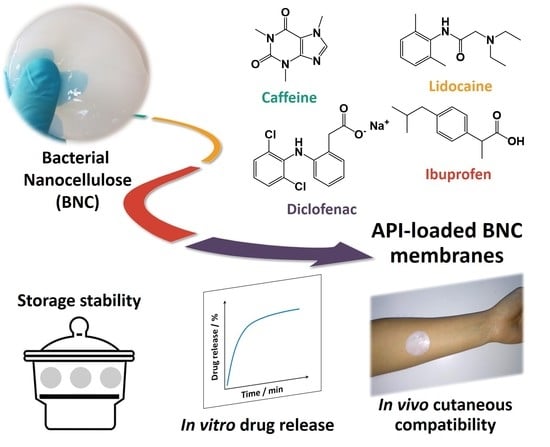
Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing
Abstract
:1. Introduction
2. Results and Discussion
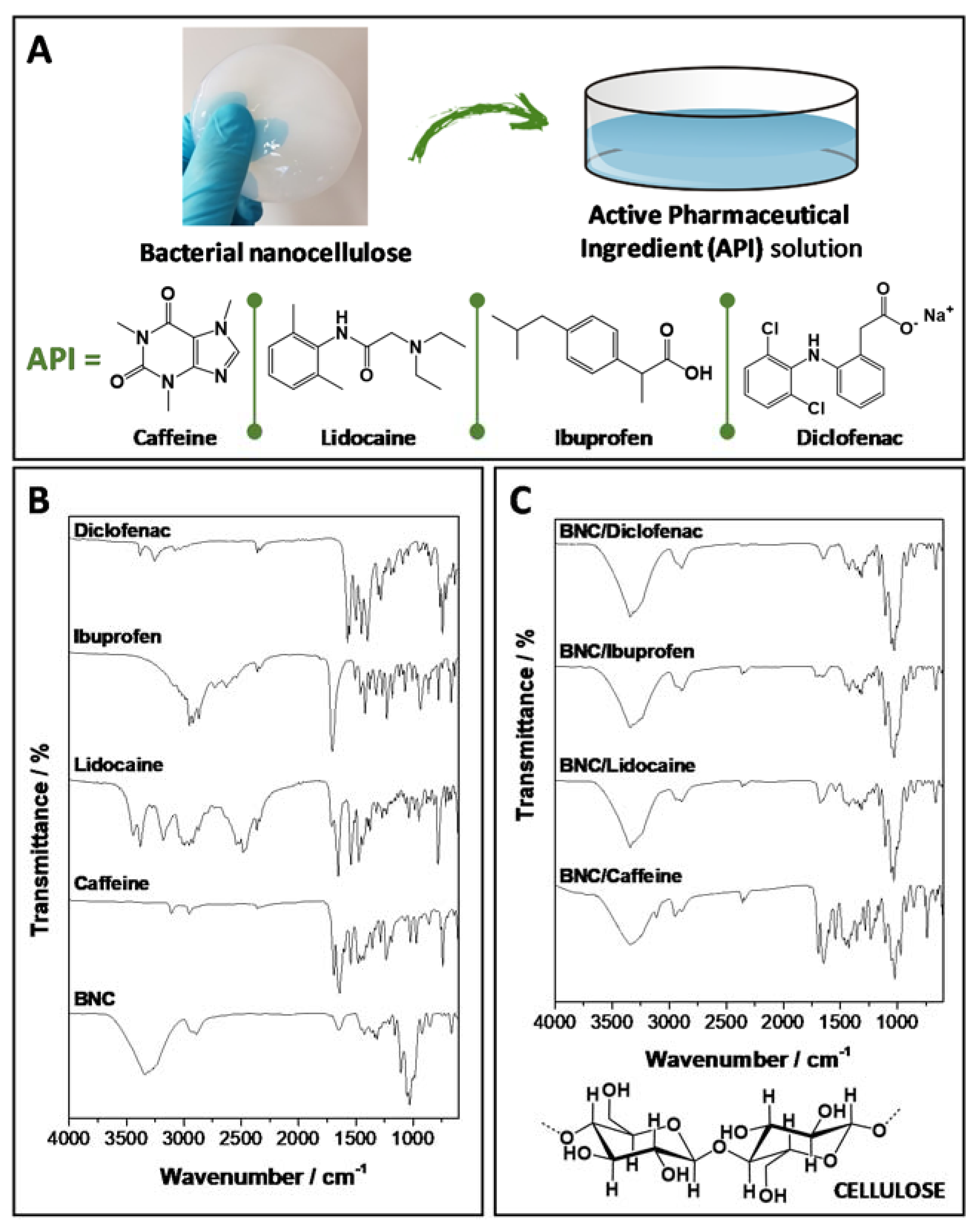
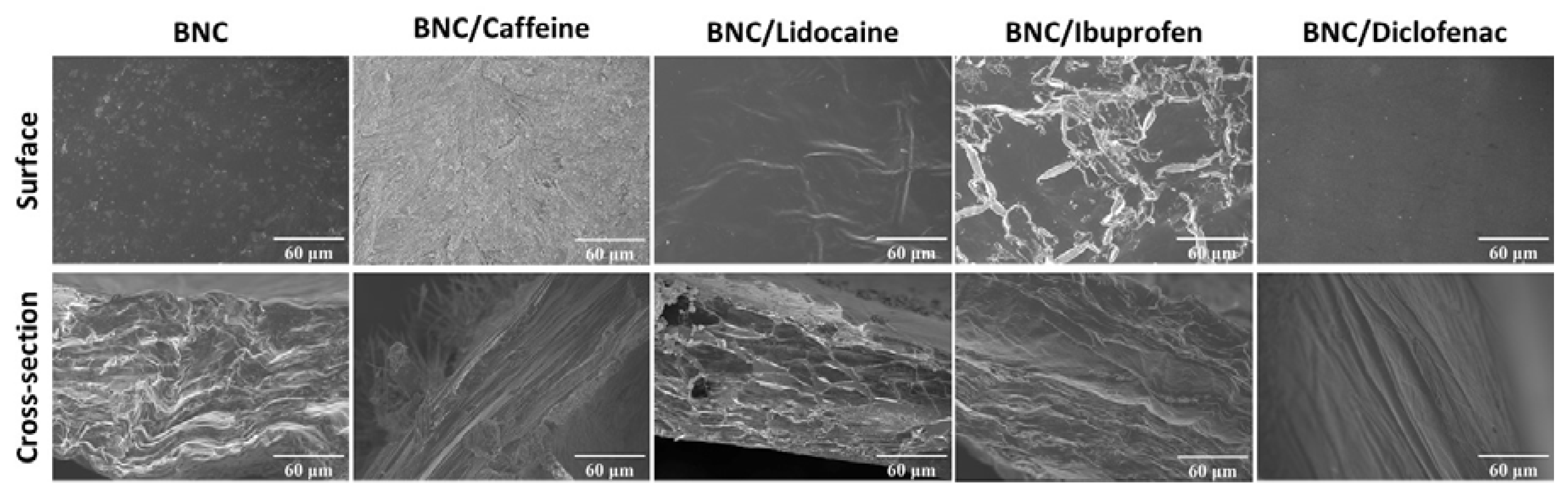
2.1. Preparation and Characterization of API-Loaded BNC Membranes
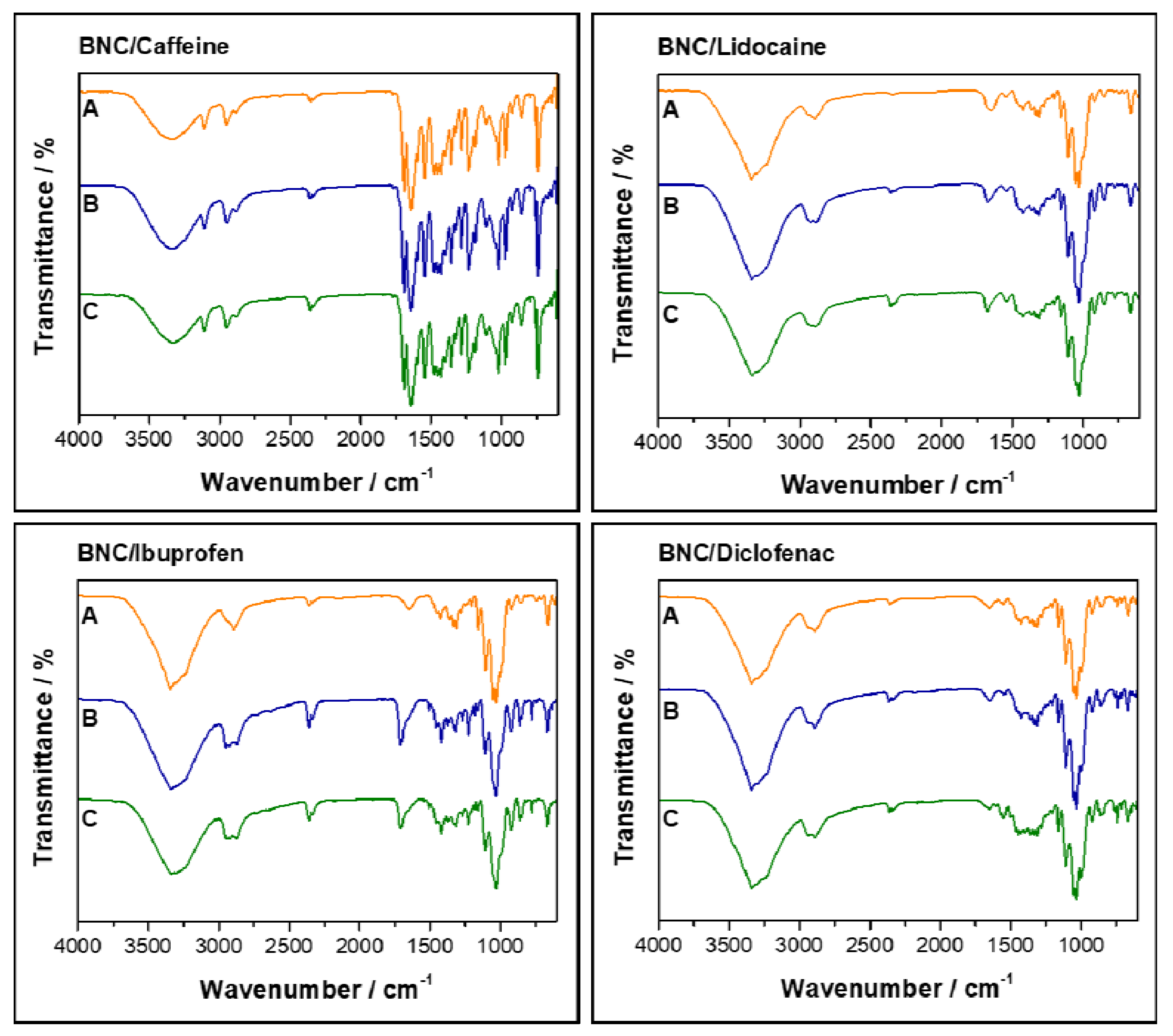
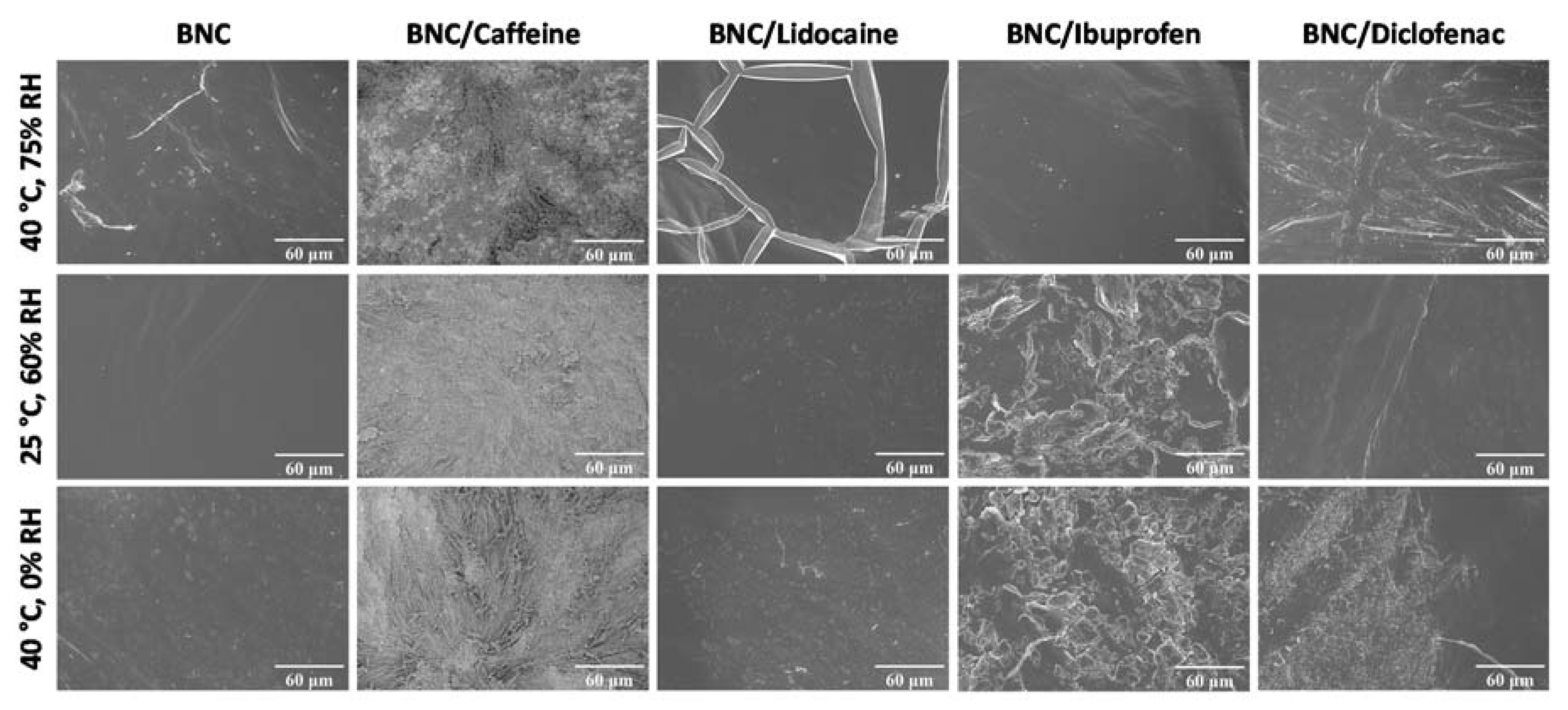
2.2. Storage Stability Studies of the API-Loaded BNC Membranes
2.3. In Vivo Assessment of Cutaneous Compatibility
3. Materials and Methods
3.1. Chemicals
3.2. Bacterial Nanocellulose Biosynthesis
3.3. Preparation of API-loaded BNC Membranes
3.4. Characterization Techniques
3.5. Storage Stability Tests
3.6. In Vitro Drug Release Assays
3.7. In Vivo Assessment of Cutaneous Compatibility of Caffeine-Loaded BNC Membranes
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APIs | Active pharmaceutical ingredients |
| BNC | Bacterial nanocellulose |
| CE | Conformité Européenne |
| FDA | Food and Drug Agency |
| FTIR-ATR | Fourier transform infrared-attenuated total reflectance |
| ICDRG | International Contact Dermatitis Research Group |
| NSAID | Non-steroidal anti-inflammatory drug |
| PBS | Phosphate buffer saline |
| RH | Relative humidity |
| SC | Stratum corneum |
| SEM | Scanning electron microscopy |
