Matrilin-3-Primed Adipose-Derived Mesenchymal Stromal Cell Spheroids Prevent Mesenchymal Stromal-Cell-Derived Chondrocyte Hypertrophy
Abstract
:1. Introduction
2. Results
2.1. Effect of Matrilin-3 Concentration on Ad-MSC Chondrogenesis in MAHA Hydrogel
2.2. Effect of Matrilin-3 on Suppression of Ad-MSC-Derived Chondrocyte Hypertrophy
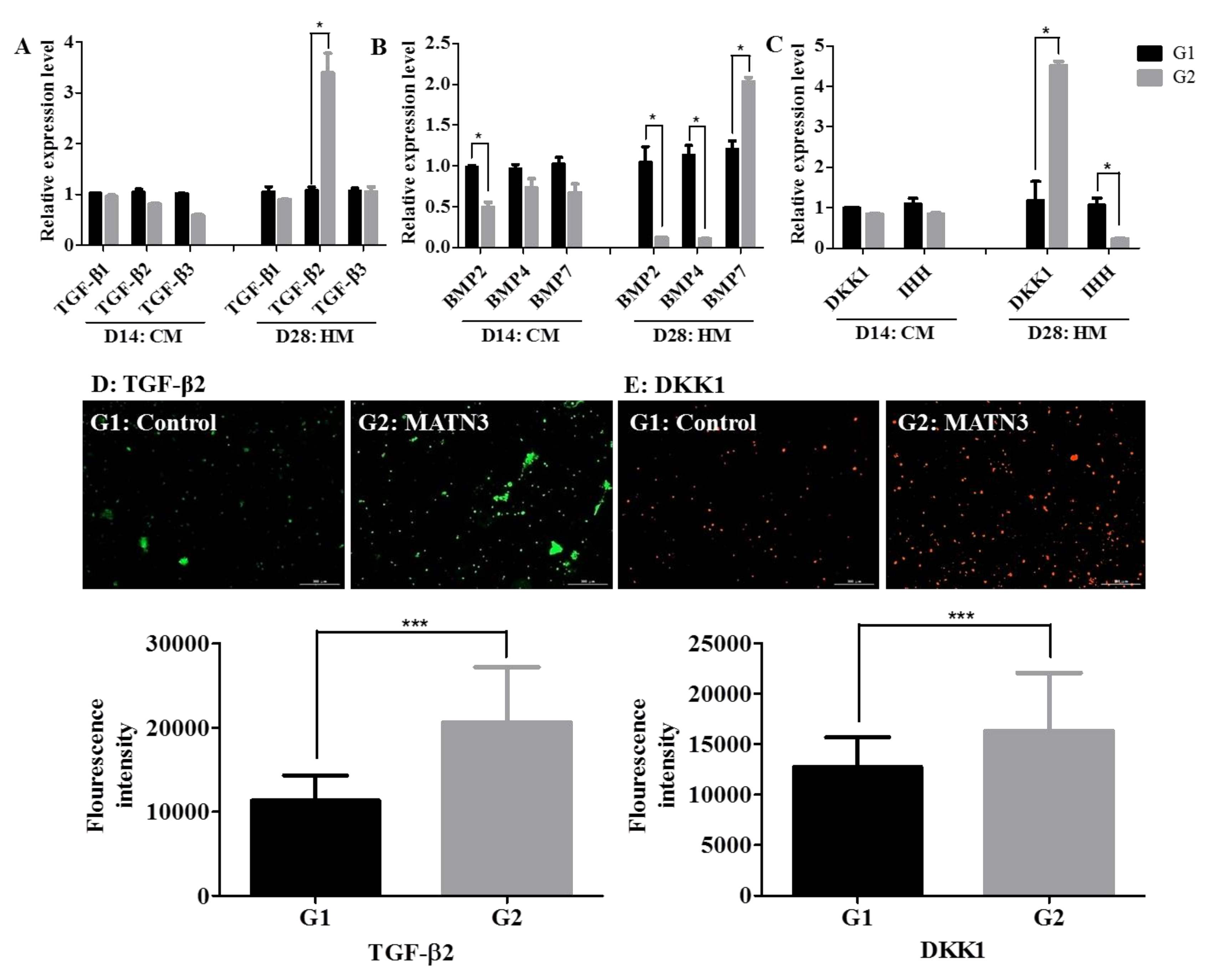
2.3. Changes in TGF-β and BMP Expression during Hypertrophic Differentiation
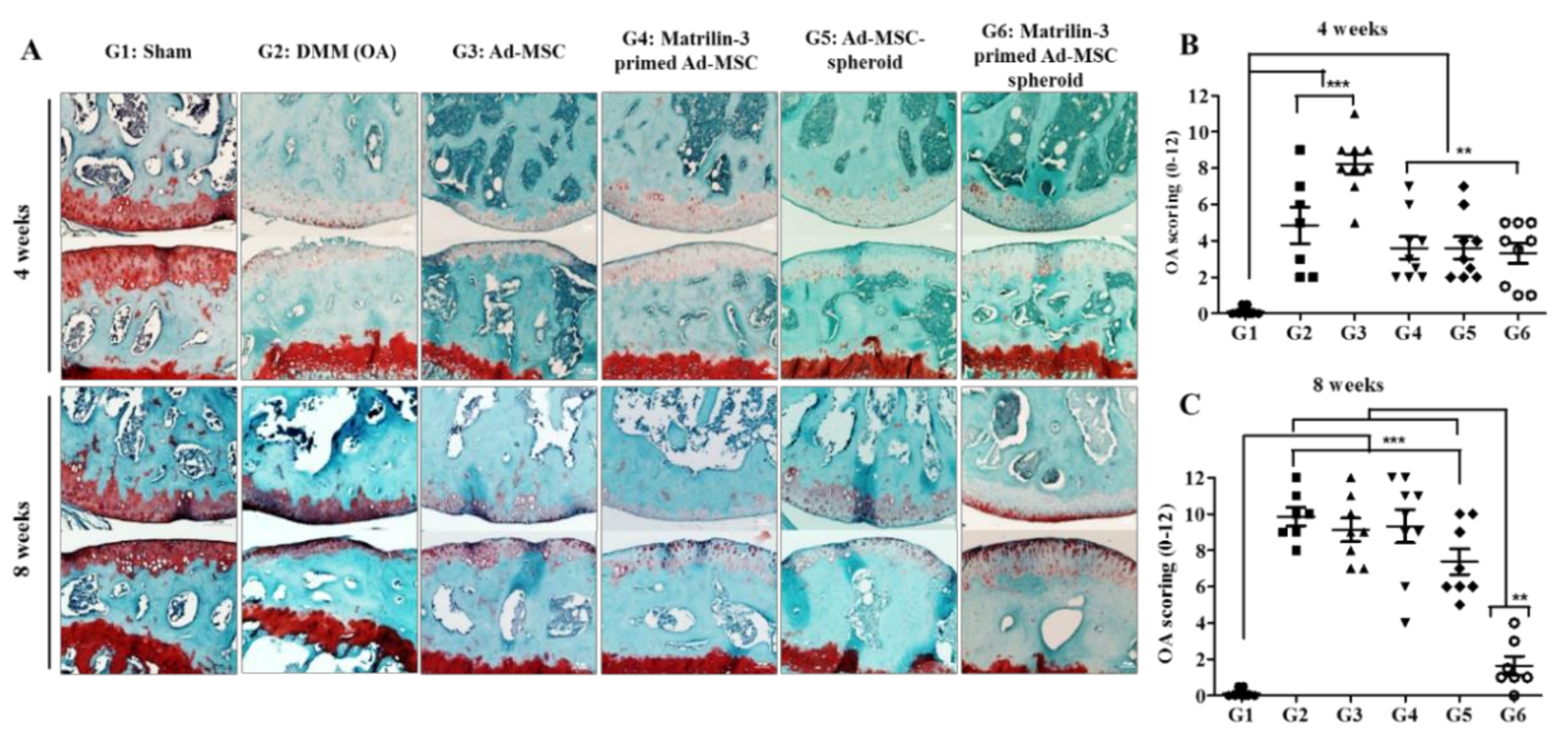
2.4. Matrilin-3 Spheroids Protect Cartilage Degeneration from OA in DMM-Induced Mice
2.5. Treatment with Matrilin-3 Spheroids Attenuates Subchondral Bone Sclerosis in a Post-Traumatic OA Model
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Ad-MSCs from Adipose Tissue
4.2. Preparation of Photopolymerizable Hyaluronic Acid
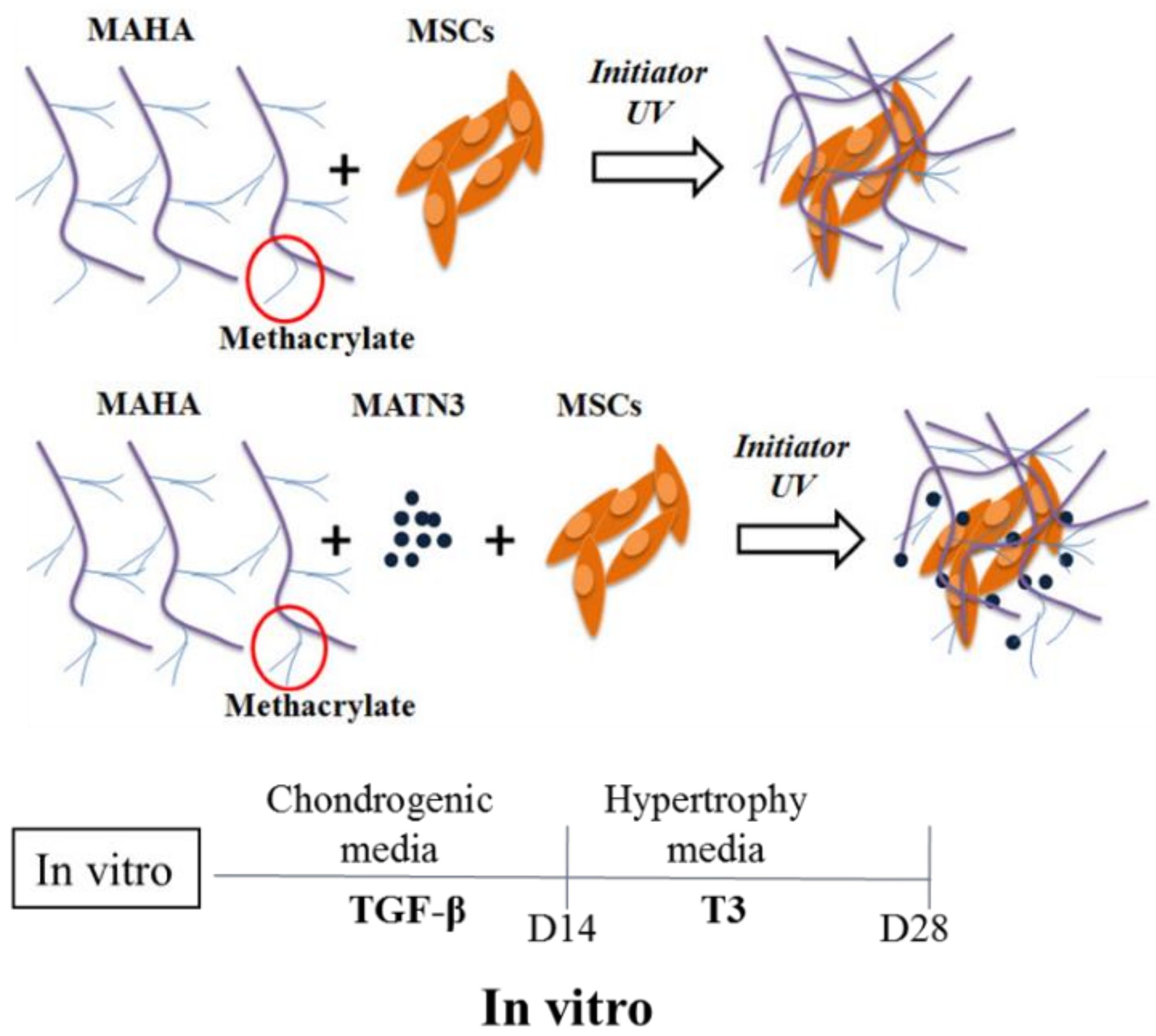
4.3. Human Ad-MSCs and Matrilin-3 Encapsulation in Hydrogel and Chondrogenesis
4.4. Ad-MSC Chondrogenesis and Hypertrophy Induction
4.5. Live and Dead Assays
4.6. RNA Extraction and mRNA Expression Analysis
4.7. Histological Analysis
4.8. Destabilization of Medial Meniscus Osteoarthritis Model
4.9. Ad-MSC Priming with Matrilin-3, Spheroid Preparation, and Transplantation
4.10. Microcomputed Tomography Analysis
4.11. Histological Analysis and Immunohistochemistry
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MATN3 | Matrilin-3 |
| MAHA | Methacrylated hyaluronic acid |
| T3 | thyroid hormone |
| TGF-β | transforming growth factor factor-β |
| BMP | bone morphogenetic protein |
| DKK1 | Dickkopf WNT Signaling Pathway Inhibitor 1 |
| IHH | Indian hedgehog |
| COL2A | Collagen 2A |
| ACAN | Aggrecan |
References
