The Predictive Value of Salt Sensitivity and Osmotic Fragility in the Development of Cerebral Small Vessel Disease
Abstract
1. Introduction
Aim
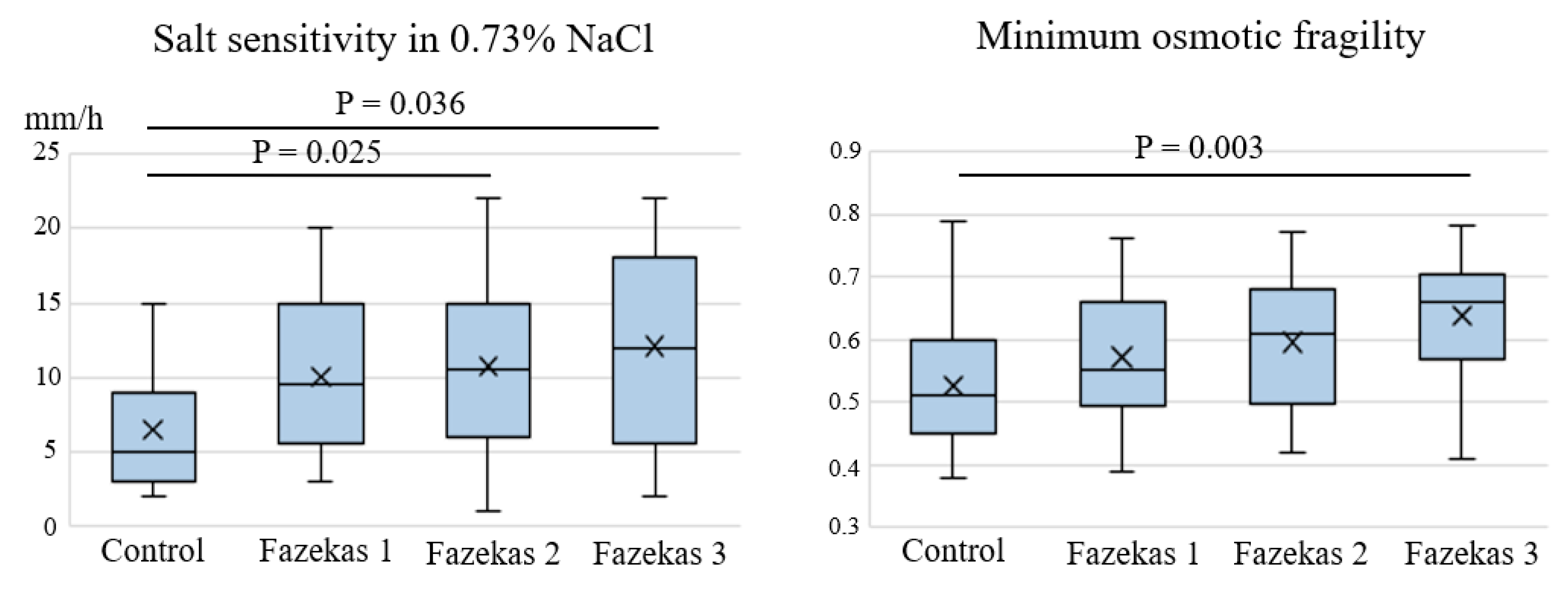
2. Results
3. Discussion
4. Materials and Methods
Exclusion Criteria
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AH | Arterial hypertension |
| AUC | Area under the curve |
| BBB | Blood–brain barrier |
| CSVD | Cerebral small vessel disease |
| DM | Diabetes mellitus |
| MRI | Magnetic resonance imaging |
| ROC | Receiver operating characteristic |
| WMHs | White matter hyperintensities |
References
- Pantoni, L.; Gorelick, P.B. (Eds.) Cerebral Small Vessel Disease; Cambridge University Press: Cambridge, UK, 2014; p. 371. [Google Scholar]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef] [PubMed]
- The LADIS Study Group; Poggesi, A.; Pantoni, L.; Inzitari, D.; Fazekas, F.; Ferro, J.; O’Brien, J.; Hennerici, M.; Scheltens, P.; Erkinjuntti, T.; et al. 2001–2011: A decade of the LADIS (Leukoaraiosis And DISability) Study: what have we learned about white matter changes and small-vessel disease? Cerebrovasc. Dis. 2011, 32, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L. American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; Stroke Council Hypertension. Salt Sensit. Blood Press. A Sci. Statement Am. Heart Assoc. 2016, 68, e7–e46. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Bailey, E.L.; Wardlaw, J.M.; Graham, D.; Dominiczak, A.F.; Sudlow, C.L.; Smith, C. Cerebral small vessel endothelial structural changes predate hypertension in stroke-prone spontaneously hypertensive rats: A blinded, controlled immunohistochemical study of 5- to 21-week-old rats. Neuropathol. Appl. Neurobiol. 2011, 37, 711–726. [Google Scholar] [CrossRef]
- Heye, A.K.; Thrippleton, M.J.; Chappell, F.M.; Hernández, M.D.C.V.; Armitage, P.A.; Makin, S.D.; Maniega, S.M.; Sakka, E.; Flatman, P.W.; Dennis, M.S.; et al. Blood pressure and sodium: Association with MRI markers in cerebral small vessel disease. Br. J. Pharmacol. 2015, 36, 264–274. [Google Scholar] [CrossRef]
- Makin, S.D.J.; Mubki, G.F.; Doubal, F.N.; Shuler, K.; Staals, J.; Dennis, M.S.; Wardlaw, J.M. Small vessel disease and dietary salt intake: Cross-sectional study and systematic review. J. Stroke Cerebrovasc. Dis. 2017, 26, 3020–3028. [Google Scholar] [CrossRef]
- Oberleithner, H.; Peters, W.; Kusche-Vihrog, K.; Korte, S.; Schillers, H.; Kliche, K.; Oberleithner, K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflüg. Arch.-Eur. J. Physiol. 2011, 462, 519–528. [Google Scholar] [CrossRef]
