Vertebral Bone Marrow-Derived Mesenchymal Stromal Cells from Osteoporotic and Healthy Patients Possess Similar Differentiation Properties In Vitro
Abstract
:1. Introduction
2. Results
2.1. Morphology and Proliferation Rate of BMSCs from Osteoporotic and Non-Osteoporotic Control Donors
2.2. Phenotypic Analysis and Immunomodulatory Capacity
2.3. Osteogenic, Adipogenic, and Chondrogenic Differentiation
2.4. Alkaline Phosphatase Intensity and Activity during Osteogenic Differentiation Process
2.5. Assessment of Osteogenic Differentiation
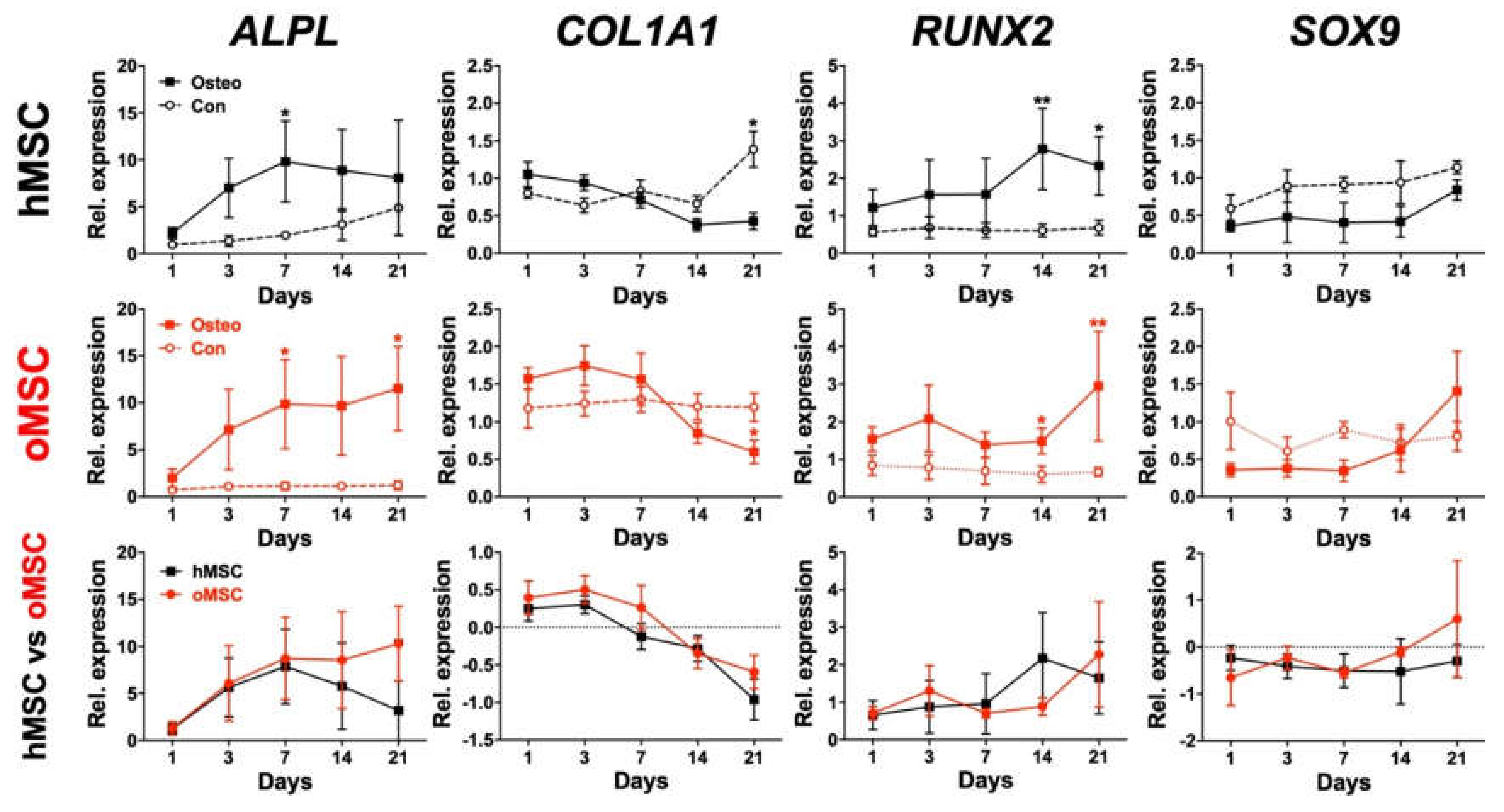
2.6. Osteoblast Marker Gene Expression
3. Discussion
4. Materials and Methods
4.1. Tissue Donors and Isolation of Bone Marrow-Derived MSCs
4.2. Morphologic Analysis
4.3. MTT Assay
4.4. Flow Cytometric Analysis
4.5. Immunomodulatory Capacity
4.6. Adipogenic Differentiation
4.7. Chondrogenic Differentiation
4.8. Osteogenic Differentiation
4.9. Alkaline Phosphatase Measurement, Optical Density Measurement, and Free Phosphate Assay
4.10. Real-Time Polymerase Chain Reaction
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALPL | Alkaline phosphatase gene |
| BMSC | Bone marrow-derived mesenchymal stromal cells |
| CD | Cluster of differentiation |
| cDNA | Complementary deoxyribonucleic acid |
| CFSE | Carboxyfluorescein succinimidyl ester |
| COL1A1 | Collagen, type I, alpha 1 |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| ddCT | Delta-delta-Ct |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| hMSC | Healthy mesenchymal stromal cells |
| ISCT | International Society for Cellular Therapy |
| mRNA | Messenger ribonucleic acid |
| MSC | Mesenchymal stromal cells |
| OD | Optical density |
| oMSC | Osteoporotic mesenchymal stromal cells |
| PBMC | Peripheral blood mononuclear cell |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| RT-PCR | Real-time polymerase chain reaction |
| RUNX2 | Runt-related transcription factor 2 |
| SOX9 | SRY-Box Transcription Factor 9 |
References
- Cauley, J.A. Public health impact of osteoporosis. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antebi, B.; Pelled, G.; Gazit, D. Stem cell therapy for osteoporosis. Curr. Osteoporos. Rep. 2014, 12, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Invest. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front. Immunol. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teitelbaum, S.L. Stem cells and osteoporosis therapy. Cell Stem Cell 2010, 7, 553–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., De Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, H.J., Kaltsas, G., et al., Eds.; MDText.com Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bianco, P.; Robey, P.G. Skeletal stem cells. Development 2015, 142, 1023–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuzawa, Y.; Yagi, K.; Yamashita, Y.; Nakachi, Y.; Nikaido, I.; Bono, H.; Ninomiya, Y.; Kanesaki-Yatsuka, Y.; Akita, M.; Motegi, H.; et al. Id4, a new candidate gene for senile osteoporosis, acts as a molecular switch promoting osteoblast differentiation. PLoS Genet. 