- Sekiya, I.; Vuoristo, J.T.; Larson, B.L.; Prockop, D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 4397–4402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Jt. Surg. Am. Vol. 1998, 80, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Mwale, F.; Girard-Lauriault, P.L.; Wang, H.T.; Lerouge, S.; Antoniou, J.; Wertheimer, M.R. Suppression of genes related to hypertrophy and osteogenesis in committed human mesenchymal stem cells cultured on novel nitrogen-rich plasma polymer coatings. Tissue Eng. 2006, 12, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Stachura, D.; Roughley, P.; Antoniou, J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2006, 24, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004, 50, 142–150. [Google Scholar] [CrossRef]
- Dell’Accio, F.; De Bari, C.; Luyten, F.P. Microenvironment and phenotypic stability specify tissue formation by human articular cartilage-derived cells in vivo. Exp. Cell Res. 2003, 287, 16–27. [Google Scholar] [CrossRef]
- Pfander, D.; Swoboda, B.; Kirsch, T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am. J. Pathol. 2001, 159, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, T.; Swoboda, B.; Nah, H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthr. Cartil. 2000, 8, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.D.; Fischer, M.; Gannon, J.; Thompson, R.C., Jr.; Oegema, T.R., Jr. Expression of type-X collagen in osteoarthritis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1995, 13, 4–12. [Google Scholar] [CrossRef]
- Shibakawa, A.; Yudoh, K.; Masuko-Hongo, K.; Kato, T.; Nishioka, K.; Nakamura, H. The role of subchondral bone resorption pits in osteoarthritis: MMP production by cells derived from bone marrow. Osteoarthr. Cartil. 2005, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.O.; Oreffo, R.O.; Clarke, N.M.; Roach, H.I. Changes in the antiangiogenic properties of articular cartilage in osteoarthritis. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2003, 8, 849–857. [Google Scholar] [CrossRef]
