Unfavorable Reduction in the Ratio of Endothelin B to A Receptors in Experimental 5/6 Nephrectomy and Adenine Models of Chronic Renal Insufficiency
Abstract
:1. Introduction
2. Results
2.1. Twelve-Weeks of Chronic Renal Insufficiency Induced by 5/6 Nephrectomy
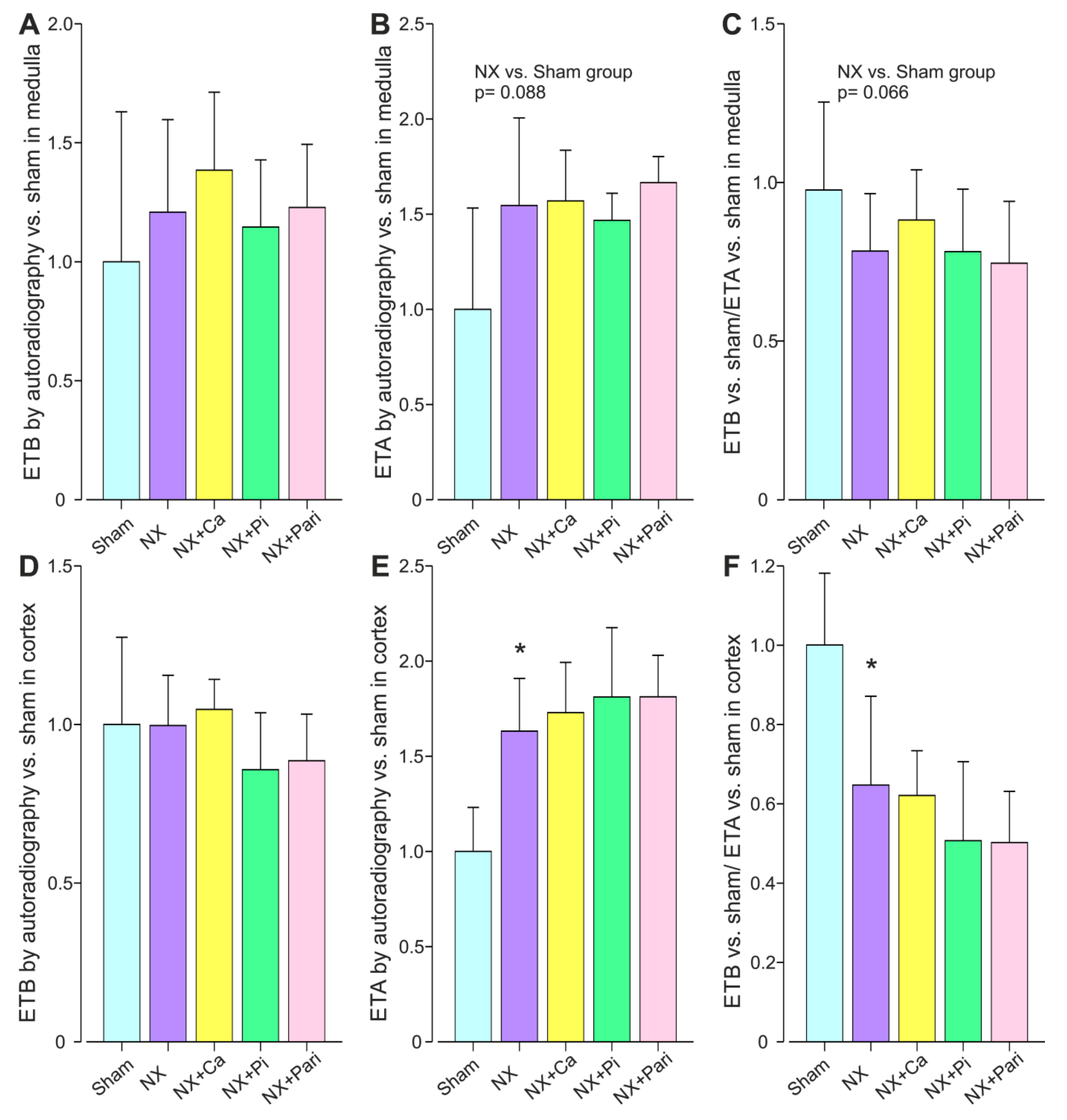
2.2. Twenty-Seven Weeks of Chronic Renal Insufficiency Induced by 5/6 Nephrectomy
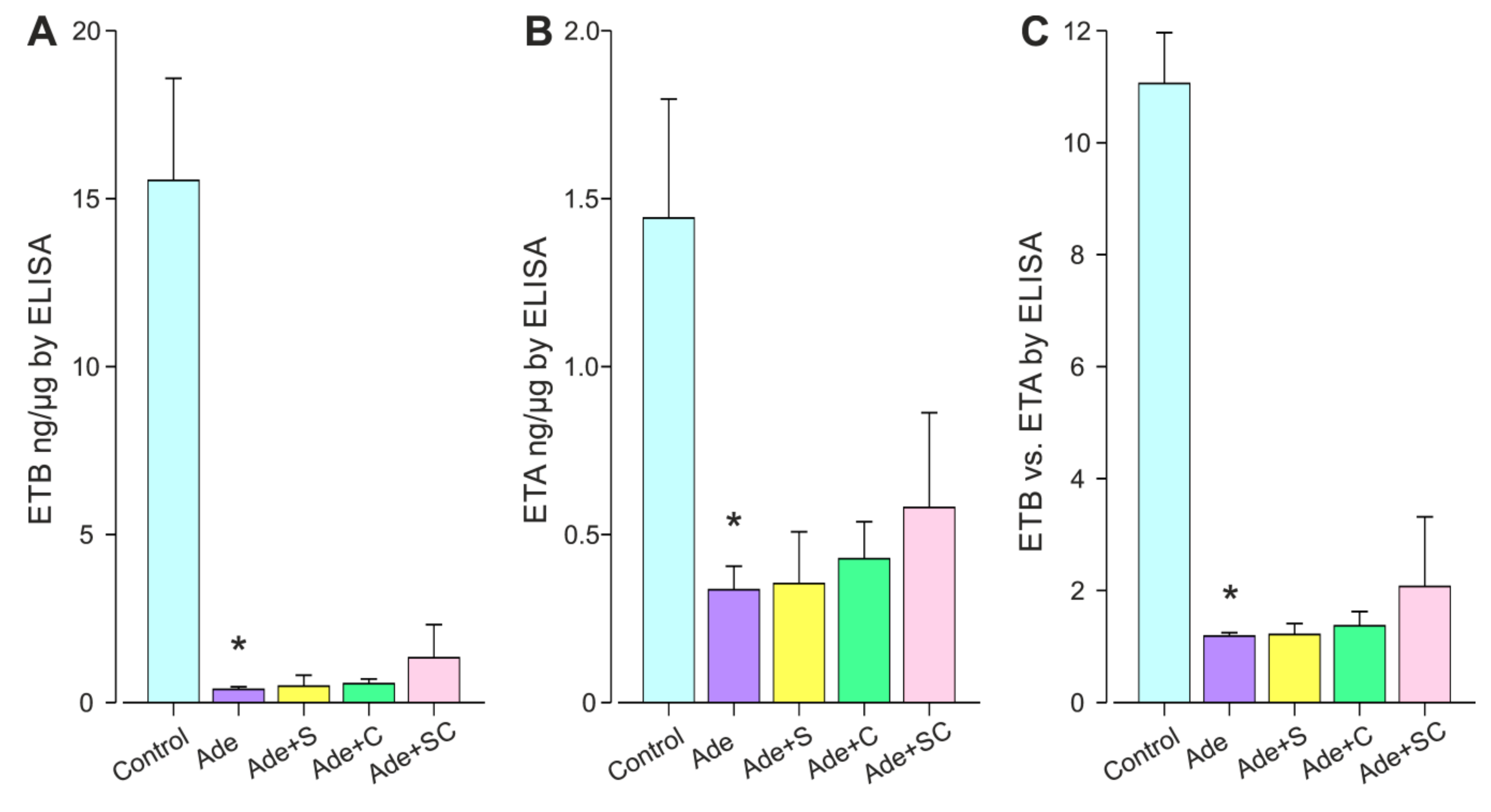
2.3. Twelve Weeks of Chronic Renal Insufficiency Induced by 0.3% Adenine Diet
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.1.1. Twelve-Week 5/6 Nephrectomy Model with Oxonic Acid Diet Modulation
4.1.2. Twenty-Seven-Week 5/6 Nephrectomy Model with Calcium and Phosphate Diets and Intraperitoneal Paricacitol Injections
4.1.3. Twelve-Week Adenine Model with Endothelin a Receptor Antagonist and Calcimimetic
4.2. Plasma and Urine Determinations
4.3. Real-Time Quantitative RT-PCR
4.4. Kidney Autoradiography
4.5. ELISA
4.6. Tubulointerstitial Kidney Histology
4.7. Data Presentation and Analysis of Results
Author Contributions
Funding
Conflicts of Interest
References
- Eckardt, K.U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Kottgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Kohan, D.E.; Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014, 86, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Longaretti, L.; Benigni, A. Endothelin receptor selectivity in chronic renal failure. Eur. J. Clin. Invest. 2009, 39, 32–37. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Parving, H.H.; Andress, D.L.; Bakris, G.; Correa-Rotter, R.; Hou, F.F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J.V.; et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947. [Google Scholar] [CrossRef]
- Vaneckova, I.; Hojna, S.; Kadlecova, M.; Vernerova, Z.; Kopkan, L.; Cervenka, L.; Zicha, J. Renoprotective effects of ET(A) receptor antagonists therapy in experimental non-diabetic chronic kidney disease: Is there still hope for the future? Physiol. Res. 2018, 67, S55–S67. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.M. Role of endothelin in chronic renal failure—Developments in renal involvement. Rheumatology (Oxford) 2006, 45, iii36–iii38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speed, J.S.; Pollock, D.M. Endothelin, kidney disease, and hypertension. Hypertension 2013, 61, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, W.; Pittrow, D. Endothelin receptor selectivity in chronic kidney disease: Rationale and review of recent evidence. Eur. J. Clin. Invest. 2009, 39, 50–67. [Google Scholar] [CrossRef]