2010, 6, e1001019. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, B.; Li, C.; Rong, J.S.; Tao, S.Q.; Tao, T.Z. Decreased proliferation ability and differentiation potential of mesenchymal stem cells of osteoporosis rat. Asian Pac. J. Trop. Med. 2014, 7, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.P.; Garat, S.; Gajardo, H.; Pino, A.M.; Seitz, G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J. Cell. Biochem. 1999, 75, 414–423. [Google Scholar] [CrossRef]
- Wang, Z.; Goh, J.; Das De, S.; Ge, Z.; Ouyang, H.; Chong, J.S.; Low, S.L.; Lee, E.H. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006, 12, 1753–1761. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.G.; Randau, T.M.; Hilgers, C.; Haddouti, E.M.; Masson, W.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques. Int. J. Mol. Sci. 2020, 21, 4382. [Google Scholar] [CrossRef]
- Paebst, F.; Piehler, D.; Brehm, W.; Heller, S.; Schroeck, C.; Tarnok, A.; Burk, J. Comparative immunophenotyping of equine multipotent mesenchymal stromal cells: An approach toward a standardized definition. Cytom. A 2014, 85, 678–687. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. A 2018, 93, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Mafi, P.; Mafi, R.; Khan, W. The Effects of Ageing on Differentiation and Characterisation of Human Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2018, 13, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, J.; Davies, J.E.; Stanford, W.L. Concise Review: Musculoskeletal Stem Cells to Treat Age-Related Osteoporosis. Stem Cells Transl. Med. 2017, 6, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Phetfong, J.; Sanvoranart, T.; Nartprayut, K.; Nimsanor, N.; Seenprachawong, K.; Prachayasittikul, V.; Supokawej, A. Osteoporosis: The current status of mesenchymal stem cell-based therapy. Cell Mol. Biol. Lett. 2016, 21, 12. [Google Scholar] [CrossRef] [Green Version]
- Aghebati-Maleki, L.; Dolati, S.; Zandi, R.; Fotouhi, A.; Ahmadi, M.; Aghebati, A.; Nouri, M.; Kazem Shakouri, S.; Yousefi, M. Prospect of mesenchymal stem cells in therapy of osteoporosis: A review. J. Cell. Physiol. 2019, 234, 8570–8578. [Google Scholar] [CrossRef]
- Bieback, K.; Brinkmann, I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J. Stem Cells 2010, 2, 81–92. [Google Scholar]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Haddouti, E.M.; Randau, T.M.; Hilgers, C.; Masson, W.; Walgenbach, K.J.; Pflugmacher, R.; Burger, C.; Gravius, S.; Schildberg, F.A. Characterization and Comparison of Human and Ovine Mesenchymal Stromal Cells from Three Corresponding Sources. Int. J. Mol. Sci. 2020, 21, 2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schildberg, F.A.; Donnenberg, V.S. Stromal cells in health and disease. Cytom. A 2018, 93, 871–875. [Google Scholar] [CrossRef]
- Tome-Bermejo, F.; Pinera, A.R.; Alvarez-Galovich, L. Osteoporosis and the Management of Spinal Degenerative Disease (I). Arch. Bone Jt. Surg. 2017, 5, 272–282. [Google Scholar]
