) |
| control | 7.9 (±1.2) | 7.8 (±1.1) | 9.1 (±1.2) | 9.1 (±1.0) | 5.1 (±1.0) | |
| p | 0.4371 | 0.2343 | 0.1448 | 0.0486 | 0.1070 | |
| (7) α,γ-dimethylenelactone | treated | 3.5 (±0.7) | 4.5 (±0.7) | 5.1 (±0.8) | 3.9 (±0.7) | 1.8 (±0.5) |
| control | 8.0 (±0.6) | 9.0 (±0.7) | 8.4 (±0.9) | 9.0 (±0.5) | 4.4 (±0.7) | |
| p | 0.0002 | 0.0003 | 0.0170 | 0.0001 | 0.0086 | |
| Aphid Behavior | Control | (1) Citral | (4) β,γ-Dimethyllactone | (5) α-Methylenelactone | (7) α,γ-Dimethylenelactone |
|---|---|---|---|---|---|
| Total time on the leaf (min) | 14.6 (±0.2) a | 5.2 (±1.3) b | 13.9 (±0.5) a | 11.4 (±1.2) ab | 13.3 (±1.1) a |
| Total probing time (min) | 9.2 (±0.8) a | 3.0 (±1.2) b | 8.8 (±0.9) a | 6.6 (±4.5) ab | 10.0 (±1.0) a |
| Probe duration (min) | 2.1 (±0.4) a | 0.7 (±0.2) b | 1.8 (±0.3) ab | 1.6 (±0.4) ab | 3.8 (±0.7) a |
| Number of probes (min) | 5.6 (±0.6) a | 3.8 (±0.9) ab | 6.5 (±0.6) ac | 5.3 (±0.7) abc | 3.0 (±0.3) b |
| Time to the first probe (sec) | 14.4 (±2.7) a | 11.6 (±2.2) a | 13.1 (±1.8) a | 11.8 (±3.6) a | 20.9 (±3.4) a |
| Aphid Probing Behavior | Control | (1) Citral | (4) β,γ-Dimethyllactone | (5) α-Methylenelactone | (7) α,γ-Dimethylenelactone |
|---|---|---|---|---|---|
| General Aspects of Aphid Probing Behavior 1 | |||||
| Total duration of probing C + E1 + E2 + F + G (h) | 7.4 (±0.2) a n =16 | 4.2 (±0.4) b n = 16 | 7.1 (±0.3) a n = 16 | 4.1 (±0.5) bc n = 16 | 6.5 (±0.3) ac n = 16 |
| Total duration of pathway phase C + F (h) | 2.6 (±0.5) n = 16 | 2.9 (±0.3) n = 16 | 2.5 (±0.6) n = 16 | 3.3 (±0.4) n = 16 | 3.4 (±0.4) n = 16 |
| Total duration of phloem phase E1 + E2 (h) | 4.5 (±0.7) a n = 16 | 1.3 (±0.3) b n = 16 | 4.6 (±0.7) ab n = 16 | 0.5 (±0.4) bc n = 16 | 3.0 (±0.4) ab n = 16 |
| Total duration of sap ingestion phase E2 (h) | 4.5 (±0.7) a n = 16 | 1.2 (±0.3) b n = 16 | 4.6 (±0.7) ab n = 16 | 0.5 (±0.1) bc n = 16 | 2.8 (±0.4) ab n = 16 |
| Total duration of xylem phase G (min) | 11.4 (±6.0) n = 16 | 3.7 (±3.6) n = 16 | 4.8 (±3.3) n = 16 | 13.3 (±13.3) n = 16 | 8.2 (±3.3) n = 16 |
| Phloem phase index (E1 + E2)/(C + E + G) | 0.62 (±0.09) a n = 16 | 0.31 (±0.06) ab n = 16 | 0.64 (±0.09) a n = 16 | 0.13 (±0.05) b n = 16 | 0.46 (±0.06) a n = 16 |
| Phloem salivation index E1/(E1 + E2) | 0.01 (±0.00) a n = 16 | 0.08 (±0.02) ab n = 16 | 0.01 (±0.00) ac n = 16 | 0.19 (±0.09) b n = 16 | 0.05 (±0.03) ab n = 16 |
| Number of probes (#) | 11.5 (±2.7) a n = 16 | 38.7 (±3.1) b n = 16 | 12.4 (±3.4) a n = 16 | 37.2 (±5.0) b n = 16 | 23.2 (±3.2) ab n = 16 |
| Aphid Probing Behavior before the First Phloem Phase | |||||
