Effect of Embryo Vitrification on the Steroid Biosynthesis of Liver Tissue in Rabbit Offspring
Abstract
:1. Introduction
2. Results
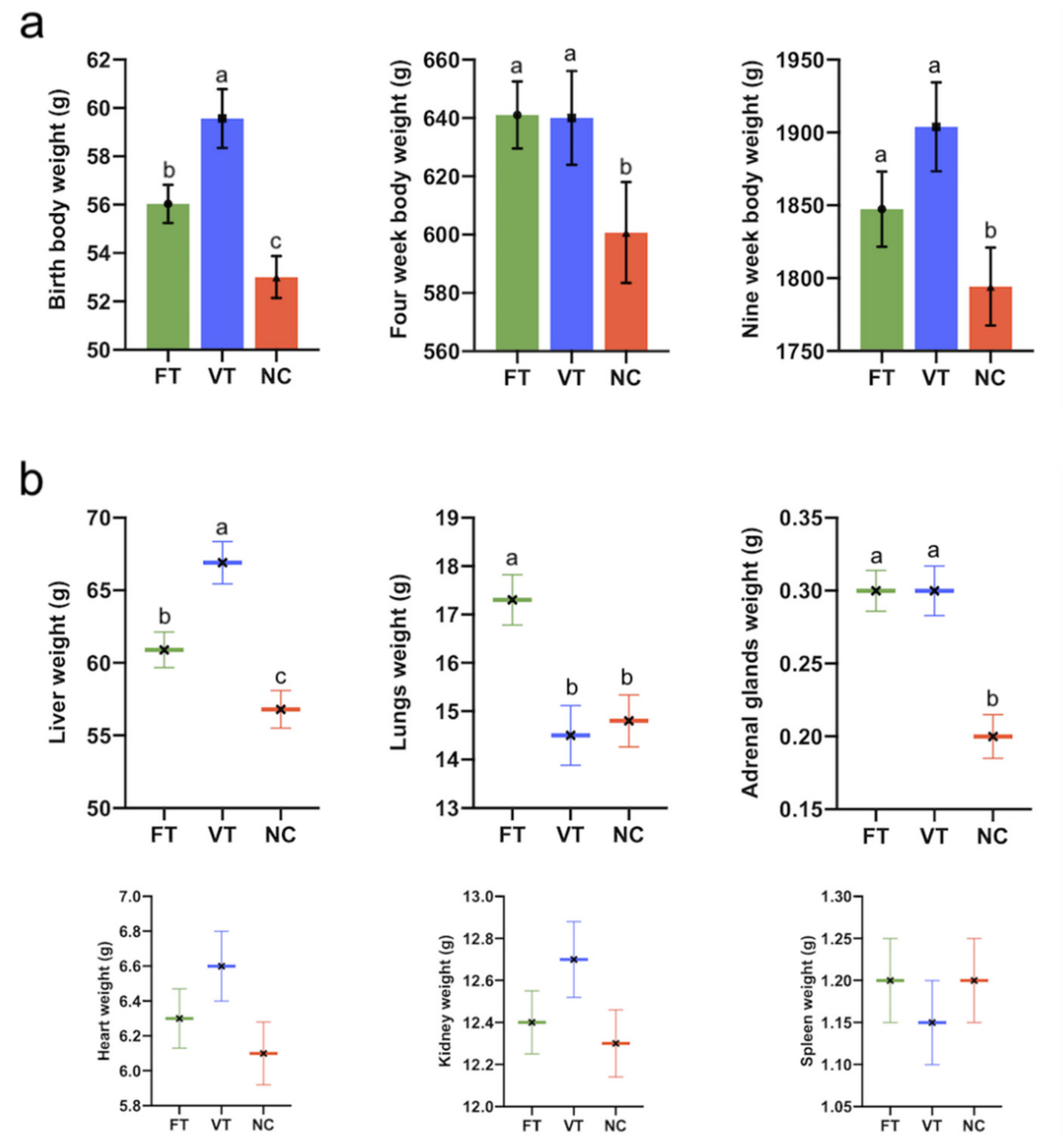
2.1. Foetal and Postnatal Growth Performance, Body Weight and Organ Phenotype Study
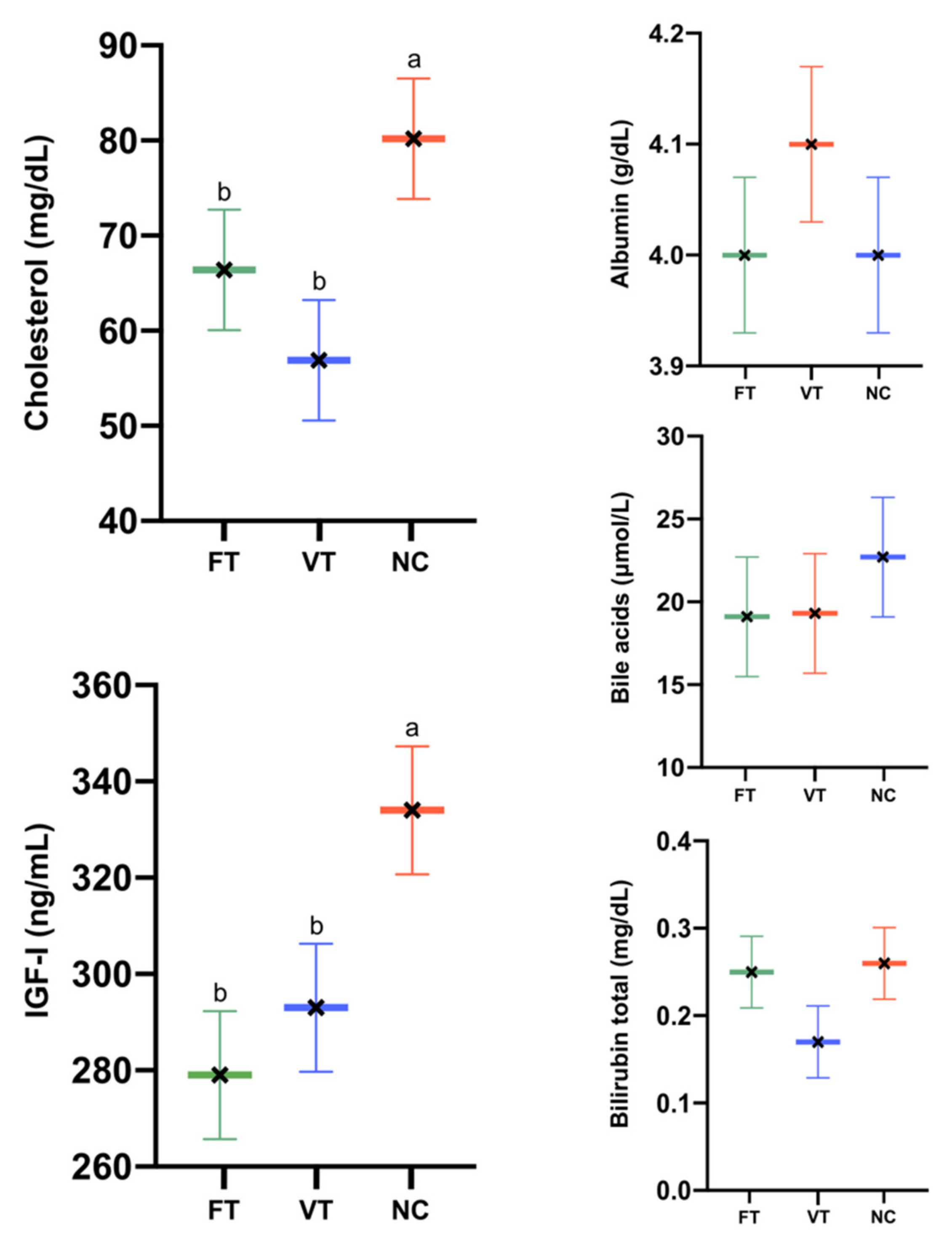
2.2. Hepatic Functionality Assessment
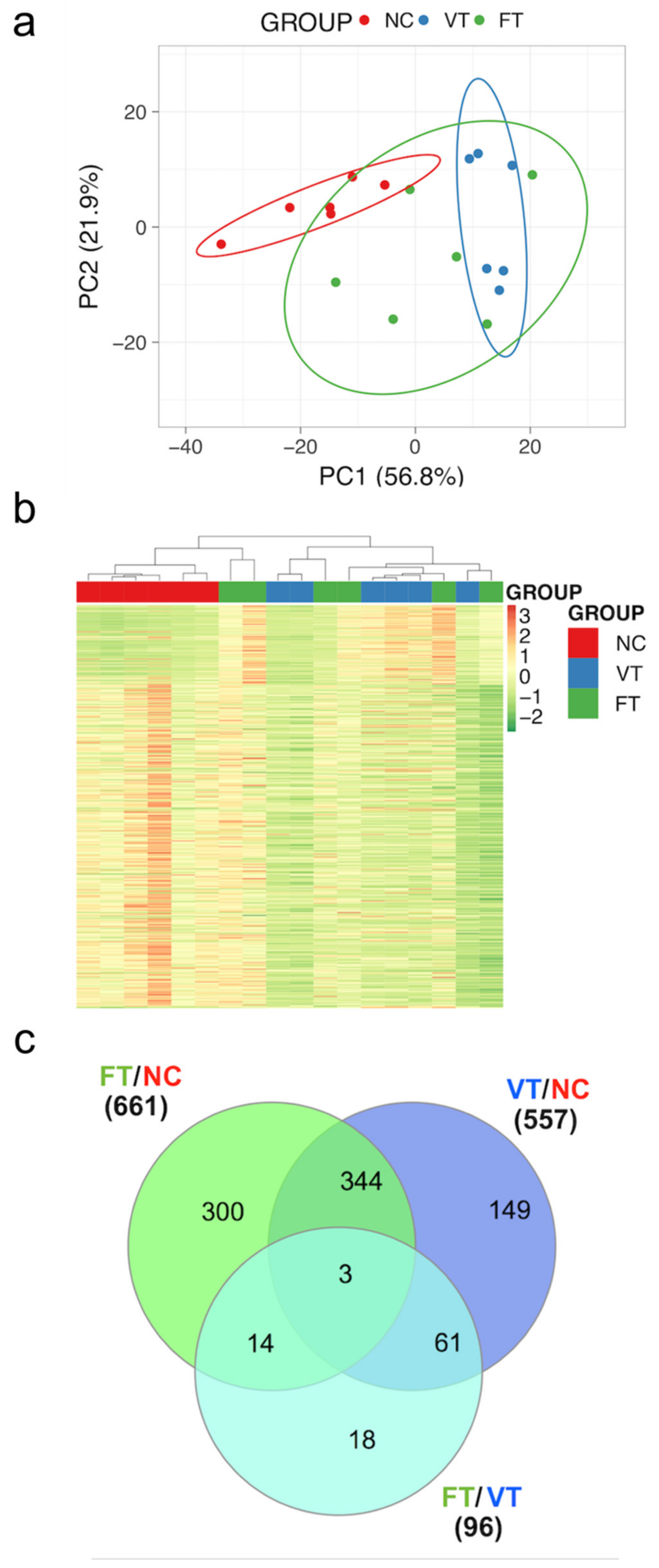
2.3. Comparative Study of Liver mRNA Expression
2.4. Comparative Study of Liver mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Statements
4.2. Experimental Design
4.3. Embryo Collection Procedure
4.4. Embryo Vitrification
4.5. Embryo Transfer Procedure
4.6. Foetal Growth Study
4.7. Growth, Body Weight and Organs Weight Study
4.8. Hepatic Functionality Assessment
4.9. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.10. The Hepatic Metabolomic Approach in Response to ART Stressors
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| APOA4 | Apolipoprotein A-IV |
| ART | Assisted reproductive technologies |
| BSA | Bovine serum albumin |
| CLN6 | Transmembrane ER Protein |
| CRL | Crown-rump length |
| DAMs | Differentially accumulated metabolites |
| DMSO | Dimethyl sulphoxide |
| EG | Ethylene glycol |
| ELOVL4 | Fatty Acid Elongase 4 |
| FT | Embryo exposure to the transfer procedure |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GLM | General linear model |
| H2AFZ | Histone family member Z |
| HM | Heat-Maps |
| IGF-I | Insulin-like growth factor I |
| LC-APCI-MS | Liquid chromatography–positive ion atmospheric pressure chemical ionization-mass spectrometry |
| LIPC | Lipase C |
| NC | Naturally conceived animals |
| PBS | Phosphate-buffered saline |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| RT-qPCR | Real-time polymerase chain reaction |
| VT | Embryo exposure to the cryopreservation-transfer procedure |
References
- Novakovic, B.; Lewis, S.; Halliday, J.; Kennedy, J.; Burgner, D.P.; Czajko, A.; Kim, B.; Sexton-Oates, A.; Juonala, M.; Hammarberg, K.; et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat. Commun. 2019, 10, 3922. [Google Scholar] [CrossRef]