- Chin, D.K.; Park, J.Y.; Yoon, Y.S.; Kuh, S.U.; Jin, B.H.; Kim, K.S.; Cho, Y.E. Prevalence of osteoporosis in patients requiring spine surgery: Incidence and significance of osteoporosis in spine disease. Osteoporos. Int. 2007, 18, 1219–1224. [Google Scholar] [CrossRef]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.O.; Loebel, C.; Bara, J.J.; Fussinger, M.A.; Duttenhoefer, F.; Alini, M.; Stoddart, M.J. Effect of Short-Term Stimulation with Interleukin-1beta and Differentiation Medium on Human Mesenchymal Stromal Cell Paracrine Activity in Coculture with Osteoblasts. BioMed Res. Int. 2015, 2015, 714230. [Google Scholar] [CrossRef] [Green Version]
- Loebel, C.; Czekanska, E.M.; Bruderer, M.; Salzmann, G.; Alini, M.; Stoddart, M.J. In vitro osteogenic potential of human mesenchymal stem cells is predicted by Runx2/Sox9 ratio. Tissue Eng. Part A 2015, 21, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, W.L.; Luyten, F.P.; Roberts, S.J. From skeletal development to the creation of pluripotent stem cell-derived bone-forming progenitors. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018, 373, 20170218. [Google Scholar] [CrossRef] [Green Version]
- Dalle Carbonare, L.; Mottes, M.; Cheri, S.; Deiana, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Salvagno, G.L.; Lippi, G.; Valenti, M.T. Increased Gene Expression of RUNX2 and SOX9 in Mesenchymal Circulating Progenitors Is Associated with Autophagy during Physical Activity. Oxid. Med. Cell Longev. 2019, 2019, 8426259. [Google Scholar] [CrossRef]
- Pino, A.M.; Rosen, C.J.; Rodriguez, J.P. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol. Res. 2012, 45, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Camernik, K.; Mihelic, A.; Mihalic, R.; Haring, G.; Herman, S.; Marolt Presen, D.; Janez, A.; Trebse, R.; Marc, J.; Zupan, J. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res. Ther. 2020, 11, 146. [Google Scholar] [CrossRef]
- Chen, D.; Shen, H.; He, Y.; Chen, Y.; Wang, Q.; Lu, J.; Jiang, Y. Synergetic effects of hBMSCs and hPCs in osteogenic differentiation and their capacity in the repair of critical-sized femoral condyle defects. Mol. Med. Rep. 2015, 11, 1111–1119. [Google Scholar] [CrossRef]
- Igarashi, M.; Kamiya, N.; Hasegawa, M.; Kasuya, T.; Takahashi, T.; Takagi, M. Inductive effects of dexamethasone on the gene expression of Cbfa1, Osterix and bone matrix proteins during differentiation of cultured primary rat osteoblasts. J. Mol. Histol. 2004, 35, 3–10. [Google Scholar] [CrossRef]
- Park, B.W.; Hah, Y.S.; Kim, D.R.; Kim, J.R.; Byun, J.H. Osteogenic phenotypes and mineralization of cultured human periosteal-derived cells. Arch. Oral Biol. 2007, 52, 983–989. [Google Scholar] [CrossRef]
- Loebel, C.; Czekanska, E.M.; Staudacher, J.; Salzmann, G.; Richards, R.G.; Alini, M.; Stoddart, M.J. The calcification potential of human MSCs can be enhanced by interleukin-1beta in osteogenic medium. J. Tissue Eng. Regen. Med. 2017, 11, 564–571. [Google Scholar] [CrossRef]
- Sawa, N.; Fujimoto, H.; Sawa, Y.; Yamashita, J. Alternating Differentiation and Dedifferentiation between Mature Osteoblasts and Osteocytes. Sci. Rep. 2019, 9, 13842. [Google Scholar] [CrossRef] [Green Version]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. Paris 2009, 57, 318–323. [Google Scholar] [CrossRef]