| TEWL | Transepidermal water loss |
| WHO | World Health Organization |
References
- Vilela, C.; Pinto, R.J.B.; Figueiredo, A.R.P.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Development and applications of cellulose nanofibers based polymer composites. In Advanced Composite Materials: Properties and Applications; Bafekrpour, E., Ed.; De Gruyter Open: Berlin, Germany, 2017; pp. 1–65. [Google Scholar]
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.A.A.P.; Silvestre, A.J.D.; Freire, C.S.R. Polysaccharide Based Hybrid Materials, 1st ed.; Springer: Berlin, Germany, 2018; ISBN 978-3-030-00346-3. [Google Scholar]
- Vilela, C.; Freire, C.S.R.; Marques, P.A.A.P.; Trindade, T.; Pascoal Neto, C.; Fardim, P. Synthesis and characterization of new CaCO3/cellulose nanocomposites prepared by controlled hydrolysis of dimethylcarbonate. Carbohydr. Polym. 2010, 79, 1150–1156. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced materials through assembly of nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Bejoy, T.; Midhun, C.R.; Athira, K.B.; Rubiyah, M.H.; Jithin, J.; Audrey, M.; Glenna, L.D.; Clément, S. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef] [Green Version]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Faria, M.; Vilela, C.; Mohammadkazemi, F.; Silvestre, A.J.D.; Freire, C.S.R.; Cordeiro, N. Poly(glycidyl methacrylate)/bacterial cellulose nanocomposites: Preparation, characterization and post-modification. Int. J. Biol. Macromol. 2019, 127, 618–627. [Google Scholar] [CrossRef]
- Faria, M.; Vilela, C.; Silvestre, A.J.D.; Deepa, B.; Resnik, M.; Freire, C.S.R.; Cordeiro, N. Physicochemical surface properties of bacterial cellulose/polymethacrylate nanocomposites: An approach by inverse gas chromatography. Carbohydr. Polym. 2019, 206, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and conductive nanocellulose-based films for active and intelligent food packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef]
- Moniri, M.; Moghaddam, A.B.; Azizi, S.; Rahim, R.A.; Bin Ariff, A.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [Green Version]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Freire, C.S.R.; Neto, C.P. Do bacterial cellulose membranes have potential in drug-delivery systems? Expert Opin. Drug Deliv. 2014, 11, 1113–1124. [Google Scholar] [CrossRef]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose membranes as supports for dermal release of lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Pascoal Neto, C.