UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model
Abstract
:1. Introduction
2. Results
2.1. Generation of Hydrophilic Surface on UV-Treated Titanium
2.2. Osteoblast Attachment to Titanium with or Without UV Treatment
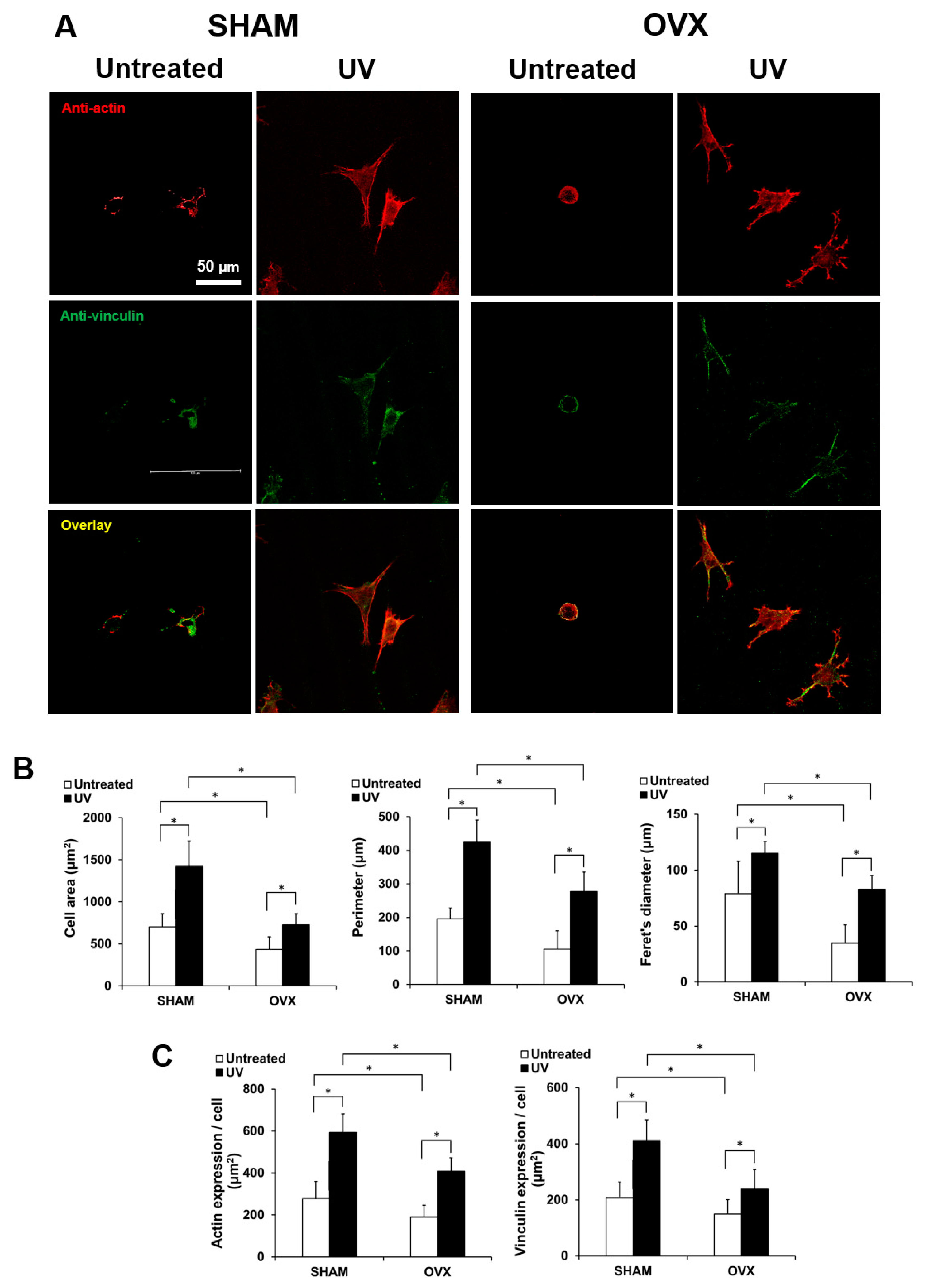
2.3. Spreading Behavior of Osteoblasts on Titanium with or Without UV Treatment
2.4. Osteoblast Proliferation on Titanium with or Without UV Treatment
2.5. Osteoblastic Differentiation on Titanium with or Without UV Treatment
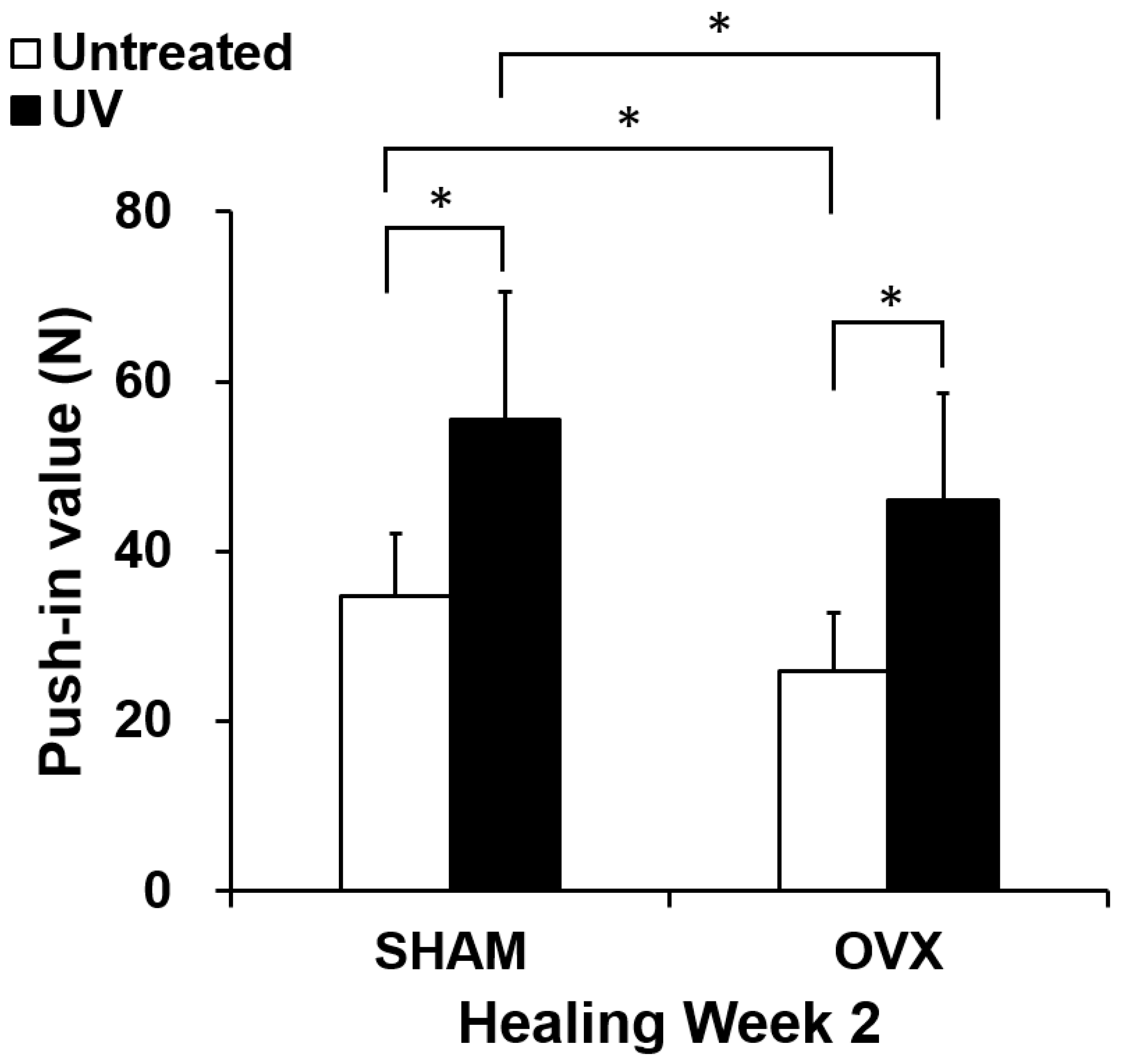
2.6. In Vivo Mechanical Anchorage of Titanium with or Without UV Treatment
3. Discussion
4. Materials and Methods
4.1. Titanium Samples Preparation
4.2. Animal Experiments
4.3. Osteoblastic Cell Culture
4.4. Cell Attachment and Proliferation Assays
4.5. Alkaline Phosphatase (ALP) Activity
4.6. Mineralization Staining
4.7. Morphology and Morphometry of Osteoblastic Cells
4.8. Gene Expression Analysis (Quantitative Real-Time PCR)
4.9. Implant Surgery
4.10. Biomechanical Implant Push-in Test
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| BMP-2 | Bone morphogenetic protein-2 |
| BrdU | 5-bromo-2′ deoxyuridine |
| cDNA | Complementary DNA |
| Col1a1 | collagen type I alpha 1 chain |
| ddH2O | double-distilled H2O |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| OVX | Ovariectomy |
| PCR | Polymerase chain reaction |
| WST | Water-soluble tetrazolium salts |
References
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, D.K.; Park, J.Y.; Yoon, Y.S.; Kuh, S.U.; Jin, B.H.; Kim, K.S.; Cho, Y.E. Prevalence of osteoporosis in patients requiring spine surgery: Incidence and significance of osteoporosis in spine disease. Osteoporos. Int. 2007, 18, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Inoue, G.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine 2013, 38, E487–E492. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, K.E.; Iyer, S.; Gupta, G.; Khanna, A.J. Instrumentation of the osteoporotic spine: Biomechanical and clinical considerations. Spine J. 2011, 11, 54–63. [Google Scholar] [CrossRef]
- Wu, Z.X.; Gong, F.T.; Liu, L.; Ma, Z.S.; Zhang, Y.; Zhao, X.; Yang, M.; Lei, W.; Sang, H.X. A comparative study on screw loosening in osteoporotic lumbar spine fusion between expandable and conventional pedicle screws. Arch. Orthop. Trauma Surg. 2012, 132, 471–476. [Google Scholar] [CrossRef]