- Roseboom, T.J. Developmental plasticity and its relevance to assisted human reproduction. Hum. Reprod. 2018, 33, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Dulioust, E.; Toyama, K.; Busnel, M.C.; Moutier, R.; Carlier, M.; Marchaland, C.; Ducot, B.; Roubertoux, P.; Auroux, M. Long-term effects of embryo freezing in mice. Proc. Natl. Acad. Sci. USA 1995, 92, 589–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auroux, M.; Cerutti, I.; Ducot, B.; Loeuillet, A. Is embryo-cryopreservation really neutral? A new long-term effect of embryo freezing in mice: Protection of adults from induced cancer according to strain and sex. Reprod. Toxicol. 2004, 18, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.S.; Saenz-de-Juano, M.D.; Jiménez-Trigos, E.; Viudes-de-Castro, M.P.; Peñaranda, D.S.; Marco-Jiménez, F. Rabbit morula vitrification reduces early foetal growth and increases losses throughout gestation. Cryobiology 2013, 67, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Saenz-De-Juano, M.D.; Marco-Jimenez, F.; Schmaltz-Panneau, B.; Jimenez-Trigos, E.; Viudes-De-Castro, M.P.; Penaranda, D.S.; Jouneau, L.; Lecardonnel, J.; Lavara, R.; Naturil-Alfonso, C.; et al. Vitrification alters rabbit foetal placenta at transcriptomic and proteomic level. Reproduction 2014, 147, 789–801. [Google Scholar] [CrossRef] [Green Version]
- Saenz-de-Juano, M.D.; Vicente, J.S.; Hollung, K.; Marco-Jiménez, F. Effect of embryo vitrification on rabbit foetal placenta proteome during pregnancy. PLoS ONE 2015, 10, e0125157. [Google Scholar] [CrossRef]
- Berntsen, S.; Pinborg, A. Large for gestational age and macrosomia in singletons born after frozen/thawed embryo transfer (FET) in assisted reproductive technology (ART). Birth Defects Res. 2018, 110, 630–643. [Google Scholar] [CrossRef]
- Maheshwari, A.; Pandey, S.; Raja, E.A.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum. Reprod. Update 2018, 24, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Dominguez, X.; Vicente, J.S.; Marco-Jiménez, F. Developmental plasticity in response to embryo cryopreservation: The importance of the vitrification device in rabbits. Animals 2020, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Kohda, T. Effects of embryonic manipulation and epigenetics. J. Hum. Genet. 2013, 58, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Canovas, S.; Ross, P.J.; Kelsey, G.; Coy, P. DNA Methylation in Embryo Development: Epigenetic Impact of ART (Assisted Reproductive Technologies). BioEssays 2017, 39, 1700106. [Google Scholar] [CrossRef] [PubMed]
- Canovas, S.; Ivanova, E.; Romar, R.; García-Martínez, S.; Soriano-Úbeda, C.; García-Vázquez, F.A.; Saadeh, H.; Andrews, S.; Kelsey, G.; Coy, P. DNA methylation and gene expression changes derived from assisted reproductive technologies can be decreased by reproductive fluids. eLife 2017, 6, e23670. [Google Scholar] [CrossRef]
