Design of Polymeric and Biocompatible Delivery Systems by Dissolving Mesoporous Silica Templates
Abstract
:1. Introduction
2. Results
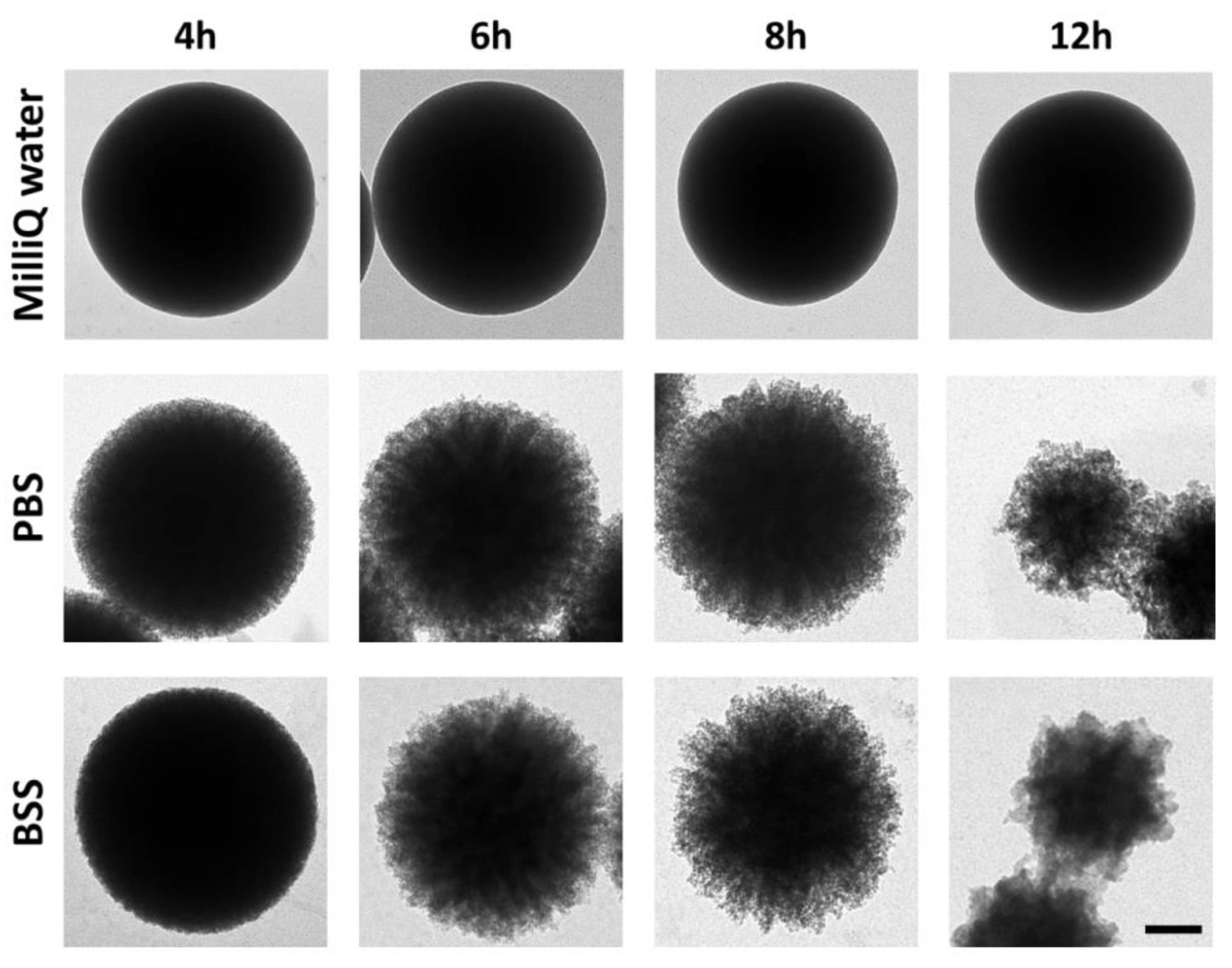
2.1. Optimization of the MSP Dissolution Process
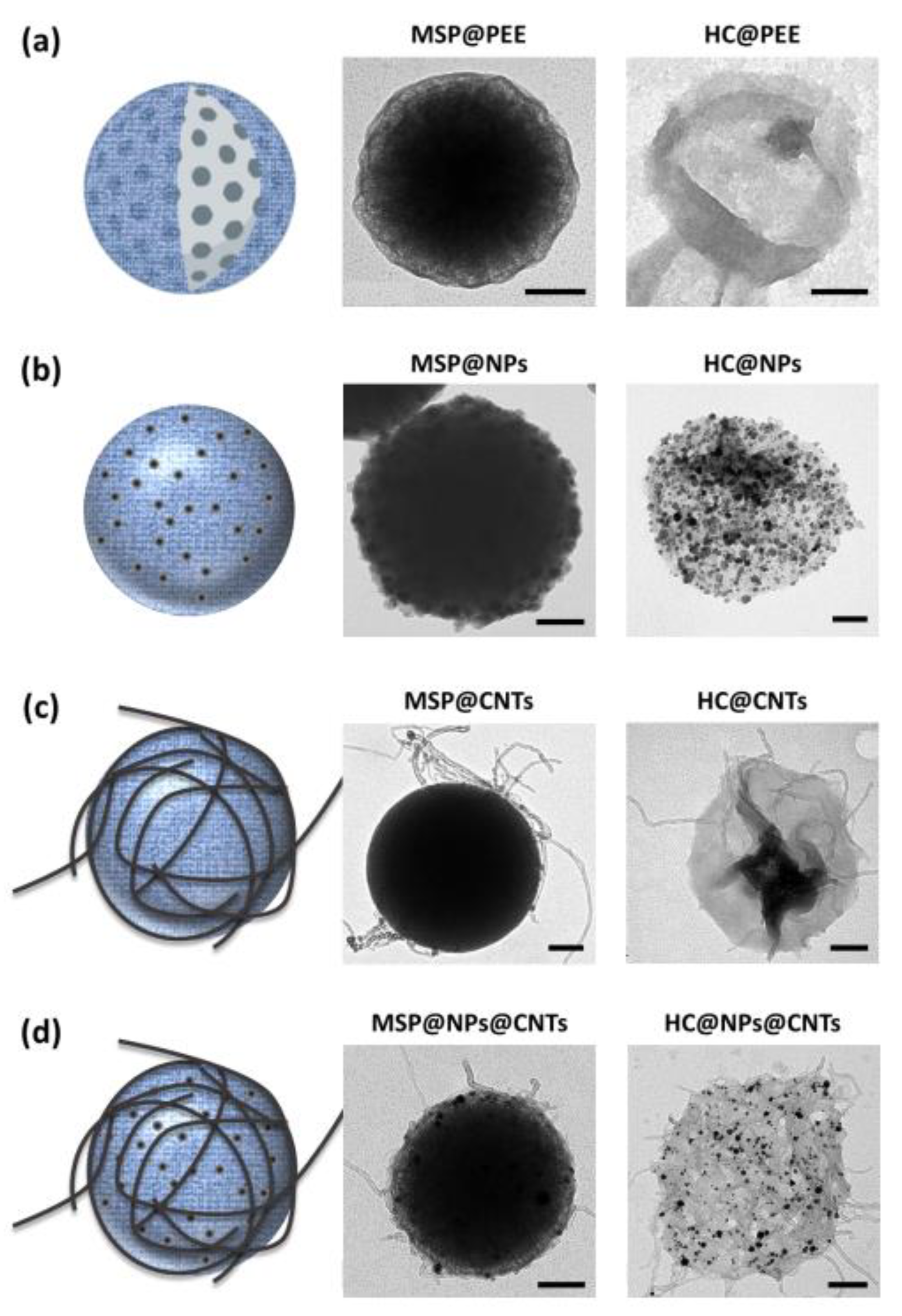
2.2. Producing and Customizing Polymeric Capsules by Dissolving the MSP Core
2.3. Producing Protein Capsules Using MSP Sacrificial Cores
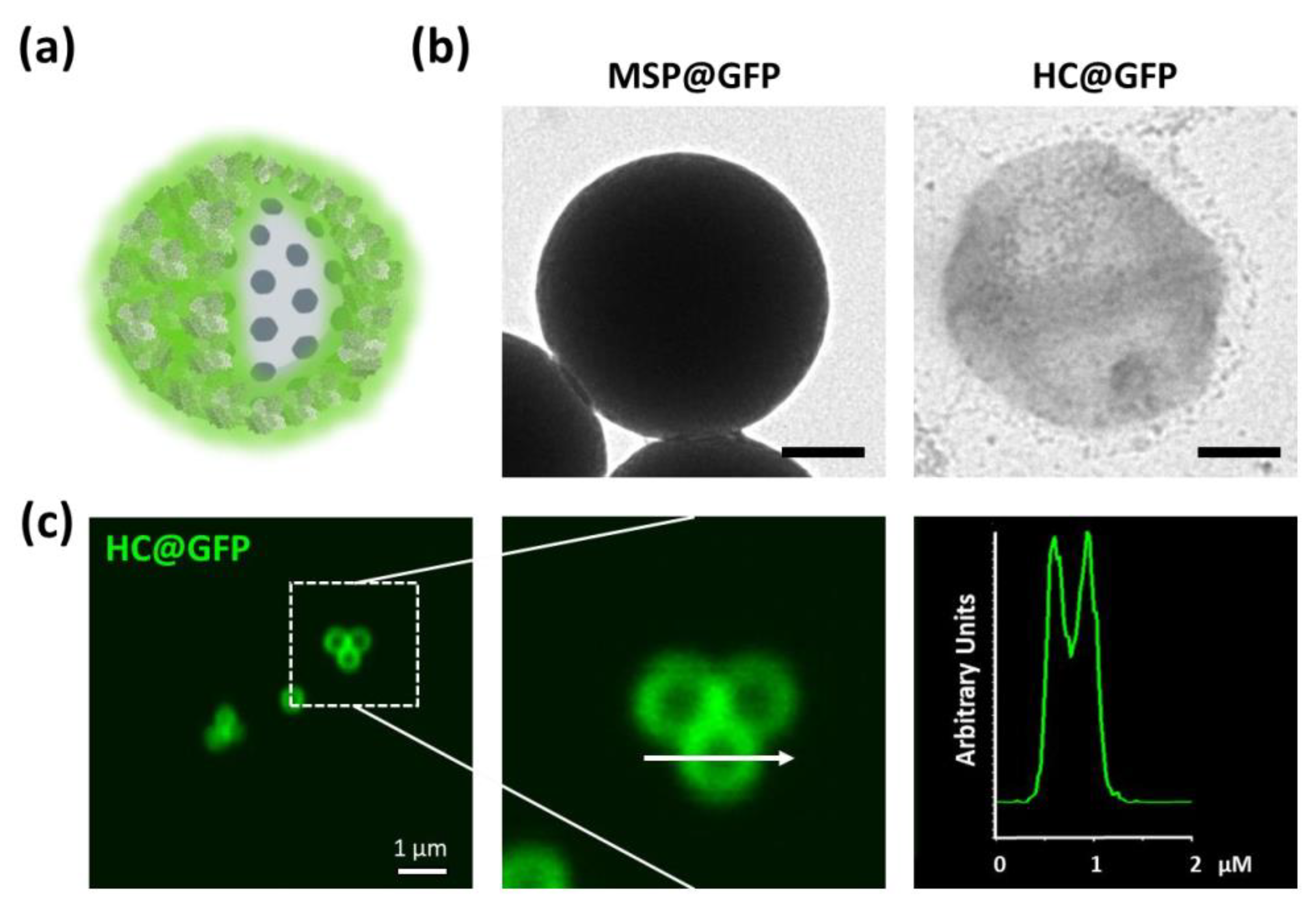
2.4. Loading the Polymeric/Protein Capsules
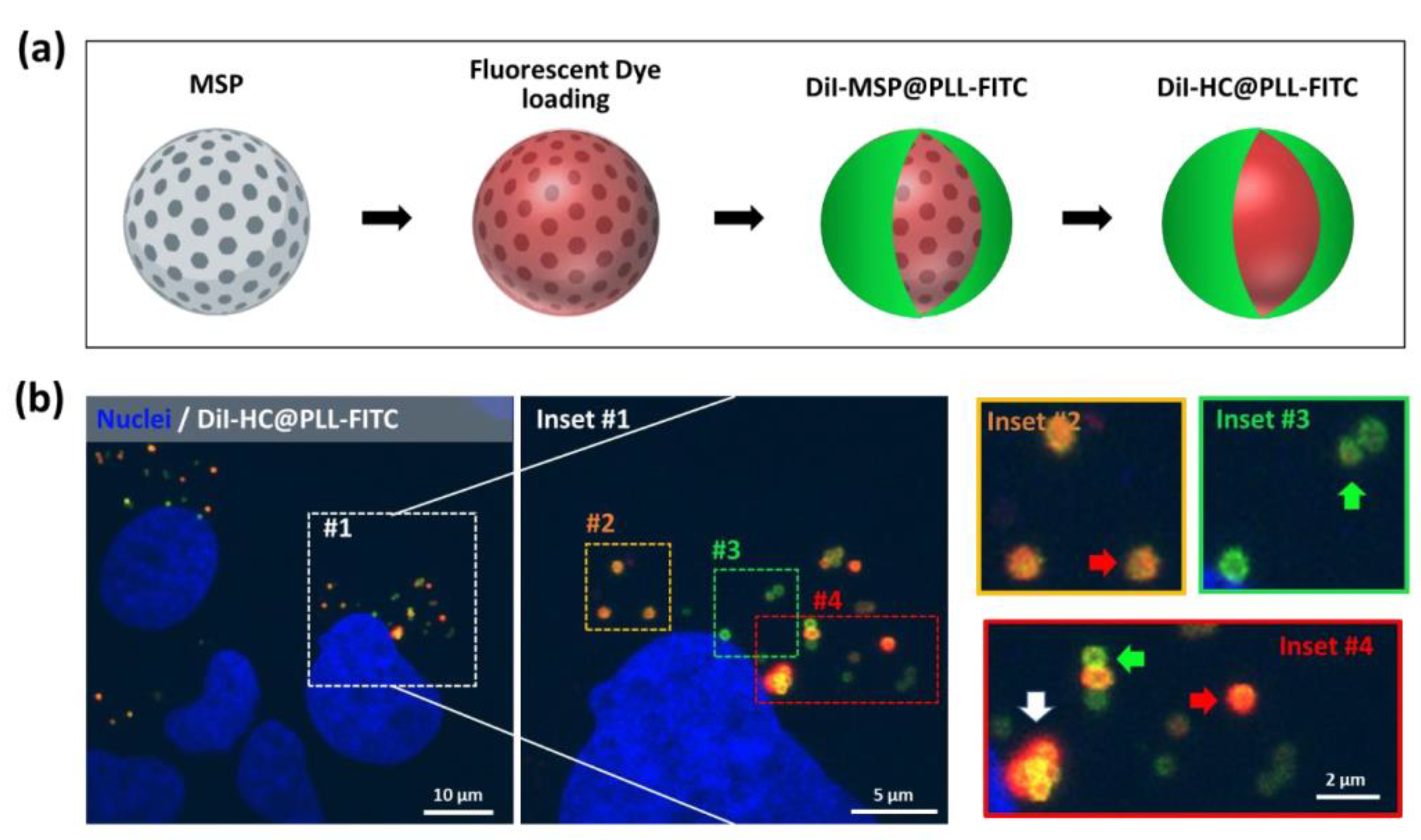
2.5. Polymeric Capsules are Engulfed by HeLa Cells and Deliver Their Contents
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis and Characterization of MSPs
4.3. MSP Coating
4.4. MSPs Loading
4.5. Dissolution of the Silica Core
4.6. Cell Culture, Fluorescent Cell Labeling, Confocal Microscopy, and Viability
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mo, R.; Jiang, T.; Gu, Z. Enhanced Anticancer Efficacy by ATP-Mediated Liposomal Drug Delivery. Angew. Int. Ed. Chem. 2014, 53, 1–7. [Google Scholar] [CrossRef]
- Dicheva, B.M.; ten Hagen, T.L.M.; Seynhaeve, A.L.B.; Amin, M.; Eggermont, A.M.M.; Koning, G.A. Enhanced Specificity and Drug Delivery in Tumors by cRGD-Anchoring Thermosensitive Liposomes. Pharm. Res. 2015, 32, 3862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.X.; Redemann, C.T.; Szoka, F.C. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem. 1996, 7, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, D.; Luo, K.; She, W.; Guo, C.; Yang, Y. Peptide Dendrimer–Doxorubicin Conjugate-Based Nanoparticle as an Enzyme-Responsive Drug Delivery System for Cancer Therapy. Adv. Healthc. Mater. 2014, 3, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Pehlivan, S.B.; Vural, İ.; Ünlü, N. In Vitro/In Vivo Evaluation of Dexamethasone—PAMAM Dendrimer Complexes for Retinal Drug Delivery. Pharm. Drug Deliv. Pharm. Technol. 2015, 104, 3814–3823. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Barz, M.; Rijcken, C.J.F.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, A.M.; Vladimir, P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki Dizaj, S.; Barzegar-Jalali, M.; Hossein Zarrintan, M.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef]