- Slagter, K.W.; Raghoebar, G.M.; Vissink, A. Osteoporosis and edentulous jaws. Int. J. Prosthodont. 2008, 21, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Giro, G.; Chambrone, L.; Goldstein, A.; Rodrigues, J.A.; Zenobio, E.; Feres, M.; Figueiredo, L.C.; Cassoni, A.; Shibli, J.A. Impact of osteoporosis in dental implants: A systematic review. World J. Orthop. 2015, 6, 311–315. [Google Scholar] [CrossRef]
- Manzano, G.; Montero, J.; Martin-Vallejo, J.; del Fabbro, M.; Bravo, M.; Testori, T. Risk Factors in Early Implant Failure: A Meta-Analysis. Implant. Dent. 2016, 25, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T. Ultraviolet photofunctionalization of titanium implants. Int. J. Oral Maxillofac. Implant. 2014, 29, e95–e102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent degradation of the protein adsorption capacity of titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Ogawa, T. Biological aging of implant surfaces and their restoration with ultraviolet light treatment: A novel understanding of osseointegration. Int. J. Oral Maxillofac. Implant. 2012, 27, 753–761. [Google Scholar] [CrossRef]
- Lee, J.H.; Ogawa, T. The biological aging of titanium implants. Implant. Dent. 2012, 21, 415–421. [Google Scholar] [CrossRef]
- Friedlander, A.H. The physiology, medical management and oral implications of menopause. J. Am. Dent. Assoc. 2002, 133, 73–81. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.D.; Simmons, H.A.; Pirie, C.M.; Ke, H.Z. FDA Guidelines and animal models for osteoporosis. Bone 1995, 17, S125–S133. [Google Scholar] [CrossRef]
- Cho, P.; Schneider, G.B.; Krizan, K.; Keller, J.C. Examination of the bone-implant interface in experimentally induced osteoporotic bone. Implant. Dent. 2004, 13, 79–87. [Google Scholar] [CrossRef]
- Yamazaki, M.; Shirota, T.; Tokugawa, Y.; Motohashi, M.; Ohno, K.; Michi, K.; Yamaguchi, A. Bone reactions to titanium screw implants in ovariectomized animals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 87, 411–418. [Google Scholar] [CrossRef]
- Giro, G.; Goncalves, D.; Sakakura, C.E.; Pereira, R.M.; Marcantonio Junior, E.; Orrico, S.R. Influence of estrogen deficiency and its treatment with alendronate and estrogen on bone density around osseointegrated implants: Radiographic study in female rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Goergen, J.; Wenisch, S.; Raabe, O.; Moritz, A.; Schlewitz, G.; Schnettler, R.; Hempel, U.; Heiss, C.; Arnhold, S. Characterization of Bone-Marrow-Derived Stem Cells in Osteoporotic Models of the Rat. ISRN Stem Cells 2013, 2013, 262451. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.P.; Montecinos, L.; Rios, S.; Reyes, P.; Martinez, J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J. Cell. Biochem. 2000, 79, 557–565. [Google Scholar] [CrossRef]