- Hocher, B.; Thone-Reineke, C.; Rohmeiss, P.; Schmager, F.; Slowinski, T.; Burst, V.; Siegmund, F.; Quertermous, T.; Bauer, C.; Neumayer, H.H.; et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J. Clin. Invest. 1997, 99, 1380–1389. [Google Scholar] [CrossRef]
- Liefeldt, L.; Schonfelder, G.; Bocker, W.; Hocher, B.; Talsness, C.E.; Rettig, R.; Paul, M. Transgenic rats expressing the human ET-2 gene: A model for the study of endothelin actions in vivo. J. Mol. Med. (Berl) 1999, 77, 565–574. [Google Scholar] [CrossRef]
- Maguire, J.J.; Davenport, A.P. Endothelin receptors and their antagonists. Semin. Nephrol. 2015, 35, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhaun, N.; Webb, D.J. Endothelins in cardiovascular biology and therapeutics. Nat. Rev. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ohkita, M.; Takaoka, M.; Shiota, Y.; Nojiri, R.; Matsumura, Y. Nitric oxide inhibits endothelin-1 production through the suppression of nuclear factor kappa B. Clin. Sci. (Lond) 2002, 103, 68S–71S. [Google Scholar] [CrossRef] [PubMed]
- Vila Cuenca, M.; Hordijk, P.L.; Vervloet, M.G. Most exposed: The endothelium in chronic kidney disease. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef] [PubMed]
- Dhaun, N.; Goddard, J.; Webb, D.J. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Wang, X.; Zheng, L.; Li, J.; Xu, M.; Rong, R.; Zhu, T.; Jia, Y. Downregulation of endothelin A receptor (ETaR) ameliorates renal ischemia reperfusion injury by increasing nitric oxide production. Life Sci. 2019, 228, 295–304. [Google Scholar] [CrossRef]
- Tormanen, S.; Porsti, I.; Lakkisto, P.; Tikkanen, I.; Niemela, O.; Paavonen, T.; Mustonen, J.; Eraranta, A. Endothelin A receptor blocker and calcimimetic in the adenine rat model of chronic renal insufficiency. BMC Nephrol. 2017, 18, 323. [Google Scholar] [CrossRef] [Green Version]
- Anand, R.; Harry, D.; Holt, S.; Milner, P.; Dashwood, M.; Goodier, D.; Jarmulowicz, M.; Moore, K. Endothelin is an important determinant of renal function in a rat model of acute liver and renal failure. Gut 2002, 50, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Arfian, N.; Emoto, N.; Vignon-Zellweger, N.; Nakayama, K.; Yagi, K.; Hirata, K. ET-1 deletion from endothelial cells protects the kidney during the extension phase of ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2012, 425, 443–449. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Prince, S.; Fadel, F.; El Moghrabi, S.; Warnock, D.G.; Kolkhof, P.; Jaisser, F. Sulfenic Acid Modification of Endothelin B Receptor is Responsible for the Benefit of a Nonsteroidal Mineralocorticoid Receptor Antagonist in Renal Ischemia. J. Am. Soc. Nephrol. 2016, 27, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Benigni, A.; Zola, C.; Corna, D.; Orisio, S.; Facchinetti, D.; Benati, L.; Remuzzi, G. Blocking both type A and B endothelin receptors in the kidney attenuates renal injury and prolongs survival in rats with remnant kidney. Am. J. Kidney Dis. 1996, 27, 416–423. [Google Scholar] [CrossRef]
- Chen, S.; Evans, T.; Deng, D.; Cukiernik, M.; Chakrabarti, S. Hyperhexosemia induced functional and structural changes in the kidneys: Role of endothelins. Nephron 2002, 90, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Nakamura, T.; Ebihara, I.; Osada, S.; Tomino, Y.; Masaki, T.; Goto, K.; Furuichi, Y.; Koide, H. Gene expression for endothelins and their receptors in glomeruli of diabetic rats. J. Lab. Clin. Med. 1993, 122, 149–156. [Google Scholar] [PubMed]
- Nakamura, T.; Ebihara, I.; Fukui, M.; Osada, S.; Tomino, Y.; Masaki, T.; Goto, K.; Furuichi, Y.; Koide, H. Increased endothelin and endothelin receptor mRNA expression in polycystic kidneys of cpk mice. J. Am. Soc. Nephrol. 1993, 4, 1064–1072. [Google Scholar]
- Nakamura, T.; Ebihara, I.; Fukui, M.; Osada, S.; Tomino, Y.; Masaki, T.; Goto, K.; Furuichi, Y.; Koide, H. Renal expression of mRNAs for endothelin-1, endothelin-3 and endothelin receptors in NZB/W F1 mice. Ren. Physiol. Biochem. 1993, 16, 233–243. [Google Scholar] [CrossRef]
- Nakamura, T.; Ebihara, I.; Fukui, M.; Osada, S.; Tomino, Y.; Masaki, T.; Goto, K.; Furuichi, Y.; Koide, H. Modulation of glomerular endothelin and endothelin receptor gene expression in aminonucleoside-induced nephrosis. J. Am. Soc. Nephrol. 1995, 5, 1585–1590. [Google Scholar]
- Shimizu, T.; Hata, S.; Kuroda, T.; Mihara, S.; Fujimoto, M. Different roles of two types of endothelin receptors in partial ablation-induced chronic renal failure in rats. Eur. J. Pharmacol. 1999, 381, 39–49. [Google Scholar] [CrossRef]
- Yoshimura, A.; Iwasaki, S.; Inui, K.; Ideura, T.; Koshikawa, S.; Yanagisawa, M.; Masaki, T. Endothelin-1 and endothelin B type receptor are induced in mesangial proliferative nephritis in the rat. Kidney Int. 1995, 48, 1290–1297. [Google Scholar] [CrossRef] [Green Version]