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Drumond, I.; Almeida, I.F.; Costa, P.; Rosado, C.F.; Neto, C.P.; Freire, C.S.R.; Silvestre, A.J.D. Topical caffeine delivery using biocellulose membranes: A potential innovative system for cellulite treatment. Cellulose 2014, 21, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.H.C.S.; Rodrigues, A.F.; Almeida, I.F.; Costa, P.C.; Rosado, C.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial cellulose membranes as transdermal delivery systems for diclofenac: In vitro dissolution and permeation studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pavaloiu, R.D.; Stoica, A.; Stroescu, M.; Dobre, T. Controlled release of amoxicillin from bacterial cellulose membranes. Cent. Eur. J. Chem. 2014, 12, 962–967. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Neves, B.M.; Sèbe, G.; Coma, V.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Design of nonsteroidal anti-inflammatory drug-based ionic liquids with improved water solubility and drug delivery. ACS Sustain. Chem. Eng. 2019, 7, 14126–14134. [Google Scholar] [CrossRef]
- Morais, E.S.; Silva, N.H.C.S.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sá, I.; Ventura, S.P.M.; Silvestre, A.J.D.; et al. Anti-inflammatory and antioxidant nanostructured cellulose membranes loaded with phenolic-based ionic liquids for cutaneous application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef]
- Saïdi, L.; Vilela, C.; Oliveira, H.; Silvestre, A.J.D.; Freire, C.S.R. Poly(N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr. Polym. 2017, 169, 357–365. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Islan, G.A.; Drachemberg, M.F.; Alvarez, V.A.; Bartel, L.C.; Bolzán, A.D.; Castro, G.R. Hybrid bacterial cellulose-pectin films for delivery of bioactive molecules. New J. Chem. 2018, 42, 7457–7467. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.D.; Freire, C.S.R.; Sousa Lobo, J.M.; Costa, P.C. Bacterial cellulose membranes as drug delivery systems: An in vivo skin compatibility study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef]
- WHO. Guidelines for stability testing of pharmaceutical products containing well established drug substances in conventional dosage forms (Annex 5). In World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1996; pp. 65–80. [Google Scholar]
- Herman, A.; Herman, A.P. Caffeine’s mechanism of action and its cosmetic use. Skin Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef]
- The DrugBank Database (version 5.1.4). Available online: https://www.drugbank.ca/ (accessed on 15 November 2019).