- Oursler, M.J.; Osdoby, P.; Pyfferoen, J.; Riggs, B.L.; Spelsberg, T.C. Avian osteoclasts as estrogen target cells. Proc. Natl. Acad. Sci. USA 1991, 88, 6613–6617. [Google Scholar] [CrossRef] [Green Version]
- Eghbali-Fatourechi, G.; Khosla, S.; Sanyal, A.; Boyle, W.J.; Lacey, D.L.; Riggs, B.L. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Investig. 2003, 111, 1221–1230. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Jilka, R.L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef]
- Kimble, R.B.; Srivastava, S.; Ross, F.P.; Matayoshi, A.; Pacifici, R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J. Biol. Chem. 1996, 271, 28890–28897. [Google Scholar] [CrossRef] [Green Version]
- Kimble, R.B.; Vannice, J.L.; Bloedow, D.C.; Thompson, R.C.; Hopfer, W.; Kung, V.T.; Brownfield, C.; Pacifici, R. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J. Clin. Investig. 1994, 93, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Ammann, P.; Rizzoli, R.; Bonjour, J.P.; Bourrin, S.; Meyer, J.M.; Vassalli, P.; Garcia, I. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J. Clin. Investig. 1997, 99, 1699–1703. [Google Scholar] [CrossRef]
- Kousteni, S.; Bellido, T.; Plotkin, L.I.; O’Brien, C.A.; Bodenner, D.L.; Han, L.; Han, K.; Di Gregorio, G.B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 2001, 104, 719–730. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, A.; Ishijima, M.; Hasegawa, M.; Mohammadzadeh Rezaei, N.; Nakhaei, K.; Sekiya, T.; Torii, Y.; Hirota, M.; Park, W.; Miley, D.D.; et al. Biological and Physicochemical Characteristics of 2 Different Hydrophilic Surfaces Created by Saline-Storage and Ultraviolet Treatment. Implant. Dent. 2018, 27, 405–414. [Google Scholar] [CrossRef]
- Hirota, M.; Ikeda, T.; Sugita, Y.; Ishijima, M.; Hirota, S.; Ogawa, T. Impaired osteoblastic behavior and function on saliva-contaminated titanium and its restoration by UV treatment. Mater. Sci. Eng. C 2019, 100, 165–177. [Google Scholar] [CrossRef]
- Okubo, T.; Tsukimura, N.; Taniyama, T.; Ishijima, M.; Nakhaei, K.; Rezaei, N.M.; Hirota, M.; Park, W.; Akita, D.; Tateno, A.; et al. Ultraviolet treatment restores bioactivity of titanium mesh plate degraded by contact with medical gloves. J. Oral Sci. 2018, 60, 567–573. [Google Scholar] [CrossRef]
- Hirota, M.; Ozawa, T.; Iwai, T.; Ogawa, T.; Tohnai, I. Effect of Photofunctionalization on Early Implant Failure. Int. J. Oral Maxillofac. Implant. 2018, 33, 1098–1102. [Google Scholar] [CrossRef]