- Zager, R.A.; Johnson, A.C.; Andress, D.; Becker, K. Progressive endothelin-1 gene activation initiates chronic/end-stage renal disease following experimental ischemic/reperfusion injury. Kidney Int. 2013, 84, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Eraranta, A.; Kurra, V.; Tahvanainen, A.M.; Vehmas, T.I.; Koobi, P.; Lakkisto, P.; Tikkanen, I.; Niemela, O.J.; Mustonen, J.T.; Porsti, I.H. Oxonic acid-induced hyperuricemia elevates plasma aldosterone in experimental renal insufficiency. J. Hypertens 2008, 26, 1661–1668. [Google Scholar] [CrossRef]
- Kurra, V.; Eraranta, A.; Jolma, P.; Vehmas, T.I.; Riutta, A.; Moilanen, E.; Tahvanainen, A.; Kalliovalkama, J.; Niemela, O.; Myllymaki, J.; et al. Hyperuricemia, oxidative stress, and carotid artery tone in experimental renal insufficiency. Am. J. Hypertens 2009, 22, 964–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurra, V.; Vehmas, T.; Eraranta, A.; Jokihaara, J.; Pirttiniemi, P.; Ruskoaho, H.; Tokola, H.; Niemela, O.; Mustonen, J.; Porsti, I. Effects of oxonic acid-induced hyperuricemia on mesenteric artery tone and cardiac load in experimental renal insufficiency. BMC Nephrol. 2015, 16, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eraranta, A.; Riutta, A.; Fan, M.; Koskela, J.; Tikkanen, I.; Lakkisto, P.; Niemela, O.; Parkkinen, J.; Mustonen, J.; Porsti, I. Dietary phosphate binding and loading alter kidney angiotensin-converting enzyme mRNA and protein content in 5/6 nephrectomized rats. Am. J. Nephrol. 2012, 35, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Eraranta, A.; Tormanen, S.; Koobi, P.; Vehmas, T.I.; Lakkisto, P.; Tikkanen, I.; Moilanen, E.; Niemela, O.; Mustonen, J.; Porsti, I. Phosphate binding reduces aortic angiotensin-converting enzyme and enhances nitric oxide bioactivity in experimental renal insufficiency. Am. J. Nephrol. 2014, 39, 400–408. [Google Scholar] [CrossRef]
- Porsti, I.; Fan, M.; Koobi, P.; Jolma, P.; Kalliovalkama, J.; Vehmas, T.I.; Helin, H.; Holthofer, H.; Mervaala, E.; Nyman, T.; et al. High calcium diet down-regulates kidney angiotensin-converting enzyme in experimental renal failure. Kidney Int. 2004, 66, 2155–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karavalakis, E.; Eraranta, A.; Vehmas, T.I.; Koskela, J.K.; Koobi, P.; Mustonen, J.; Niemela, O.; Rysa, J.; Ruskoaho, H.; Porsti, I. Paricalcitol treatment and arterial tone in experimental renal insufficiency. Nephron. Exp. Nephrol. 2008, 109, e84–e93. [Google Scholar] [CrossRef]
- Wolf, S.C.; Sauter, G.; Risler, T.; Brehm, B.R. Effects of combined endothelin and angiotensin II antagonism on growth factor-induced proliferation of vascular smooth muscle cells isolated from uremic rats. Ren. Fail. 2005, 27, 465–474. [Google Scholar] [CrossRef]