- Kusche-Vihrog, K.; Jeggle, P.; Oberleithner, H. The role of ENaC in vascular endothelium. Pflüg. Arch.-Eur. J. Physiol. 2013, 466, 851–859. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Plesnila, N. (Eds.) Brain Edema: From Molecular Mechanisms to Clinical Practice; Academic Press: London, UK, 2017; 554p. [Google Scholar]
- Ando, Y.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Tomita, H.; Zaikokuji, R.; Hotta, Y.; Miyazaki, N.; Yano, H.; et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Scientific reports. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Kutuzov, N.; Flyvbjerg, H.; Lauritzen, M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier. Proc. Natl. Acad. Sci. USA 2018, 115, E9429–E9438. [Google Scholar] [CrossRef]
- Mewes, M.; Nedele, J.; Schelleckes, K.; Bondareva, O.; Lenders, M.; Kusche-Vihrog, K.; Schnittler, H.J.; Brand, S.M.; Schmitz, B.; Brand, E. Salt-induced Na+/K+-ATPase-α/β expression involves soluble adenylyl cyclase in endothelial cells. Pflüg. Arch.-Eur. J. Physiol. 2017, 469, 1401–1412. [Google Scholar] [CrossRef]
- Oberleithner, H.; Wilhelmi, M. Determination of erythrocyte sodium sensitivity in man. Pflüg. Arch.-Eur. J. Physiol. 2013, 465, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H. Sodium selective erythrocyte glycocalyx and salt sensitivity in man. Pflüg. Arch.-Eur. J. Physiol. 2014, 467, 1319–1325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bain, B.J.; Bates, I.; Laffan, M.A.; Lewis, S.M. Dacie and Lewis Practical Haematology, 11th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011; p. 668. [Google Scholar]
- Joiner, C.H.; Platt, O.S.; Lux, S.E. Cation depletion by the sodium pump in red cells with pathologic cation leaks. Sickle cells and xerocytes. J. Clin. Investig. 1986, 78, 1487–1496. [Google Scholar] [CrossRef]
- Izumo, H.; Lear, S.; Williams, M.; Rosa, R.; Epstein, F.H. Sodium-potassium pump, ion fluxes, and cellular dehydration in sickle cell anemia. J. Clin. Investig. 1987, 79, 1621–1628. [Google Scholar] [CrossRef]
- King, M.J.; Zanella, A. Hereditary red cell membrane disorders and laboratory diagnostic testing. Int. J. Lab. Hematol. 2013, 35, 237–243. [Google Scholar] [CrossRef]
- Siddon, A.J.; Tormey, C.A. The chemical and laboratory investigation of hemolysis. Adv. Clin. Chem. 2019, 89, 215–258. [Google Scholar] [CrossRef]
- Postnov, Y.U.; Orlov, S.; Gulak, P.; Shevchenko, A. Altered permeability of the erythrocyte membrane for sodium and potassium ions in spontaneously hypertensive rats. Pflüg. Arch.-Eur. J. Physiol. 1976, 365, 257–263. [Google Scholar] [CrossRef]