- Kitajima, H.; Ogawa, T. The Use of Photofunctionalized Implants for Low or Extremely Low Primary Stability Cases. Int. J. Oral Maxillofac. Implant. 2016, 31, 439–447. [Google Scholar] [CrossRef]
- Yamada, M.; Ueno, T.; Minamikawa, H.; Sato, N.; Iwasa, F.; Hori, N.; Ogawa, T. N-acetyl cysteine alleviates cytotoxicity of bone substitute. J. Dent. Res. 2010, 89, 411–416. [Google Scholar] [CrossRef]
- Yamada, M.; Kojima, N.; Att, W.; Minamikawa, H.; Sakurai, K.; Ogawa, T. Improvement in the osteoblastic cellular response to a commercial collagen membrane and demineralized freeze-dried bone by an amino acid derivative: An in vitro study. Clin. Oral Implant. Res. 2011, 22, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Ikeda, T.; Tsukimura, N.; Ishijima, M.; Minamikawa, H.; Sugita, Y.; Yamada, M.; Wakabayashi, N.; Ogawa, T. Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials 2016, 108, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, P.; Ghassemi, A.; Ishijima, M.; Tanaka, M.; Park, W.; Iwasaki, C.; Hirota, M.; Ogawa, T. Success rate and strength of osseointegration of immediately loaded UV-photofunctionalized implants in a rat model. J. Prosthet. Dent. 2017, 118, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, M.; Ghassemi, A.; Soltanzadeh, P.; Tanaka, M.; Nakhaei, K.; Park, W.; Hirota, M.; Tsukimura, N.; Ogawa, T. Effect of UV Photofunctionalization on Osseointegration in Aged Rats. Implant. Dent. 2016, 25, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Ikeda, T.; Hirota, M.; Nakagawa, K.; Park, W.; Miyazawa, K.; Goto, S.; Ogawa, T. Effect of UV Photofunctionalization on Biologic and Anchoring Capability of Orthodontic Miniscrews. Int. J. Oral Maxillofac. Implant. 2015, 30, 868–879. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, P.; Rai, D.V.; Garg, M.L. Zinc as a nutritional approach to bone loss prevention in an ovariectomized rat model. Menopause 2013, 20, 1184–1193. [Google Scholar] [CrossRef]
- Okamura, A.; Ayukawa, Y.; Iyama, S.; Koyano, K. Effect of the difference of bone turnover on peri-titanium implant osteogenesis in ovariectomized rats. J. Biomed. Mater. Res. A 2004, 70, 497–505. [Google Scholar] [CrossRef]
- Ocarino, N.M.; Boeloni, J.N.; Goes, A.M.; Silva, J.F.; Marubayashi, U.; Serakides, R. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide synthase inhibition. Nitric Oxide 2008, 19, 320–325. [Google Scholar] [CrossRef]