- Walsh, D.A. Angiogenesis in osteoarthritis and spondylosis: Successful repair with undesirable outcomes. Curr. Opin. Rheumatol. 2004, 16, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wagener, R.; Ehlen, H.W.; Ko, Y.P.; Kobbe, B.; Mann, H.H.; Sengle, G.; Paulsson, M. The matrilins--adaptor proteins in the extracellular matrix. FEBS Lett. 2005, 579, 3323–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt, A.R.; Becker, A.K.; Neacsu, C.D.; Paulsson, M.; Wagener, R. The matrilins: Modulators of extracellular matrix assembly. Int. J. Biochem. Cell Biol. 2011, 43, 320–330. [Google Scholar] [CrossRef]
- Mostert, A.K.; Dijkstra, P.F.; Jansen, B.R.; van Horn, J.R.; de Graaf, B.; Heutink, P.; Lindhout, D. Familial multiple epiphyseal dysplasia due to a matrilin-3 mutation: Further delineation of the phenotype including 40 years follow-up. Am. J. Med. Genet. A 2003, 120A, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Borochowitz, Z.U.; Scheffer, D.; Adir, V.; Dagoneau, N.; Munnich, A.; Cormier-Daire, V. Spondylo-epi-metaphyseal dysplasia (SEMD) matrilin 3 type: Homozygote matrilin 3 mutation in a novel form of SEMD. J. Med. Genet. 2004, 41, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabuchi, A.; Haga, N.; Maeda, K.; Nakashima, E.; Manabe, N.; Hiraoka, H.; Kitoh, H.; Kosaki, R.; Nishimura, G.; Ohashi, H.; et al. Novel and recurrent mutations clustered in the von Willebrand factor A domain of MATN3 in multiple epiphyseal dysplasia. Hum. Mutat. 2004, 24, 439–440. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q. Changes of matrilin forms during endochondral ossification. Molecular basis of oligomeric assembly. J. Biol. Chem. 2000, 275, 32628–32634. [Google Scholar] [CrossRef] [Green Version]
- Jayasuriya, C.T.; Goldring, M.B.; Terek, R.; Chen, Q. Matrilin-3 induction of IL-1 receptor antagonist is required for up-regulating collagen II and aggrecan and down-regulating ADAMTS-5 gene expression. Arthritis Res. Ther. 2012, 14, R197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, M.; Luo, J.; Chen, Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthr. Cartil. 