- Ivanova, E.; Canovas, S.; Garcia-Martínez, S.; Romar, R.; Lopes, J.S.; Rizos, D.; Sanchez-Calabuig, M.J.; Krueger, F.; Andrews, S.; Perez-Sanz, F.; et al. DNA methylation changes during preimplantation development reveal inter-species differences and reprogramming events at imprinted genes. Clin. Epigenetics 2020, 12, 64. [Google Scholar] [CrossRef]
- García-Martínez, S.; Sánchez Hurtado, M.; Gutiérrez, S.; Sánchez Margallo, F.M.; Romar, R.; Latorre, R.; Coy, P.; López Albors, O. Mimicking Physiological O2 Tension in the Female Reproductive Tract Improves Assisted Reproduction Outcomes in Pig. Mol. Hum. Reprod. 2018, 24, 260–270. [Google Scholar] [CrossRef]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y.C. In Vivo Oxygen, Temperature and pH Dynamics in the Female Reproductive Tract and Their Importance in Human Conception: A Systematic Review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef]
- Marchesi, D.E.; Qiao, J.; Feng, H.L. Embryo manipulation and imprinting. Semin. Reprod. Med. 2012, 30, 323–333. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Heras, S.; Gómez-Redondo, I.; Planells, B.; Fernández-González, R.; Pericuesta, E.; Laguna-Barraza, R.; Pérez-Cerezales, S.; Gutiérrez-Adán, A. Embryo responses to stress induced by assisted reproductive technologies. Mol. Reprod. Dev. 2019, 86, 1292–1306. [Google Scholar] [CrossRef] [Green Version]
- Vrooman, L.A.; Bartolomei, M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017, 68, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Heilbronn, L.K. The health outcomes of human offspring conceived by assisted reproductive technologies (ART). J. Dev. Orig. Health Dis. 2017, 8, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Duranthon, V.; Chavatte-Palmer, P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018, 85, 348–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibo, S.P.; Sztein, J.M. Cryopreservation of mammalian embryos: Derivation of a method. Cryobiology 2019, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sparks, A.E.T. Human embryo cryopreservation-methods, timing, and other considerations for optimizing an embryo cryopreservation program. Semin. Reprod. Med. 2015, 33, 128–144. [Google Scholar] [CrossRef]
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. ART in Europe, 2015: Results Generated From European Registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoz038. [Google Scholar] [CrossRef] [Green Version]