- Wu, H.; Shi, H.; Zhang, H.; Wang, X.; Yang, Y.; Yu, C.; Hao, C.; Du, J.; Hu, H.; Yang, S. Biomaterials Prostate stem cell antigen antibody-conjugated multiwalled carbon nanotubes for targeted ultrasound imaging and drug delivery. Biomaterials 2014, 35, 5369–5380. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhang, W.; Hong, C.; Pan, C. Silica Nanotubes Decorated by pH-Responsive Diblock Copolymers for Controlled Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonia, N.; Lather, V.; Pandita, D. Mesoporous silica nanoparticles: A smart nanosystem for management of breast cancer. Drug Discov. Today 2017, 23, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J.; Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Lin, V.S. Mesoporous Silica Nanoparticles for Intracellular Delivery of Membrane-Impermeable Proteins. JACS 2007, 129, 8845–8849. [Google Scholar] [CrossRef] [Green Version]
- Khosravian, P.; Ardestani, M.S.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Javar, H.A.; Amanlou, M. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. Oncol. Targets Ther. 2016, 9, 7315–7330. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.H.; Nel, A.E. Use of a Lipid-Coated Mesoporous Silica Nanoparticle Platform for Synergistic Gemcitabine and Paclitaxel Delivery to Human Pancreatic Cancer in Mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef] [Green Version]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Multifunctional Mesoporous Silica Nanoparticles for Combined Therapeutic, Diagnostic and Targeted Action in Cancer Treatment. Curr. Drugs Targets 2011, 12, 1166–1186. [Google Scholar] [CrossRef] [PubMed]