2008, 16, 1110–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Weyden, L.; Wei, L.; Luo, J.; Yang, X.; Birk, D.E.; Adams, D.J.; Bradley, A.; Chen, Q. Functional knockout of the matrilin-3 gene causes premature chondrocyte maturation to hypertrophy and increases bone mineral density and osteoarthritis. Am. J. Pathol. 2006, 169, 515–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasuriya, C.T.; Zhou, F.H.; Pei, M.; Wang, Z.; Lemme, N.J.; Haines, P.; Chen, Q. Matrilin-3 chondrodysplasia mutations cause attenuated chondrogenesis, premature hypertrophy and aberrant response to TGF-beta in chondroprogenitor cells. Int. J. Mol. Sci. 2014, 15, 14555–14573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Trehan, S.K.; Guan, Y.; Sun, C.; Moore, D.C.; Jayasuriya, C.T.; Chen, Q. Matrilin-3 inhibits chondrocyte hypertrophy as a bone morphogenetic protein-2 antagonist. J. Biol. Chem. 2014, 289, 34768–34779. [Google Scholar] [CrossRef] [Green Version]
- Muttigi, M.S.; Kim, B.J.; Choi, B.; Yoshie, A.; Kumar, H.; Han, I.; Park, H.; Lee, S.H. Matrilin-3 codelivery with adipose-derived mesenchymal stem cells promotes articular cartilage regeneration in a rat osteochondral defect model. J. Tissue Eng. Regen. Med. 2017, 12, 667–675. [Google Scholar] [CrossRef]
- Mueller, M.B.; Tuan, R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008, 58, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J.N. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int. J. Mol. Sci. 2015, 16, 19225–19247. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Kim, J.D.; Oh, E.J.; Oh, S.H.; Lee, J.H.; Im, G.I. PD98059-impregnated functional PLGA scaffold for direct tissue engineering promotes chondrogenesis and prevents hypertrophy from mesenchymal stem cells. Tissue Eng. Part A 2014, 20, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Karl, A.; Olbrich, N.; Pfeifer, C.; Berner, A.; Zellner, J.; Kujat, R.; Angele, P.; Nerlich, M.; Mueller, M.B. Thyroid hormone-induced hypertrophy in mesenchymal stem cell chondrogenesis is mediated by bone morphogenetic protein-4. Tissue Eng. Part A 2014, 20, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Steinert, A.F.; Proffen, B.; Kunz, M.; Hendrich, C.; Ghivizzani, S.C.; Noth, U.; Rethwilm, A.; Eulert, J.; Evans, C.H. Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Res. Ther. 2009, 11, R148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, R.; Timur, U.T.; Edip, S.; Haak, E.; Wruck, C.; Weinans, H.; Jahr, H. TGF-beta2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2015, 198, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Luvalle, P.; Leask, T.; Leboy, P.S. A BMP responsive transcriptional region in the chicken type X collagen gene. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1998, 13, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Kaartinen, V.M.; Mishina, Y. Loss-of-function of ACVR1 in osteoblasts increases bone mass and activates canonical Wnt signaling through suppression of Wnt inhibitors SOST and DKK1. Biochem. Biophys. Res. Commun. 2011, 414, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Caron, M.M.; Emans, P.J.; Cremers, A.; Surtel, D.A.; Coolsen, M.M.; van Rhijn, L.W.; Welting, T.J. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthr. Cartil. 2013, 21, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.K.; Kronenberg, H.M.; Chuang, P.T.; Mackem, S.; Yang, Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development 2008, 135, 1947–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Soegiarto, D.W.; Yang, Y.; Lanske, B.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Investig. 2005, 115, 1734–1742. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef]
- Pan, J.; Wang, B.; Li, W.; Zhou, X.; Scherr, T.; Yang, Y.; Price, C.; Wang, L. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone 2012, 51, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Deberg, M.A.; Piccardi, N.; Msika, P.; Reginster, J.Y.; Henrotin, Y.E. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, -1beta and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthr. Cartil. 