- Schreiber, S.; Bueche, C.Z.; Garz, C.; Kropf, S.; Angenstein, F.; Goldschmidt, J.; Neumann, J.; Heinze, H.-J.; Goertler, M.; Reymann, K.G.; et al. The pathologic cascade of cerebrovascular lesions in SHR-SP: is erythrocyte accumulation an early phase? J. Cereb. Blood Flow Metab. 2012, 32, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Farrall, A.J.; Wardlaw, J.M. Blood brain barrier: Ageing and microvascular disease—Systemic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.; Makin, S.D.; Hernández, M.C.V.; Armitage, P.A.; Heye, A.K.; Chappell, F.M.; Muñoz-Maniega, S.; Sakka, E.; Shuler, K.; Dennis, M.S.; et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimer Dement. 2017, 13, 634–643. [Google Scholar] [CrossRef]
- Chakraborti, S.; Dhalla, N.S. (Eds.) Regulation of Membrane Na+-K+ ATPase; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; p. 436. [Google Scholar]
- Van Der Land, V.; Hijmans, C.T.; De Ruiter, M.; Mutsaerts, H.; Cnossen, M.H.; Engelen, M.; Majoie, C.B.L.M.; Nederveen, A.; Grootenhuis, M.A.; Fijnvandraat, K. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br. J. Haematol. 2014, 168, 553–556. [Google Scholar] [CrossRef]
- Jorgensen, D.R.; Rosano, C.; Novelli, E.M. Can neuroimaging markers of vascular pathology explain cognitive performance in adults with sickle cell anemia? A review of the literature. Hemoglobin 2016, 40, 381–387. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Valdes Hernandez, M.C.; Munoz-Maniega, S. What are white matter hyperintensities made of? J. Am. Hear. Assoc. 2015, 4. [Google Scholar] [CrossRef]
- Fernando, M.S.; Simpson, J.E.; E Matthews, F.; Brayne, C.; Lewis, C.E.; Barber, R.; Kalaria, R.N.; Forster, G.; Esteves, F.; Wharton, S.B.; et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006, 37, 1391–1398. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.W.; Dubois, B.; Feldman, H.H.; Fox, N.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cífková, R.; De Backer, G.; Dominiczak, A.F.; et al. Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.; Alavi, A.; Hurtig, H.; Zimmerman, R. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]



| Parameters | CSVD (n = 73) | Control (n = 19) | p |
|---|---|---|---|
| Age, years | 60.1 ± 6.5 | 56.9 ± 6.4 | 0.061 |
| Sex Women (n, %) | 48 (65.8%) | 14 (73.7%) | 0.592 |
| Arterial hypertension (AH) (n, %) | 64 (87.7%) | 9 (47.4%) | <0.001 |
| Stage of AH (n, %) | |||
| 1 | 15 (20.5%) | 5 (26.3%) | |
| 2 | 13 (17.9%) | 3 (15.8%) | |
| 3 | 36 (49.3%) | 1 (5.3%) | |
| Diabetes mellitus (DM) type 2 (n, %) | 15 (20.5%) | 0 (0.0%) | 0.034 |