- Saenz-de-Juano, M.; Marco-Jimenez, F.; Viudes-de-Castro, M.; Lavara, R.; Vicente, J. Direct Comparison of the Effects of Slow Freezing and Vitrification on Late Blastocyst Gene Expression, Development, Implantation and Offspring of Rabbit Morulae. Reprod. Domest. Anim. 2014, 49, 505–511. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Marco-Jiménez, F.; Peñaranda, D.S.; Vicente, J.S. Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model. Animals 2020, 10, 1043. [Google Scholar] [CrossRef]
- Lavara, R.; Baselga, M.; Marco-Jiménez, F.; Vicente, J.S. Embryo vitrification in rabbits: Consequences for progeny growth. Theriogenology 2015, 84, 674–680. [Google Scholar] [CrossRef]
- Lavara, R.; Baselga, M.; Marco-Jiménez, F.; Vicente, J.S. Long-term and transgenerational effects of cryopreservation on rabbit embryos. Theriogenology 2014, 81, 988–992. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Marco-Jiménez, F.; Peñaranda, D.S.; Diretto, G.; Garcia-Carpintero, V.; Cañizares, J.; Vicente, J.S. Long-term and transgenerational phenotypic, transcriptional and metabolic effects in rabbit males born following vitrified embryo transfer. Sci. Rep. 2020, 10, 11313. [Google Scholar] [CrossRef]
- Feuer, S.K.; Rinaudo, P.F. From Embryos to Adults: A DOHaD Perspective on In Vitro Fertilization and Other Assisted Reproductive Technologies. Healthcare 2016, 4, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandstra, H.; Brentjens, L.B.P.M.; Spauwen, B.; Touwslager, R.N.H.; Bons, J.A.P.; Mulder, A.L.; Smits, L.J.M.; van der Hoeven, M.A.H.B.M.; van Golde, R.J.T.; Evers, J.L.H.; et al. Association of Culture Medium With Growth, Weight and Cardiovascular Development of IVF Children at the Age of 9 Years. Hum. Reprod. 2018, 33, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Zheng, Z.; Yu, H.; Wang, H.; Qin, J. Birth prevalence of congenital malformations in singleton pregnancies resulting from in vitro fertilization/intracytoplasmic sperm injection worldwide: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2018, 297, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Tierney, E.S.S.; Chen, A.C.; Ling, A.Y.; Fleischmann, R.R.; Baker, V.L. Vascular Health of Children Conceived via In Vitro Fertilization. J. Pediatrics 2019, 214, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, X.M.; Jin, L.; Wang, T.T.; Ullah, K.; Sheng, J.Z.; Huang, H.F. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: A systematic review and meta-analysis. Fertil. Steril. 2017, 107, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Feuer, S.K.; Liu, X.; Donjacour, A.; Simbulan, R.; Maltepe, E.; Rinaudo, P. Transcriptional signatures throughout development: The effects of mouse embryo manipulation in vitro. Reproduction 2017, 153, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Feuer, S.K.; Rinaudo, P.F. Physiological, metabolic and transcriptional postnatal phenotypes of in vitro fertilization (IVF) in the mouse. J. Dev. Orig. Health Dis. 2017, 8, 403–410. [Google Scholar] [CrossRef]
- Ecker, D.J.; Stein, P.; Xu, Z.; Williams, C.J.; Kopf, G.S.; Bilker, W.B.; Abel, T.; Schultz, R.M. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 1595–1600. [Google Scholar] [CrossRef] [Green Version]
- Fauque, P.; Mondon, F.; Letourneur, F.; Ripoche, M.A.; Journot, L.; Barbaux, S.; Dandolo, L.; Patrat, C.; Wolf, J.P.; Jouannet, P.; et al. In vitro fertilization and embryo culture strongly impact the placental transcriptome in the mouse model. PLoS ONE 2010, 5, e9218. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, R.; Ramirez, M.A.; Pericuesta, E.; Calle, A.; Gutierrez-Adan, A. Histone Modifications at the Blastocyst Axin1Fu Locus Mark the Heritability of In Vitro Culture-Induced Epigenetic Alterations in Mice1. Biol. Reprod. 2010, 83, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Gonzalez, R.; Moreira, P.; Bilbao, A.; Jiménez, A.; Pérez-Crespo, M.; Ramírez, M.A.; De Fonseca, F.R.; Pintado, B.; Gutiérrez-Adán, A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 5880–5885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winick, M.; Noble, A. Quantitative changes in DNA, RNA, and protein during prenatal and postnatal growth in the rat. Dev. Biol. 1965, 12, 451–466. [Google Scholar] [CrossRef]
- Saenz-de-Juano, M.D.; Marco-Jiménez, F.; Vicente, J.S. Embryo transfer manipulation cause gene expression variation in blastocysts that disrupt implantation and offspring rates at birth in rabbit. European J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Delle Piane, L.D.; Lin, W.; Liu, X.; Donjacour, A.; Minasi, P.; Revelli, A.; Maltepe, E.; Rinaudo, P.F. Effect of the Method of Conception and Embryo Transfer Procedure on Mid-Gestation Placenta and Fetal Development in an IVF Mouse Model. Hum. Reprod. 2010, 25, 2039–2046. [Google Scholar] [CrossRef] [Green Version]