- Evans, J.F.; Yeh, J.K.; Aloia, J.F. Osteoblast-like cells of the hypophysectomized rat: A model of aberrant osteoblast development. Am. J. Physiol. Endocrinol. Metab. 2000, 278, 832–838. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Serguienko, A.; Wang, M.Y.; Myklebost, O. Real-Time Vital Mineralization Detection and Quantification during In Vitro Osteoblast Differentiation. Biol. Proced. Online 2018, 20, 14. [Google Scholar] [CrossRef]
- Schneider, R.K.; Puellen, A.; Kramann, R.; Raupach, K.; Bornemann, J.; Knuechel, R.; Perez-Bouza, A.; Neuss, S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials 2010, 31, 467–480. [Google Scholar] [CrossRef]
- Wiraja, C.; Yeo, D.C.; Chong, M.S.; Xu, C. Nanosensors for Continuous and Noninvasive Monitoring of Mesenchymal Stem Cell Osteogenic Differentiation. Small 2016, 12, 1342–1350. [Google Scholar] [CrossRef]
- Kaneto, C.M.; Lima, P.S.; Zanette, D.L.; Prata, K.L.; Pina Neto, J.M.; De Paula, F.J.; Silva, W.A., Jr. COL1A1 and miR-29b show lower expression levels during osteoblast differentiation of bone marrow stromal cells from Osteogenesis Imperfecta patients. BMC Med. Genet. 2014, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Shui, C.; Spelsberg, T.C.; Riggs, B.L.; Khosla, S. Changes in Runx2/Cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. J. Bone Miner. Res. 2003, 18, 213–221. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xie, R.L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; Van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; De Crombrugghe, B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003, 19, 458–466. [Google Scholar] [CrossRef]
- Murakami, S.; Lefebvre, V.; De Crombrugghe, B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J. Biol. Chem. 2000, 275, 3687–3692. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, V.; Smits, P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. C Embryo Today 2005, 75, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Jiang, L.S.; Jiang, S.D.; Dai, L.Y. Osteogenic potential and responsiveness to leptin of mesenchymal stem cells between postmenopausal women with osteoarthritis and osteoporosis. J. Orthop. Res. 2009, 27, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Barbanti Brodano, G.; Terzi, S.; Trombi, L.; Griffoni, C.; Valtieri, M.; Boriani, S.; Magli, M.C. Mesenchymal stem cells derived from vertebrae (vMSCs) show best biological properties. Eur. Spine J. 2013, 22, S979–S984. [Google Scholar] [CrossRef] [Green Version]
- Fragkakis, E.M.; El-Jawhari, J.J.; Dunsmuir, R.A.; Millner, P.A.; Rao, A.S.; Henshaw, K.T.; Pountos, I.; Jones, E.; Giannoudis, P.V. Vertebral body versus iliac crest bone marrow as a source of multipotential stromal cells: Comparison of processing techniques, tri-lineage differentiation and application on a scaffold for spine fusion. PLoS ONE 2018, 13, e0197969. [Google Scholar] [CrossRef]