- Hadipour Moghaddam, S.P.; Mohammadpour, R.; Ghandehari, H. In vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. J. Control. Release 2019, 311–312, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Giovaninni, G.; Moore, C.J.; Hall, A.J.; Byrne, H.J.; Gubala, V. pH-Dependent silica nanoparticle dissolution and cargo release. Colloids Surf. B Biointerfaces 2018, 169, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.D.; Grosso, D.; Boissiere, C.; Belamie, E.; Coradin, T.; Sanchez, C. Stability of mesoporous oxide and mixed metal oxide materials under biologically relevant conditions. Chem. Mater. 2007, 19, 4349–4356. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Zhu, M.; Chen, Y.; Chen, F. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous Mesoporous Mater. 2010, 131, 314–320. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Degradable Drug Carriers: Vanishing Mesoporous Silica Nanoparticles. Chem. Mater. 2019, 31, 4364–4378. [Google Scholar] [CrossRef]
- Navarro-Palomares, E.; González-Saiz, P.; Renero-Lecuna, C.; Martín Rodríguez, R.; Aguado, F.; González-Alonso, D.; Fernandez Barquin, L.; Gonzalez, J.A.; Bañobre-López, M.; Fanarraga, M.L.; et al. Dye-doped biodegradable nanoparticle SiO2 coating in zinc- and iron-oxide nanoparticles to improve biocompatibility and in vivo imaging studies. Nanoscale 2020, 12, 6164–6175. [Google Scholar] [CrossRef]
- Utembe, W.; Potgieter, K.; Stefaniak, A.B.; Gulumian, M. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Part. Fibre Toxicol. 2015, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Seré, S.; De Roo, B.; Vervaele, M.; Van Gool, S.; Jacobs, S.; Seo, J.W.; Locquet, J. Altering the Biodegradation of Mesoporous Silica Nanoparticles by Means of Experimental Parameters and Surface Functionalization. J. Nanomater. 2018, 2018, 7390618. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-A.A.; Choi, S.; Jeon, S.M.; Yu, J. Silica nanoparticle stability in biological media revisited. Sci. Rep. 2018, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Braun, K.; Pochert, A.; Beck, M.; Fiedler, R.; Gruber, J.; Lindén, M. Dissolution kinetics of mesoporous silica nanoparticles in different simulated body fluids. J. Sol-Gel Sci. Technol. 2016, 79, 319–327. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.Á.; Valiente, R.; Fanarraga, M.L. Engineering sub-cellular targeting strategies to enhance safe cytosolic silica particle dissolution in cells. Pharmaceutics 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Correa-Duarte, M.A.; Kosiorek, A.; Kandulski, W.; Giersig, M.; Liz-Marzán, L.M. Layer-by-layer assembly of multiwall carbon nanotubes on spherical colloids. Chem. Mater. 2005, 17, 3268–3272. [Google Scholar] [CrossRef]
- Feng, W.; Zhou, X.; He, C.; Qiu, K.; Nie, W.; Chen, L.; Wang, H.; Mo, X.; Zhanga, Y. Polyelectrolyte multilayer functionalized mesoporous silica nanoparticles for pH-responsive drug delivery: Layer thickness-dependent release profiles and biocompatibility. J. Mater. Chem. B 2013, 1, 5886–5898. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Sun, C.; Zhang, X.; Wu, Z.; Wang, Z.; Li, C. Hollow and degradable polyelectrolyte nanocapsules for protein drug delivery. Acta Biomater. 2010, 6, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Dove, P.M.; Han, N.; Wallace, A.F.; De Yoreo, J.J. Kinetics of amorphous silica dissolution and the paradox of the silica polymorphs. Proc. Natl. Acad. Sci. USA 2008, 105, 9903–9908. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.L.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M.; Vallet-Regí, M. Tuning mesoporous silica dissolution in physiological environments: A review. J. Mater. Sci. 2017, 52, 8761–8771. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Li, Z.; Wei, L.; Gao, M.; Lei, H. One-pot reaction to synthesize biocompatible magnetite nanoparticles. Adv. Mater. 2005, 17, 1001–1005. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Tung, L.D.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; González-Domínguez, E.; González-Lavado, E.; Marín-Caba, L.; Vaz, B.; Pérez-Lorenzo, M.M.; Correa-Duarte, M.A.; Fanarraga, M.L. A Biomimetic Escape Strategy for Cytoplasm Invasion by Synthetic Particles. Angew. Chem.-Int. Ed. 2017, 56, 13736–13740. [Google Scholar] [CrossRef] [PubMed]
- Padín-González, E.; Navarro-Palomares, E.; Valdivia, L.; Iturrioz-Rodríguez, N.; Correa, M.A.; Valiente, R.; Fanarraga, M.L. A custom-made functionalization method to control the biological identity of nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102268. [Google Scholar] [CrossRef] [PubMed]
- Baseer, A.; Koenneke, A.; Zapp, J.; Khan, S.A.; Schneider, M. Design and Characterization of Surface-Crosslinked Gelatin Nanoparticles for the Delivery of Hydrophilic Macromolecular Drugs. Macromol. Chem. Phys. 2019, 220, 1900260. [Google Scholar] [CrossRef] [Green Version]
- Chaibi, S.; Benachour, D.; Merbah, M.; Esperanza Cagiao, M.; Baltá Calleja, F.J. The role of crosslinking on the physical properties of gelatin based films. Colloid Polym. Sci. 2015, 293, 2741–2752. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Wu, Y.H.; Tsai, W.B.; Tsai, C.C.; Chen, W.S.; Wu, C.S. Core-shell silica@chitosan nanoparticles and hollow chitosan nanospheres using silica nanoparticles as templates: Preparation and ultrasound bubble application. Carbohydr. Polym. 2011, 84, 770–774. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Becker, A.L.; Johnston, A.P.R.; Wark, K.L.; Turatti, F.; Caruso, F. A general approach for DNA encapsulation in degradable polymer microcapsules. ACS Nano 2007, 1, 63–69. [Google Scholar] [CrossRef]
- Wang, Y.; Caruso, F. Nanoporous protein particles through templating mesoporous silica spheres. Adv. Mater. 2006, 18, 795–800. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, A.; Caruso, F. Nanoporous polyelectrolyte spheres prepared by sequentially coating sacrificial mesoporous silica spheres. Angew. Chem.-Int. Ed. 2005, 44, 2888–2892. [Google Scholar] [CrossRef]