- Wale, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2015, 22, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Charni-Natan, M.; Aloni-Grinstein, R.; Osher, E.; Rotter, V. Liver and steroid hormones-can a touch of p53 make a difference? Front. Endocrinol. 2019, 10, 374. [Google Scholar] [CrossRef] [Green Version]
- Yakar, S.; Liu, J.L.I.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; Leroith, D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef] [Green Version]
- Juul, A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm. IGF Res. 2003, 13, 113–170. [Google Scholar] [CrossRef]
- Miles, H.L.; Hofman, P.L.; Peek, J.; Harris, M.; Wilson, D.; Robinson, E.M.; Gluckman, P.D.; Cutfield, W.S. In vitro fertilization improves childhood growth and metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 3441–3445. [Google Scholar] [CrossRef] [Green Version]
- Juul, A.; Kastrup, K.W.; Pedersen, S.A.; Skakkebæk, N.E. Growth Hormone (GH) Provocative Retesting of 108 Young Adults with Childhood-Onset GH Deficiency and the Diagnostic Value of Insulin-Like Growth Factor I (IGF-I) and IGF-Binding Protein-3 1. J. Clin. Endocrinol. Metab. 1997, 82, 1195–1201. [Google Scholar]
- Belva, F.; Bonduelle, M.; Provyn, S.; Painter, R.C.; Tournaye, H.; Roelants, M.; De Schepper, J. Metabolic Syndrome and Its Components in Young Adults Conceived by ICSI. Int. J. Endocrinol. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, N.; Atger, V.; Paul, J.L.; Sturm, M.; Duverger, N.; Rothblat, G.H.; Moatti, N. Human ApoA-IV overexpression in transgenic mice induces camp-stimulated cholesterol efflux from J774 macrophages to whole serum. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1283–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.U.; Jiang, X.C.; Liu, R.; Liang, G.; Beyer, T.P.; Gao, H.; Ryan, T.P.; Shuyu, D.L.; Eacho, P.I.; Cao, G. Liver X receptors (LXRs) regulate apolipoprotein AIV-Implications of the antiatherosclerotic effect of LXR agonists. Mol. Endocrinol. 2004, 18, 2000–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.; Cao, Y.; Gao, J. Decreased expression of the APOA1-APOC3-APOA4 gene cluster is associated with risk of alzheimer’s disease. Drug Des. Dev. Ther. 2015, 9, 5421–5431. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Li, X.; Xie, L.; Li, S.; Liu, J.; Jia, L.; Dong, X.; Ren, X.; Xiao, J.; Yang, C.; et al. A long non-coding RNA, APOA4-AS, regulatesAPOA4 expression depending on HuR in mice. Nucleic Acids Res. 2016, 44, 6423–6433. [Google Scholar] [CrossRef] [Green Version]