2005, 13, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Yamaguchi, S.; Nagai, M.; Kiyan, W.; Zhang, X.; Kuroki, H. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthr. Cartil. 2016, 24, 1092–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronemberger, G.S.; Matsui, R.A.M.; Miranda, G.; Granjeiro, J.M.; Baptista, L.S. Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks. World J. Stem Cells 2020, 12, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Kim, D.W.; Kim, S.H.; Cho, J.H.; Lee, H.J.; Park, D.Y.; Park, S.R.; Choi, B.H.; Min, B.H. Establishment of a reliable and reproducible murine osteoarthritis model. Osteoarthr. Cartil. 2013, 21, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Choi, B.H.; Jin, L.H.; Park, S.R.; Min, B.-H. Comparison between subchondral bone change and cartilage degeneration in collagenase- and DMM- induced osteoarthritis (OA) models in mice. Tissue Eng. Regen. Med. 2013, 10, 211–217. [Google Scholar] [CrossRef]
- McNulty, M.A.; Loeser, R.F.; Davey, C.; Callahan, M.F.; Ferguson, C.M.; Carlson, C.S. A Comprehensive Histological Assessment of Osteoarthritis Lesions in Mice. Cartilage 2011, 2, 354–363. [Google Scholar] [CrossRef] [Green Version]



| Gene | Primer Sequence | Accession Number |
|---|---|---|
| 18S | 5′-GTAACCCGTTGAACCCCATT-3′ 5′-CCATCCAATCGGTAGTAGCG-3′ | NR_003286.2 |
| SOX9 | 5′-GTACCCGCACTTGCACAAC-3′ 5′-TCTCGCTCTCGTTCAGAAGTC-3′ | NM_000346.3 |
| Collagen 2a | 5′-GGGAGTAATGCAAGGACCA-3′ 5′-ATCATCACCAGGCTTTCCAG-3′ | NM_001844.4 |
| Aggrecan | 5′-GCCTGCGCTCCAATGACT-3′ 5′-ATGGAACACGATGCCTTTCAC-3′ | NM_013227.3 |
| Collagen 10 | 5′-ACGCTGAACGATACCAAATG-3′ 5′-TGCTATACCTTTACTCTTTATGGTGTA-3′ | NM_000493.3 |
| RUNX2 | 5′-CAGACCAGCAGCACTCCATA-3′ 5′-CAGCGTCAACACCATCATTC-3′ | NM_004348 |
| ALP | 5′-GACAAGAAGCCCTTCACTGC-3′ 5′-AGACTGCGCCTGGTAGTTGT-3′ | NM_000478.4 |
| TGF-β1 | 5′-ACTACTACGCCAAGGAGGTCAC-3′ 5′-TGCTTGAACTTGTCATAGATTTCG-3′ | NM_000660.5 |
| TGF-β2 | 5′-CAGATGCTTCTGGATTTATGGTATT-3′ 5′-CCAAAGGGTACAATGCCAAC-3′ | NM_003238.3 |