- Vuong, Q.V.; Roach, P.D. Caffeine in green tea: Its removal and isolation. Sep. Purif. Rev. 2014, 43, 155–174. [Google Scholar] [CrossRef]
- Filippa, M.A.; Gasull, E.I. Ibuprofen solubility in pure organic solvents and aqueous mixtures of cosolvents: Interactions and thermodynamic parameters relating to the solvation process. Fluid Phase Equilib. 2013, 354, 185–190. [Google Scholar] [CrossRef]
- Llinas, A.; Burley, J.C.; Box, K.J.; Glen, R.C.; Goodman, J.M. Diclofenac solubility: Independent determination of the intrinsic solubility of three crystal forms. J. Med. Chem. 2007, 50, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [Green Version]
- Acharya, M.; Mishra, S.; Sahoo, R.N.; Mallick, S. Infrared spectroscopy for analysis of co-processed ibuprofen and magnesium trisilicate at milling and freeze drying. Acta Chim. Slov. 2017, 64, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall, Ltd.: London, UK, 1975; ISBN 0412138506. [Google Scholar]
- Gadim, T.D.O.; Figueiredo, A.G.P.R.; Rosero-Navarro, N.C.; Vilela, C.; Gamelas, J.A.F.; Barros-Timmons, A.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanostructured bacterial cellulose-poly(4-styrene sulfonic acid) composite membranes with high storage modulus and protonic conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875. [Google Scholar] [CrossRef]
- Vilela, C.; Gadim, T.D.O.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanocellulose/poly(methacryloyloxyethyl phosphate) composites as proton separator materials. Cellulose 2016, 23, 3677–3689. [Google Scholar] [CrossRef]
- Kraft, J.N.; Lynde, C.W. Moisturizers: What they are and a practical approach to product selection. Skin Ther. Lett. 2005, 10, 1–8. [Google Scholar]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Gupta, S.V. Salts of therapeutic agents: Chemical, physicochemical, and biological considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profile. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Dubey, D.; Malviya, R.; Sharma, P.K. Mathematical Modelling and Release Behaviour of Drug. Drug Deliv. Lett. 2014, 4, 254–268. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Wagemaker, T.A.L.; Rijo, P.; Rodrigues, L.M.; Maia Campos, P.M.B.G.; Fernandes, A.S.; Rosado, C. Integrated approach in the assessment of skin compatibility of cosmetic formulations with green coffee oil. Int. J. Cosmet. Sci. 2015, 37, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, J.; Tupker, R.A.; Agner, T.; Serup, J.; Pinnagoda, J.; Tupker, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement: A report from the standardisation group of the European Society of Contact Dermatitis. Contact Dermat. 1990, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, V. EEMCO Guidance for the Assessment of Transepidermal Water Loss in Cosmetic Sciences. Skin Pharmacol. Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef]
- Piérard, G.E. EEMCO Guidance for the Assessment of Skin Colour. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 1–11. [Google Scholar] [CrossRef]