- Leese, H.J.; Guerif, F.; Allgar, V.; Brison, D.R.; Lundin, K.; Sturmey, R.G. Biological optimization, the Goldilocks principle, and how much is lagom in the preimplantation embryo. Mol. Reprod. Dev. 2016, 83, 748–754. [Google Scholar] [CrossRef]
- Marco-Jiménez, F.; Baselga, M.; Vicente, J.S. Successful re-establishment of a rabbit population from embryos vitrified 15 years ago: The importance of biobanks in livestock conservation. PLoS ONE 2018, 13, e0199234. [Google Scholar]
- Kohda, T.; Ishino, F. Embryo manipulation via assisted reproductive technology and epigenetic asymmetry in mammalian early development. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120353. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Dominguez, X.; Juarez, J.D.; Vicente, J.S.; Marco-Jiménez, F. Impact of embryo technologies on secondary sex ratio in rabbit. Cryobiology 2020, in press. [Google Scholar] [CrossRef]
- Ouhayoun, J. Croissance et qualiteés boucheères du lapin. Cuniculture 1984, 11, 181–188. [Google Scholar]
- Viudes-de-Castro, M.P.; Marco-Jiménez, F.; Cedano-Castro, J.I.; Vicente, J.S. Effect of corifollitropin alfa supplemented with or without LH on ovarian stimulation and embryo viability in rabbit. Theriogenology 2017, 98, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Marco-Jiménez, F.; Lavara, R.; Jiménez-Trigos, E.; Vicente, J.S. In vivo development of vitrified rabbit embryos: Effects of vitrification device, recipient genotype, and asynchrony. Theriogenology 2013, 79, 1124–1129. [Google Scholar]
- Vicente, J.S.; Viudes-De-Castro, M.P.; García, M.L. In vivo survival rate of rabbit morulae after vitrification in a medium without serum protein. Reprod. Nutr. Dev. 1999, 39, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dominguez, X.; Marco-Jimenez, F.; Viudes-de-Castro, M.P.; Vicente, J.S. Minimally invasive embryo transfer and embryo vitrification at the optimal embryo stage in rabbit model. J. Vis. Exp. 2019, 147, e58055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besenfelder, U.; Strouhal, C.; Brem, G. A Method for Endoscopie Embryo Collection and Transfer in the Rabbit. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 1998, 45, 577–579. [Google Scholar] [CrossRef] [PubMed]


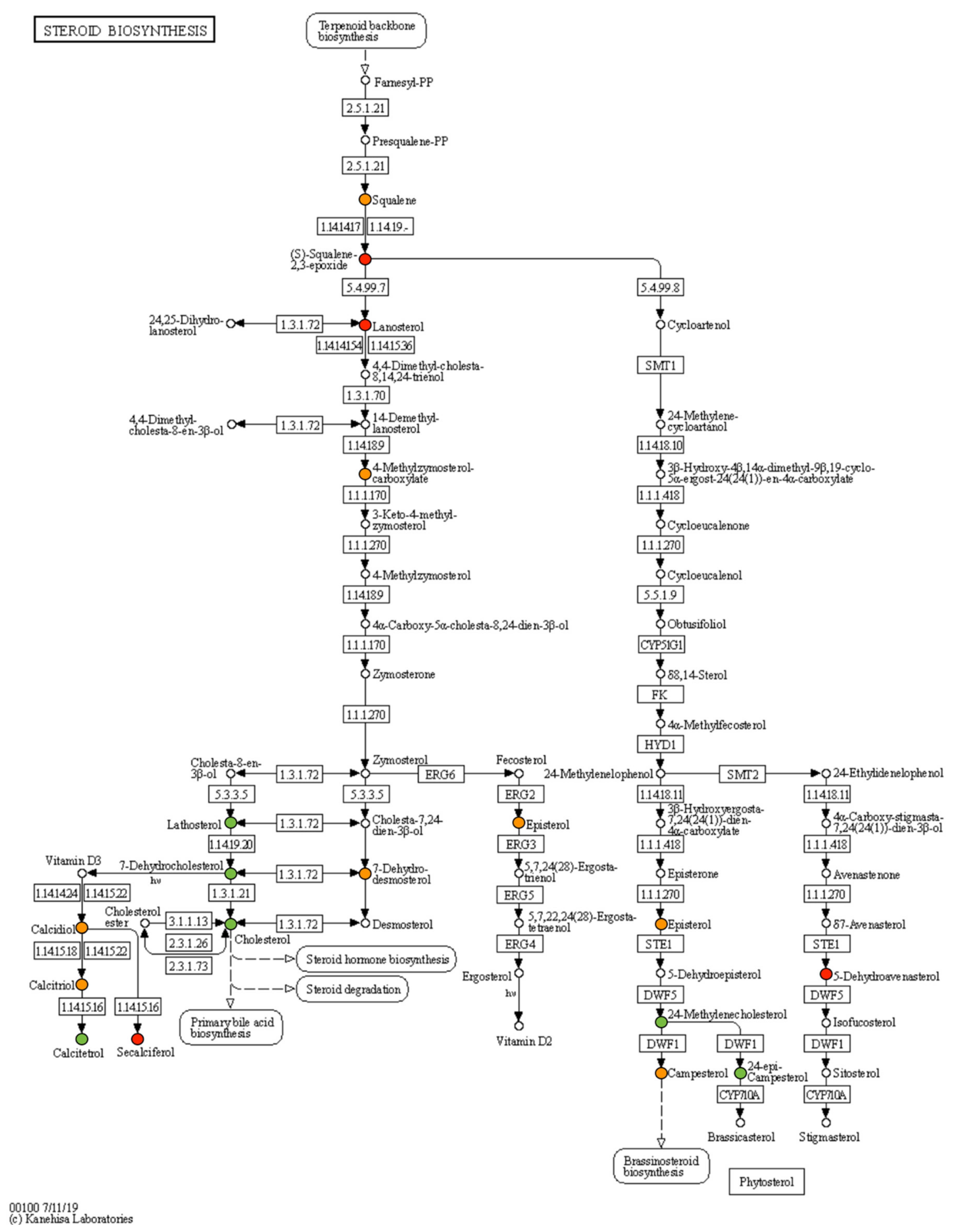

| Metabolite | Fresh-Transferred (FT/NC) | Vitrified-Transferred (VT/NC) | Vitrified-Fresh (VT/FT) |
|---|---|---|---|