| TGF-β3 | 5′-CGCACACAGCAGTTCTCC-3′ 5′-AAGAAGCGGGCTTTGGAC-3′ | NM_003239.3 |
| BMP-2 | 5′-CGGACTGCGGTCTCCTAA-3′ 5′-GGAAGCAGCAACGCTAGAAG-3′ | NM_001200.3 |
| BMP-4 | 5′-GAGGAAGGAAGATGCGAGAA-3′ 5′-GCACTACGGAATGGCTCCTA-3′ | NM_130850.3 |
| BMP-7 | 5′-TGTCGAGCAGGAAGAGATCC-3′ 5′-ACGCTTCGACAATGAGACG-3′ | NM_001719.2 |
| IHH | 5′-TGCATTGCTCGTCAAGTC-3′ 5′-CCACTCTCCAGGCGTACCT-3′ | NM_002181.3 |
| DKK1 | 5′-AATGATTTTGATCAGAAGACACACATA-3′ 5′-CAGGCGTGCAAATCTGTCT-3′ | NM_012242.2 |
| Modified Semiquantitative Grading Scale | Score |
|---|---|
| Normal cartilage | 0 |
| Loss of safranin-O with no structural lesions | 0.5 |
| Roughened articular surface and small fibrillations | 1 |
| Fibrillation below the superficial layer and some loss of lamina | 2 |
| Fibrillations extending to the calcified cartilage across less than 20% of the cartilage width | 3 |
| Fibrillation and erosions extending from 20 to 80% of the cartilage width | 5 |
| Cartilage erosion extending beyond 80% of the cartilage width | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttigi, M.S.; Kim, B.J.; Choi, B.; Han, I.; Park, H.; Lee, S.-H. Matrilin-3-Primed Adipose-Derived Mesenchymal Stromal Cell Spheroids Prevent Mesenchymal Stromal-Cell-Derived Chondrocyte Hypertrophy. Int. J. Mol. Sci. 2020, 21, 8911. https://doi.org/10.3390/ijms21238911
Muttigi MS, Kim BJ, Choi B, Han I, Park H, Lee S-H. Matrilin-3-Primed Adipose-Derived Mesenchymal Stromal Cell Spheroids Prevent Mesenchymal Stromal-Cell-Derived Chondrocyte Hypertrophy. International Journal of Molecular Sciences. 2020; 21(23):8911. https://doi.org/10.3390/ijms21238911
Chicago/Turabian StyleMuttigi, Manjunatha S., Byoung Ju Kim, Bogyu Choi, Inbo Han, Hansoo Park, and Soo-Hong Lee. 2020. "Matrilin-3-Primed Adipose-Derived Mesenchymal Stromal Cell Spheroids Prevent Mesenchymal Stromal-Cell-Derived Chondrocyte Hypertrophy" International Journal of Molecular Sciences 21, no. 23: 8911. https://doi.org/10.3390/ijms21238911