- Hadaya, D.; Gkouveris, I.; Soundia, A.; Bezouglaia, O.; Boyce, R.W.; Stolina, M.; Dwyer, D.; Dry, S.M.; Pirih, F.Q.; Aghaloo, T.L.; et al. Clinically Relevant Doses of Sclerostin Antibody Do Not Induce Osteonecrosis of the Jaw (ONJ) in Rats with Experimental Periodontitis. J. Bone Miner. Res. 2019, 34, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Yamada, M.; Sugita, Y.; Ogawa, T. Effect of photofunctionalization on fluoride-treated nanofeatured titanium. J. Biomater. Appl. 2014, 28, 1200–1212. [Google Scholar] [CrossRef]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, N.M.; Hasegawa, M.; Ishijima, M.; Nakhaei, K.; Okubo, T.; Taniyama, T.; Ghassemi, A.; Tahsili, T.; Park, W.; Hirota, M.; et al. Biological and osseointegration capabilities of hierarchically (meso-/micro-/nano-scale) roughened zirconia. Int. J. Nanomed. 2018, 13, 3381–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Watanabe, J.; Ueno, T.; Ogawa, T.; Egusa, H. Cytoprotective Preconditioning of Osteoblast-Like Cells with N-Acetyl-L-Cysteine for Bone Regeneration in Cell Therapy. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruta, J.; Sato, N.; Ishijima, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruta, J.; To, M.; Sugimoto, M.; Yamamoto, Y.; Shimizu, T.; Nakagawa, Y.; Inoue, H.; Saito, I.; Tsukinoki, K. Salivary Gland Derived BDNF Overexpression in Mice Exerts an Anxiolytic Effect. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Park, W.; Ishijima, M.; Hirota, M.; Soltanzadeh, P.; Ogawa, T. Engineering bone-implant integration with photofunctionalized titanium microfibers. J. Biomater. Appl. 2016, 30, 1242–1250. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniyama, T.; Saruta, J.; Mohammadzadeh Rezaei, N.; Nakhaei, K.; Ghassemi, A.; Hirota, M.; Okubo, T.; Ikeda, T.; Sugita, Y.; Hasegawa, M.; et al. UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model. Int. J. Mol. Sci. 2020, 21, 1235. https://doi.org/10.3390/ijms21041235
Taniyama T, Saruta J, Mohammadzadeh Rezaei N, Nakhaei K, Ghassemi A, Hirota M, Okubo T, Ikeda T, Sugita Y, Hasegawa M, et al. UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model. International Journal of Molecular Sciences. 2020; 21(4):1235. https://doi.org/10.3390/ijms21041235
Chicago/Turabian StyleTaniyama, Takashi, Juri Saruta, Naser Mohammadzadeh Rezaei, Kourosh Nakhaei, Amirreza Ghassemi, Makoto Hirota, Takahisa Okubo, Takayuki Ikeda, Yoshihiko Sugita, Masakazu Hasegawa, and et al. 2020. "UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model" International Journal of Molecular Sciences 21, no. 4: 1235. https://doi.org/10.3390/ijms21041235