| Hypercholesterolemia (total cholesterol >6.2 mmol/L or statin use) (n, %) | 39 (53.4%) | 9 (47.4%) | 0.188 |
| Smoking (n, %) | 19 (26.0%) | 8 (42.1%) | 0.258 |
| Obesity (body mass index >30 kg/m²) (n, %) | 34 (46.6%) | 5 (26.3%) | 0.127 |
| Parameters | CSVD (n = 73) |
|---|---|
| Cognitive impairment (n, %): | 73 (100%) |
| subjective | 29 (39.7%) |
| mild | 34 (46.6%) |
| dementia | 10 (13.7%) |
| Gait disturbances, unrelated to hemiparesis (n, %): | 42 (57.5%) |
| mild | 24 (32.8%) |
| moderate | 7 (9.6%) |
| severe | 11 (15.1%) |
| Urinary disorders (n, %) | 27 (37.0%) |
| History of stroke (n, %) | 15 (20.5%) |
| WMH, Fazekas Scale (n, %) | 73 (100%) |
| grade 1 | 18 (24.7%) |
| grade 2 | 25 (34.2%) |
| grade 3 | 30 (41.1%) |
| Lacunes (n, %) | 54 (73.9%) |
| Microbleeds (n, %) | 45 (61.6%) |
| Perivascular spaces (n, %) | 73 (100%) |
| >3 mm in the semiovale centres | 4 (5.5%) |
| >3 mm in the basal ganglia region | 22 (30.1%) |
| Predictors | B | p | OR | 95% CI, Boundary | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Salt sensitivity in 0.73% NaCl | −0.251 | 0.001 | 0.78 | 0.7 | 0.9 |
| Minimum osmotic fragility | −9.833 | 0.001 | 0.02 | 0.005 | 0.5 |
| Сonstant | 6.306 | 0.001 | 547.7 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrynina, L.A.; Shabalina, A.A.; Shamtieva, K.V.; Gnedovskaya, E.V.; Berdalin, A.B.; Krotenkova, M.V. The Predictive Value of Salt Sensitivity and Osmotic Fragility in the Development of Cerebral Small Vessel Disease. Int. J. Mol. Sci. 2020, 21, 2036. https://doi.org/10.3390/ijms21062036
Dobrynina LA, Shabalina AA, Shamtieva KV, Gnedovskaya EV, Berdalin AB, Krotenkova MV. The Predictive Value of Salt Sensitivity and Osmotic Fragility in the Development of Cerebral Small Vessel Disease. International Journal of Molecular Sciences. 2020; 21(6):2036. https://doi.org/10.3390/ijms21062036
Chicago/Turabian StyleDobrynina, Larisa A., Alla A. Shabalina, Kamila V. Shamtieva, Elena V. Gnedovskaya, Alexander B. Berdalin, and Marina V. Krotenkova. 2020. "The Predictive Value of Salt Sensitivity and Osmotic Fragility in the Development of Cerebral Small Vessel Disease" International Journal of Molecular Sciences 21, no. 6: 2036. https://doi.org/10.3390/ijms21062036
APA StyleDobrynina, L. A., Shabalina, A. A., Shamtieva, K. V., Gnedovskaya, E. V., Berdalin, A. B., & Krotenkova, M. V. (2020). The Predictive Value of Salt Sensitivity and Osmotic Fragility in the Development of Cerebral Small Vessel Disease. International Journal of Molecular Sciences, 21(6), 2036. https://doi.org/10.3390/ijms21062036