- Certikova Chabova, V.; Kujal, P.; Vanourkova, Z.; Skaroupkova, P.; Sadowski, J.; Kompanowska-Jezierska, E.; Tesar, V.; Hammock, B.; Imig, J.; Maxova, H.; et al. Addition of Endothelin A-Receptor Blockade Spoils the Beneficial Effect of Combined Renin-Angiotensin and Soluble Epoxide Hydrolase Inhibition: Studies on the Course of Chronic Kidney Disease in 5/6 Nephrectomized Ren-2 Transgenic Hypertensive Rats. Kidney Blood Press Res. 2019, 1–13. [Google Scholar] [CrossRef]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez Lozada, L.G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef]
- Rossi, G.P.; Cavallin, M.; Nussdorfer, G.G.; Pessina, A.C. The endothelin-aldosterone axis and cardiovascular diseases. J. Cardiovasc. Pharmacol. 2001, 38 Suppl 2, S49–S52. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Miguel, P.; Medrano-Andres, D.; Lopes-Martin, V.; Arribas-Gomez, I.; Rodriguez-Puyol, M.; Rodriguez-Puyol, D.; Lopez-Ongil, S. Regulation of endothelin-converting enzyme-1 (ECE-1) by the calcimimetic R-568. Pharmacol. Res. 2013, 76, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tang, R.N.; Liu, H.; Xu, M.; Pan, M.M.; Liu, B.C. Cinacalcet attenuates the renal endothelial-to-mesenchymal transition in rats with adenine-induced renal failure. Am. J. Physiol. Renal. Physiol. 2014, 306, F138–F146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrke, I.; Waldherr, R.; Ritz, E.; Wagner, J. Renal endothelin-1 and endothelin receptor type B expression in glomerular diseases with proteinuria. J. Am. Soc. Nephrol. 2001, 12, 2321–2329. [Google Scholar] [PubMed]
- Lariviere, R.; Gauthier-Bastien, A.; Ung, R.V.; St-Hilaire, J.; Mac-Way, F.; Richard, D.E.; Agharazii, M. Endothelin type A receptor blockade reduces vascular calcification and inflammation in rats with chronic kidney disease. J. Hypertens 2017, 35, 376–384. [Google Scholar] [CrossRef]
- Ritter, C.; Zhang, S.; Finch, J.L.; Liapis, H.; Suarez, E.; Ferder, L.; Delmez, J.; Slatopolsky, E. Cardiac and renal effects of atrasentan in combination with enalapril and paricalcitol in uremic rats. Kidney Blood Press Res. 2014, 39, 340–352. [Google Scholar] [CrossRef]