| Time from start of EPG to the first probe (min) | 1.3 (±0.4) n = 16 | 12.4 (±6.0) n = 16 | 2.9 (±2.1) n = 16 | 25.6 (±13.3) n = 16 | 1.3 (±0.6) n = 16 |
| Duration of first probe (min) | 103.1 (±47.3) n = 16 | 14.6 (±5.9) n = 16 | 77.5 (±40.6) n = 16 | 8.0 (±3.3) n = 16 | 14.7 (±7.0) n = 16 |
| Time from first probe to first phloem phase 2 (h) | 1.5 (±0.4) a n = 15 | 3.8 (±0.6) b n = 13 | 1.1 (±0.4) a n = 13 | 2.5 (±0.8) ab n = 7 | 2.0 (±0.4) ab n = 16 |
| Total duration of no probing after first probe 2 (min) | 14.7 (±5.6) a n = 15 | 123.2 (±27.8) b n = 13 | 15.6 (±6.0) a n = 13 | 66.6 (±41.7) ab n = 7 | 51.5 (±15.5) ab n = 16 |
| Number of probes 2 | 5.2 (±1.7) a n = 15 | 24.3 (±4.3) b n = 13 | 5.2 (±2.5) a n = 13 | 12.9 (±4.7) ac n = 7 | 9.9 (±0.3) ab n = 16 |
| Number of probes 2 < 2 min | 1.8 (±0.6) a n = 15 | 14.3 (±3.1) b n = 13 | 2.7 (±1.5) a n = 13 | 7.4 (±4.5) ab n = 7 | 4.8 (±1.5) ab n = 16 |
| Number of probes 2 2–10 min | 2.1 (±0.7) a n = 15 | 7.9 (±1.6) b n = 13 | 2.3 (±1.1) a n = 13 | 4.3 (±1.8) ab n = 7 | 3.8 (±1.0) ab n = 16 |
| Number of probes 2 > 10 min | 1.3 (±0.4) a n = 15 | 2.1 (±0.8) a n = 13 | 0.4 (±0.2) a n = 13 | 1.2 (±0.4) a n = 7 | 1.3 (±0.3) a n = 16 |
| Aphid Probing Behavior Associated with Phloem Phase | |||||
| Duration of first phloem phase E1 or E1+E2 2 (h) | 2.8 (±0.9) a n = 15 | 1.0 (±0.3) a n = 13 | 3.7 (±0.9) a n = 13 | 0.2 (±0.1) b n = 7 | 0.6 (±0.3) a n = 16 |
| Duration of first phloem ingestion phase E2 3 (h) | 2.8 (±0.9) a n = 15 | 1.1 (±0.3) a n = 12 | 4.0 (±0.8) ab n = 13 | 1.3 (±1.1) a n = 5 | 0.4 (±0.2) ac n = 16 |
| Duration of first sustained phloem sap ingestion phase E2 > 10 min 4 (h) | 3.8 (±0.9) a n = 14 | 1.6 (±0.3) b n = 10 | 4.5 (±0.8) a n = 13 | 1.4 (±1.1) b n = 5 | 1.5 (±04) ab n = 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C.; Gabryś, B. Repellent and Antifeedant Activities of Citral-Derived Lactones against the Peach Potato Aphid. Int. J. Mol. Sci. 2020, 21, 8029. https://doi.org/10.3390/ijms21218029
Dancewicz K, Szumny A, Wawrzeńczyk C, Gabryś B. Repellent and Antifeedant Activities of Citral-Derived Lactones against the Peach Potato Aphid. International Journal of Molecular Sciences. 2020; 21(21):8029. https://doi.org/10.3390/ijms21218029
Chicago/Turabian StyleDancewicz, Katarzyna, Antoni Szumny, Czesław Wawrzeńczyk, and Beata Gabryś. 2020. "Repellent and Antifeedant Activities of Citral-Derived Lactones against the Peach Potato Aphid" International Journal of Molecular Sciences 21, no. 21: 8029. https://doi.org/10.3390/ijms21218029