- De Geest, B.G.; Vandenbroucke, R.E.; Guenther, A.M.; Sukhorukov, G.B.; Hennink, W.E.; Sanders, N.N.; Demeester, J.; De Smedt, S.C. Intracellularly degradable polyelectrolyte microcapsules. Adv. Mater. 2006, 18, 1005–1009. [Google Scholar] [CrossRef]
- Gao, H.; Goriacheva, O.A.; Tarakina, N.V.; Sukhorukov, G.B. Intracellularly Biodegradable Polyelectrolyte/Silica Composite Microcapsules as Carriers for Small Molecules. ACS Appl. Mater. Interfaces 2016, 8, 9651–9661. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 2004, 14, 2073–2081. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Owen, A.; Rannard, S.; McDonald, T.O. Controlled synthesis of calcium carbonate nanoparticles and stimuli-responsive multi-layered nanocapsules for oral drug delivery. Int. J. Pharm. 2020, 574. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.; Ivanova, A.; Zyuzin, M.; Timin, A. Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Koker, S.; De Cock, L.J.; Rivera-Gil, P.; Parak, W.J.; Auzély Velty, R.; Vervaet, C.; Remon, J.P.; Grooten, J.; De Geest, B.G. Polymeric multilayer capsules delivering biotherapeutics. Adv. Drug Deliv. Rev. 2011, 63, 748–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Cui, X.; Cui, F.; Guo, L.; Shi, J. Size-controlled synthesis of monodispersed mesoporous silica nano-spheres under a neutral condition. Microporous Mesoporous Mater. 2009, 117, 609–616. [Google Scholar] [CrossRef]
- Marín-Caba, L.; Chariou, P.L.; Pesquera, C.; Correa-Duarte, M.A.; Steinmetz, N.F. Tobacco Mosaic Virus-Functionalized Mesoporous Silica Nanoparticles, a Wool-Ball-like Nanostructure for Drug Delivery. Langmuir 2019, 35, 203–211. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210, 831–835. [Google Scholar] [CrossRef]