- Peltonen, T.; Taskinen, P.; Napankangas, J.; Leskinen, H.; Ohtonen, P.; Soini, Y.; Juvonen, T.; Satta, J.; Vuolteenaho, O.; Ruskoaho, H. Increase in tissue endothelin-1 and ETA receptor levels in human aortic valve stenosis. Eur. Heart J. 2009, 30, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Zolk, O.; Quattek, J.; Sitzler, G.; Schrader, T.; Nickenig, G.; Schnabel, P.; Shimada, K.; Takahashi, M.; Bohm, M. Expression of endothelin-1, endothelin-converting enzyme, and endothelin receptors in chronic heart failure. Circulation 1999, 99, 2118–2123. [Google Scholar] [CrossRef] [Green Version]
- Lakkisto, P.; Palojoki, E.; Backlund, T.; Saraste, A.; Tikkanen, I.; Voipio-Pulkki, L.M.; Pulkki, K. Expression of heme oxygenase-1 in response to myocardial infarction in rats. J. Mol. Cell Cardiol. 2002, 34, 1357–1365. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Vaskonen, T.; Tikkanen, I.; Nurminen, K.; Ruskoaho, H.; Vapaatalo, H.; Muller, D.; Park, J.K.; Luft, F.C.; Mervaala, E.M. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension 2001, 37, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Dean, R.; Zhuo, J.; Alcorn, D.; Casley, D.; Mendelsohn, F.A. Cellular localization of endothelin receptor subtypes in the rat kidney following in vitro labelling. Clin. Exp. Pharmacol. Physiol. 1996, 23, 524–531. [Google Scholar] [CrossRef]
- Schwarz, U.; Amann, K.; Orth, S.R.; Simonaviciene, A.; Wessels, S.; Ritz, E. Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998, 53, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sham | Sham+Oxo | NX | NX + Oxo | Two-Way ANOVA p-Values | |||
|---|---|---|---|---|---|---|---|
| NX | Oxo | Interaction NX*Oxo | |||||
| Number of animals | 12 | 12 | 12 | 12 | |||
| Body weight (g) week 9 | 433 ± 8 | 412 ± 11 | 448 ± 10 | 411 ± 8 | 0.441 | 0.004 | 0.377 |
| Systolic blood pressure mmHg week 9 | 134 ± 7 | 136 ± 5 | 142 ± 6 | 152 ± 4 | 0.041 | 0.290 | 0.498 |
| Creatinineclearance (mL/min) | 2.89 ± 0.36 | 1.99 ± 0.16 | 1.21 ± 0.06* | 1.19 ± 0.09 † | ND | ND | ND |
| Plasma determinations | (n = 9) | (n = 12) | (n = 11) | (n= 12) | |||
| Creatinine (µmol/L) | 40.2 ± 5.1 | 48.5 ± 3.0 | 81.9 ± 3.1 | 83.1 ± 8.1 | <0.001 | 0.204 | 0.128 |
| Urea (mmol/L) | 6.6 ± 0.3 | 8.3 ± 0.4 | 13.5 ± 0.9 | 14.5 ± 2.0 | <0.001 | 0.099 | 0.171 |
| Uric acid (µmol/L) | 36.0 ± 10.7 | 117.3 ± 20.9 * | 62.9 ± 19.0 | 152.3 ± 19.0 # | ND | ND | ND |
| Calcium (mmol/L) | 2.42 ± 0.02 | 2.36 ± 0.02 | 2.43 ± 0.04 | 2.42 ± 0.02 | 0.280 | 0.235 | 0.399 |
| Phosphate (mmol/L) | 1.38 ± 0.07 | 1.51 ± 0.06 | 1.88 ± 0.17 | 1.92 ± 0.18 | 0.002 | 0.427 | 0.618 |
| Urine protein (mg/24 h) | 70.5 ± 10.1 | 44.0 ± 8.6 | 323.2 ± 31.6 | 247.0 ± 44.0 | <0.001 | 0.006 | 0.616 |