| Fold Change | Fold Change | Fold Change | |
| 3-alpha,7-alpha,12-alpha,26-Tetrahydroxy-5beta-cholestane | 0.11 | −0.65 | −0.75 |
| 3-alpha,7-alpha,26-Trihydroxy-5-beta-cholestane | −1.29 | −0.84 | 0.45 |
| 3-alpha,7-alpha-Dihydroxy-5-beta-cholestanate | −3.71 | −2.47 | 1.23 |
| 3-alpha,7-alpha-Dihydroxy-5-beta-cholestane | −2.67 | −1.87 | 0.80 |
| 4-alpha-Methylzymosterol | −0.77 | −0.31 | 0.46 |
| 4-alpha-Methylzymosterol-4-carboxylate | 0.57 | 0.03 | −0.54 |
| 5-Dehydroavenasterol | 0.85 | 1.45 | 0.61 |
| 7-alpha,24-Dihydroxy-4-cholesten-3-one | −1.20 | −0.81 | 0.38 |
| 7-Dehydrocholesterol | −0.90 | −0.59 | 0.31 |
| 7-Dehydrodesmosterol | −0.62 | −0.30 | 0.32 |
| 14-Demethyllanosterol | −0.02 | −0.07 | −0.05 |
| 20-alpha,22-beta-Dihydroxycholesterol | 15.11 * | 58.84 * | 1.96 |
| 22(R)-Hydroxycholesterol | −2.76 | −2.15 | 0.61 |
| 24-epi-Campesterol | −2.68 | −2.93 | −0.25 |
| 24-Methylenecholesterol | −2.75 | −3.16 | −0.41 |
| Calcidiol | −0.10 | −1.64 | −1.54 |
| Calcitetrol | −2.91 | −2.45 | 0.46 |
| Calcitriol | −0.10 | −0.39 | −0.29 |
| Campesterol | −0.50 | −0.41 | 0.10 |
| Cholesterol | −0.68 | −0.84 | −0.15 |
| Episterol | 0.27 | 0.03 | −0.24 |
| Lanosterol | 12.39 * | 10.00 * | −0.31 |
| Lathosterol | −0.69 | −1.38 | −0.69 |
| Pregnenolone | −1.76 | −2.00 | −0.24 |
| Secalciferol | 1.14 | 0.45 | −0.69 |
| Squalene | 0.45 | 0.27 | −0.18 |
| S-squalene 2,3-epoxide | 53.84 * | 30.82 * | −0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco-Jiménez, F.; Garcia-Dominguez, X.; Domínguez-Martínez, M.; Viudes-de-Castro, M.P.; Diretto, G.; Peñaranda, D.S.; Vicente, J.S. Effect of Embryo Vitrification on the Steroid Biosynthesis of Liver Tissue in Rabbit Offspring. Int. J. Mol. Sci. 2020, 21, 8642. https://doi.org/10.3390/ijms21228642
Marco-Jiménez F, Garcia-Dominguez X, Domínguez-Martínez M, Viudes-de-Castro MP, Diretto G, Peñaranda DS, Vicente JS. Effect of Embryo Vitrification on the Steroid Biosynthesis of Liver Tissue in Rabbit Offspring. International Journal of Molecular Sciences. 2020; 21(22):8642. https://doi.org/10.3390/ijms21228642
Chicago/Turabian StyleMarco-Jiménez, Francisco, Ximo Garcia-Dominguez, Marta Domínguez-Martínez, María Pilar Viudes-de-Castro, Gianfranco Diretto, David S. Peñaranda, and José S. Vicente. 2020. "Effect of Embryo Vitrification on the Steroid Biosynthesis of Liver Tissue in Rabbit Offspring" International Journal of Molecular Sciences 21, no. 22: 8642. https://doi.org/10.3390/ijms21228642