- González-Domínguez, E.; Iturrioz-Rodríguez, N.; Padín-González, E.; Villegas, J.C.; García-Hevia, L.; Pérez-Lorenzo, M.; Parak, W.J.W.; Correa-Duarte, M.A.; Fanarraga, M.L. Carbon nanotubes gathered onto silica particles lose their biomimetic properties with the cytoskeleton becoming biocompatible. Int. J. Nanomed. 2017, 12, 6317–6328. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.; Autenrieth, T.; Hempelmann, R. Core shell particles consisting of cobalt ferrite and silica as model ferrofluids [CoFe2O4-SiO2 core shell particles]. J. Magn. Magn. Mater. 2002, 252, 4–6. [Google Scholar] [CrossRef]
- Sanles-Sobrido, M.; Correa-Duarte, A.; Carregal-Romero, S.; Rodríguez-González, B.; Álvarez-Puebla, R.A.; Hervés, P.; Liz-Marzán, L.M. Highly catalytic single-crystal dendritic pt nanostructures supported on carbon nanotubes. Chem. Mater. 2009, 21, 1531–1535. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ramos, A.; Marín-Caba, L.; Iturrioz-Rodríguez, N.; Padín-González, E.; García-Hevia, L.; Mêna Oliveira, T.; Corea-Duarte, M.A.; Fanarraga, M.L. Design of Polymeric and Biocompatible Delivery Systems by Dissolving Mesoporous Silica Templates. Int. J. Mol. Sci. 2020, 21, 9573. https://doi.org/10.3390/ijms21249573
Rodríguez-Ramos A, Marín-Caba L, Iturrioz-Rodríguez N, Padín-González E, García-Hevia L, Mêna Oliveira T, Corea-Duarte MA, Fanarraga ML. Design of Polymeric and Biocompatible Delivery Systems by Dissolving Mesoporous Silica Templates. International Journal of Molecular Sciences. 2020; 21(24):9573. https://doi.org/10.3390/ijms21249573
Chicago/Turabian StyleRodríguez-Ramos, Ana, Laura Marín-Caba, Nerea Iturrioz-Rodríguez, Esperanza Padín-González, Lorena García-Hevia, Teresa Mêna Oliveira, Miguel A. Corea-Duarte, and Mónica L. Fanarraga. 2020. "Design of Polymeric and Biocompatible Delivery Systems by Dissolving Mesoporous Silica Templates" International Journal of Molecular Sciences 21, no. 24: 9573. https://doi.org/10.3390/ijms21249573