| Tubulointerstitial damage index | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.3 ± 0.4* | 0.7 ± 0.3†# | ND | ND | ND |
| Kidney Endothelin receptors | |||||||
| ETB mRNA copies × 103/ng total RNA | 4.02 ± 0.29 | 2.61 ± 0.39 | 0.97 ± 0.15 | 0.90 ± 0.13 | <0.001 | 0.037 | 0.085 |
| ETA mRNA copies × 102/ng total RNA | 2.49 ± 0.26 | 1.72 ± 0.26 | 0.70 ± 0.14 | 0.81 ± 0.18 | <0.001 | 0.281 | 0.159 |
| ETB vs. ETA mRNA | 17.2 ± 1.1 | 16.7 ± 1.4 | 15.4 ± 1.6 | 15.7 ± 2.8 | 0.156 | 0.474 | 0.786 |
| Sham | NX | NX+Ca | NX+Pi | NX+Pari | |
|---|---|---|---|---|---|
| Number of animals | 13 | 7 | 11 | 7 | 9 |
| Body weight (g) | 565 ± 8 | 507 ± 41 | 481 ± 15 | 431 ± 38 | 503 ± 32 |
| Systolic blood pressure mmHg | 130 ± 2 | 171 ± 5 * | 143 ± 4 # | 161 ± 4 | 167 ± 5 |
| Creatinineclearance (mL/min) | 1.84 ± 0.11 | 0.85 ± 0.17 * | 0.84 ± 0.07 | 0.69 ± 0.18 | 0.71 ± 0.15 |
| Plasma determinations | |||||
| Creatinine (µmol/L) | 66.6 ± 2.2 | 170.0 ± 37.7 * | 116.5 ± 7.3 | 178.0 ± 30.9 | 209.0 ± 41.7 |
| Urea (mmol/L) | 5.3 ± 0.2 | 23.3 ± 8.7 * | 12.9 ± 1.1 | 34.9 ± 11.5 | 33.8 ± 9.8 |
| 25OH-D3 (nmol/L) | 33.4 ± 2.8 | 19.8 ± 4.0 * | 13.8 ± 0.9 | 16.0 ± 1.3 | 13.0 ± 0.9 |
| 1,25(OH)2D3 (pmol/L) | 273.0 ± 27.8 | 70.5 ± 22.9 * | 105.8 ± 22.0 | 105.5 ± 42.9 | 6.2 ± 1.2# |
| Ionized calcium (mmol/L) | 1.35 ± 0.01 | 1.34 ± 0.03 | 1.59 ± 0.04 # | 0.93 ± 0.09 # | 1.31 ± 0.05 |
| Phosphate (mmol/L) | 1.19 ± 0.05 | 2.52 ± 0.54 * | 0.70 ± 0.06 # | 5.47 ± 1.21 | 3.03 ± 0.43 |
| PTH (pg/mL) | 102.4 ± 52.7 | 1172.8 ± 369.8 * | 3.7 ± 0.5 # | 3619.7 ± 255.0 # | 618.9 ± 253.8 |
| Urine protein (mg/24 h) | 132.7 ± 21.0 | 359.1 ± 49.6 * | 522.5 ± 50.6 | 626.8 ± 39.5 # | 432.5 ± 56.6 |
| Tubulointerstitial damage index | 0.6 ± 0.1 | 2.9 ± 0.3 * | 1.9 ± 0.1# | 3.2 ± 0.3 | 3.5 ± 0.3 |
| Kidney Endothelin receptors | |||||
| ETB mRNA copies × 104/ng total RNA | 0.84 ± 0.07 | 1.41 ± 0.10 * | 1.52 ± 0.07 | 1.23 ± 0.10 | 1.28 ± 0.06 |
| ETA mRNA copies × 103/ng total RNA | 0.93 ± 0.08 | 2.43 ± 0.39 * | 2.12 ± 0.11 | 2.38 ± 0.29 | 1.85 ± 0.19 |
| ETB vs. ETA mRNA | 9.9 ± 1.4 | 6.7 ± 1.4 | 7.5 ± 0.8 | 5.7 ± 1.0 | 8.3 ± 2.0 |
| Control | Ade | Ade+S | Ade+C | Ade+SC | Adenine Groups Two-Way ANOVA p-Values | |||
|---|---|---|---|---|---|---|---|---|
| Sita | Cina | Interaction Sita*Cina | ||||||
| Number of animals | 12 | 20 | 16 | 16 | 16 | |||
| Body weight (g) | ||||||||
| week 12 | 450 ± 11 | 339 ± 6 * | 341 ± 6 | 340 ± 7 | 359 ± 8 | 0.136 | 0.160 | 0.253 |
| Systolic Blood Pressure (mmHg) | ||||||||
| week 9 | 137 ± 2 | 138 ± 1 | 130 ± 2 † | 138 ± 1 ‡ | 129 ± 2 | <0.001 | 0.624 | 0.722 |