- Sánchez-Borges, M.; Caballero-Fonseca, F.; Capriles-Hulett, A.; González-Aveledo, L. Hypersensitivity reactions to nonsteroidal anti-inflammatory drugs: An update. Pharmaceuticals 2010, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borges, M.; Capriles-Hulett, A.; Caballero-Fonseca, F. Risk of skin reactions when using ibuprofen-based medicines. Expert Opin. Drug Saf. 2005, 4, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Taylor-Gjevre, R. A review of topical diclofenac use in musculoskeletal disease. Pharmaceuticals 2010, 3, 1892–1908. [Google Scholar] [CrossRef]
- Devos, S.A.; Van Der Valk, P.G.M. Epicutaneous patch testing. Eur. J. Dermatol. 2002, 12, 506–513. [Google Scholar]
- Björklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. Glycerol and urea can be used to increase skin permeability in reduced hydration conditions. Eur. J. Pharm. Sci. 2013, 50, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Gluconacetobacter sacchari: An efficient bacterial cellulose cell-factory. Carbohydr. Polym. 2011, 86, 1417–1420. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. The United States Pharmacopoeia: The National Formulary; Rockville, M., Ed.; USP Convention: Rockville, MD, USA, 2007; ISBN 9781889788470. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Membrane | Glycerol 1 | Drug Dose 2 | ||
|---|---|---|---|---|
| % (w/v) | mg cm−2 | % (w/v) | mg cm−2 | |
| BNC1 3 | 1.0 | 2.1 | – | – |
| BNC/Caffeine | 1.0 | 2.1 | 3.8 | 8.0 |
| BNC/Lidocaine | 1.0 | 2.1 | 2.0 | 4.2 |
| BNC/Ibuprofen | 1.0 | 2.1 | 0.9 | 1.9 |
| BNC5 3 | 5.0 | 10.4 | – | – |
| BNC/Diclofenac | 5.0 | 10.4 | 1.0 | 2.1 |
| Membrane 1 | Moisture-Uptake/% 2 | Weight-Loss/% 2 | |
|---|---|---|---|
| 75% RH at 40 °C | 60% RH at 25 °C | 0%RH at 40 °C | |
| BNC1 | 22.3 ± 0.4 | 9.9 ± 3.1 | 27.0 ± 8.0 |
| BNC/Caffeine | 26.0 ± 4.5 | 16.5 ± 0.7 * | 11.8 ± 5.0 * |
| BNC/Lidocaine | 36.3 ± 2.1 * | 19.8 ± 4.1 * | 7.8 ± 3.0 * |
| BNC/Ibuprofen | 12.3 ± 2.9 * | 4.1 ± 1.4 * | 5.2 ± 0.6 * |
| BNC5 | 35.7 ± 1.9 | 10.3 ± 1.4 | 37.2 ± 10.1 |
| BNC/Diclofenac | 31.3 ± 7.3 | 9.2 ± 1.0 | 25.4 ± 0.7 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.H.C.S.; Mota, J.P.; Santos de Almeida, T.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. https://doi.org/10.3390/ijms21041262
Silva NHCS, Mota JP, Santos de Almeida T, Carvalho JPF, Silvestre AJD, Vilela C, Rosado C, Freire CSR. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. International Journal of Molecular Sciences. 2020; 21(4):1262. https://doi.org/10.3390/ijms21041262
Chicago/Turabian StyleSilva, Nuno H. C. S., Joana P. Mota, Tânia Santos de Almeida, João P. F. Carvalho, Armando J. D. Silvestre, Carla Vilela, Catarina Rosado, and Carmen S. R. Freire. 2020. "Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing" International Journal of Molecular Sciences 21, no. 4: 1262. https://doi.org/10.3390/ijms21041262
APA StyleSilva, N. H. C. S., Mota, J. P., Santos de Almeida, T., Carvalho, J. P. F., Silvestre, A. J. D., Vilela, C., Rosado, C., & Freire, C. S. R. (2020). Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. International Journal of Molecular Sciences, 21(4), 1262. https://doi.org/10.3390/ijms21041262