- Jing, H.; Liao, L.; An, Y.; Su, X.; Liu, S.; Shuai, Y.; Zhang, X.; Jin, Y. Suppression of EZH2 Prevents the Shift of Osteoporotic MSC Fate to Adipocyte and Enhances Bone Formation During Osteoporosis. Mol. Ther. 2016, 24, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Schildberg, F.A.; Hegenbarth, S.I.; Schumak, B.; Scholz, K.; Limmer, A.; Knolle, P.A. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur. J. Immunol. 2008, 38, 957–967. [Google Scholar] [CrossRef]
- Schildberg, F.A.; Wojtalla, A.; Siegmund, S.V.; Endl, E.; Diehl, L.; Abdullah, Z.; Kurts, C.; Knolle, P.A. Murine hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism. Hepatology 2011, 54, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Berr, K.; Naujoks, C.; Hassel, A.; Hentschel, M.; Depprich, R.; Kubler, N.R.; Meyer, U.; Wiesmann, H.P.; Kogler, G.; et al. Generation and differentiation of microtissues from multipotent precursor cells for use in tissue engineering. Nat. Protoc. 2011, 6, 1726–1735. [Google Scholar] [CrossRef]
- Kalaszczynska, I.; Ruminski, S.; Platek, A.E.; Bissenik, I.; Zakrzewski, P.; Noszczyk, M.; Lewandowska-Szumiel, M. Substantial differences between human and ovine mesenchymal stem cells in response to osteogenic media: How to explain and how to manage? Biores. Open Access 2013, 2, 356–363. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Gene | Primer Sequence | Product Length | Accession Number |
|---|---|---|---|
| GAPDH | fwd: 5′CTCTGCTCCTCCTGTTCGAC3′ rev: 5′ACCAAATCCGTTGACTCCGA3‘ | 109 bp | NM_002046.5 |
| ALPL | fwd: 5′TTTATAAGGCGGCGGGGGTG3′ rev: 5′AGCCCAGAGATGCAATCGAC3′ | 198 bp | NM_000478.5 |
| COL1A1 | fwd: 5′TGCTCGTGGAAATGATGGTG3′ rev: 5′CCTCGCTTTCCTTCCTCTCC3′ | 449 bp | NM_000088.3 |
| RUNX2 | fwd: 5′GCGCATTCCTCATCCCAGTA3′ rev: 5′GGCTCAGGTAGGAGGGGTAA3′ | 176 bp | NM_001024630.3 |
| SOX9 | fwd:5′AGGAAGTCGGTGAAGAACGG3′ rev: 5′AAGTCGATAGGGGGCTGTCT3′ | 275 bp | NM_000346.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddouti, E.-M.; Randau, T.M.; Hilgers, C.; Masson, W.; Pflugmacher, R.; Burger, C.; Gravius, S.; Schildberg, F.A. Vertebral Bone Marrow-Derived Mesenchymal Stromal Cells from Osteoporotic and Healthy Patients Possess Similar Differentiation Properties In Vitro. Int. J. Mol. Sci. 2020, 21, 8309. https://doi.org/10.3390/ijms21218309
Haddouti E-M, Randau TM, Hilgers C, Masson W, Pflugmacher R, Burger C, Gravius S, Schildberg FA. Vertebral Bone Marrow-Derived Mesenchymal Stromal Cells from Osteoporotic and Healthy Patients Possess Similar Differentiation Properties In Vitro. International Journal of Molecular Sciences. 2020; 21(21):8309. https://doi.org/10.3390/ijms21218309
Chicago/Turabian StyleHaddouti, El-Mustapha, Thomas M. Randau, Cäcilia Hilgers, Werner Masson, Robert Pflugmacher, Christof Burger, Sascha Gravius, and Frank A. Schildberg. 2020. "Vertebral Bone Marrow-Derived Mesenchymal Stromal Cells from Osteoporotic and Healthy Patients Possess Similar Differentiation Properties In Vitro" International Journal of Molecular Sciences 21, no. 21: 8309. https://doi.org/10.3390/ijms21218309