| Creatinine clearance (mL/min) | 1.70 ± 0.12 | 0.28 ± 0.02 * | 0.41 ± 0.03 † | 0.33 ± 0.04 | 0.44 ± 0.05 | <0.001 | 0.494 | 0.743 |
| Plasma determinations | ||||||||
| Creatinine (µmol/l) | 61.3 ± 4.0 | 212.6 ± 9.0 * | 160.2 ± 9.2 † | 202.4 ± 16.6 | 154.2 ± 10.2 | <0.001 | 0.309 | 0.794 |
| Urea (mmol/L) | 9.0 ± 0.4 | 40.8 ± 1.4 * | 32.5 ± 1.3 † | 39.8 ± 2.2 | 30.8 ± 1.8 | <0.001 | 0.270 | 0.728 |
| Uric acid (µmol/l) | 93.8 ± 12.0 | 30.1 ± 4.6 * | 18.6 ± 1.4 † | 31.7 ± 4.5 ‡ | 18.0 ± 1.5 | <0.001 | 0.915 | 0.650 |
| Calcium (mmol/L) | 2.31 ± 0.02 | 2.27 ± 0.05 | 2.37 ± 0.12 ‡ | 2.20 ± 0.03 | 2.10 ± 0.03 | ND | ND | ND |
| Phosphate (mmol/L) | 1.10 ± 0.09 | 1.83 ± 0.09 * | 1.53 ± 0.08 ‡ | 1.95 ± 0.12 | 2.06 ± 0.08 | ND | ND | ND |
| PTH (pg/mL) | 31.6 ± 6.6 | 558.2 ± 51.2 * | 324.7 ± 63.1 †,‡ | 89.6 ± 28.2 † | 47.9 ± 9.4 | ND | ND | ND |
| Urine protein (mg/24 h) | 14.7 ± 1.1 | 13.9 ± 1.7 | 11.2 ± 1.1 | 12.5 ± 2.6 | 11.7 ± 1.4 | 0.710 | 0.522 | 0.416 |
| Kidney Endothelin receptors | ||||||||
| ETB mRNA copies × 104/ng total RNA | 2.69 ± 0.17 | 1.70 ± 0.10 * | 1.37 ± 0.12 | 1.67 ± 0.13 | 1.51 ± 0.10 | ND | ND | ND |
| ETA mRNA copies × 103/ng total RNA | 1.22 ± 0.13 | 5.78 ± 0.34 * | 5.41 ± 0.51 | 5.50 ± 0.43 | 4.47 ± 0.34 | 0.090 | 0.137 | 0.417 |
| ETB vs. ETA mRNA | 23.8 ± 1.9 | 3.0 ± 0.2 * | 2.6 ± 0.2 | 3.1 ± 0.2 | 3.7 ± 0.4 | 0.715 | 0.034 | 0.080 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törmänen, S.; Lakkisto, P.; Eräranta, A.; Kööbi, P.; Tikkanen, I.; Niemelä, O.; Mustonen, J.; Pörsti, I. Unfavorable Reduction in the Ratio of Endothelin B to A Receptors in Experimental 5/6 Nephrectomy and Adenine Models of Chronic Renal Insufficiency. Int. J. Mol. Sci. 2020, 21, 936. https://doi.org/10.3390/ijms21030936
Törmänen S, Lakkisto P, Eräranta A, Kööbi P, Tikkanen I, Niemelä O, Mustonen J, Pörsti I. Unfavorable Reduction in the Ratio of Endothelin B to A Receptors in Experimental 5/6 Nephrectomy and Adenine Models of Chronic Renal Insufficiency. International Journal of Molecular Sciences. 2020; 21(3):936. https://doi.org/10.3390/ijms21030936
Chicago/Turabian StyleTörmänen, Suvi, Päivi Lakkisto, Arttu Eräranta, Peeter Kööbi, Ilkka Tikkanen, Onni Niemelä, Jukka Mustonen, and Ilkka Pörsti. 2020. "Unfavorable Reduction in the Ratio of Endothelin B to A Receptors in Experimental 5/6 Nephrectomy and Adenine Models of Chronic Renal Insufficiency" International Journal of Molecular Sciences 21, no. 3: 936. https://doi.org/10.3390/ijms21030936





