Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS
Abstract
:1. Introduction
2. Results
2.1. Comprehensive Profiling of IgG N-Glycans by ESI-MS
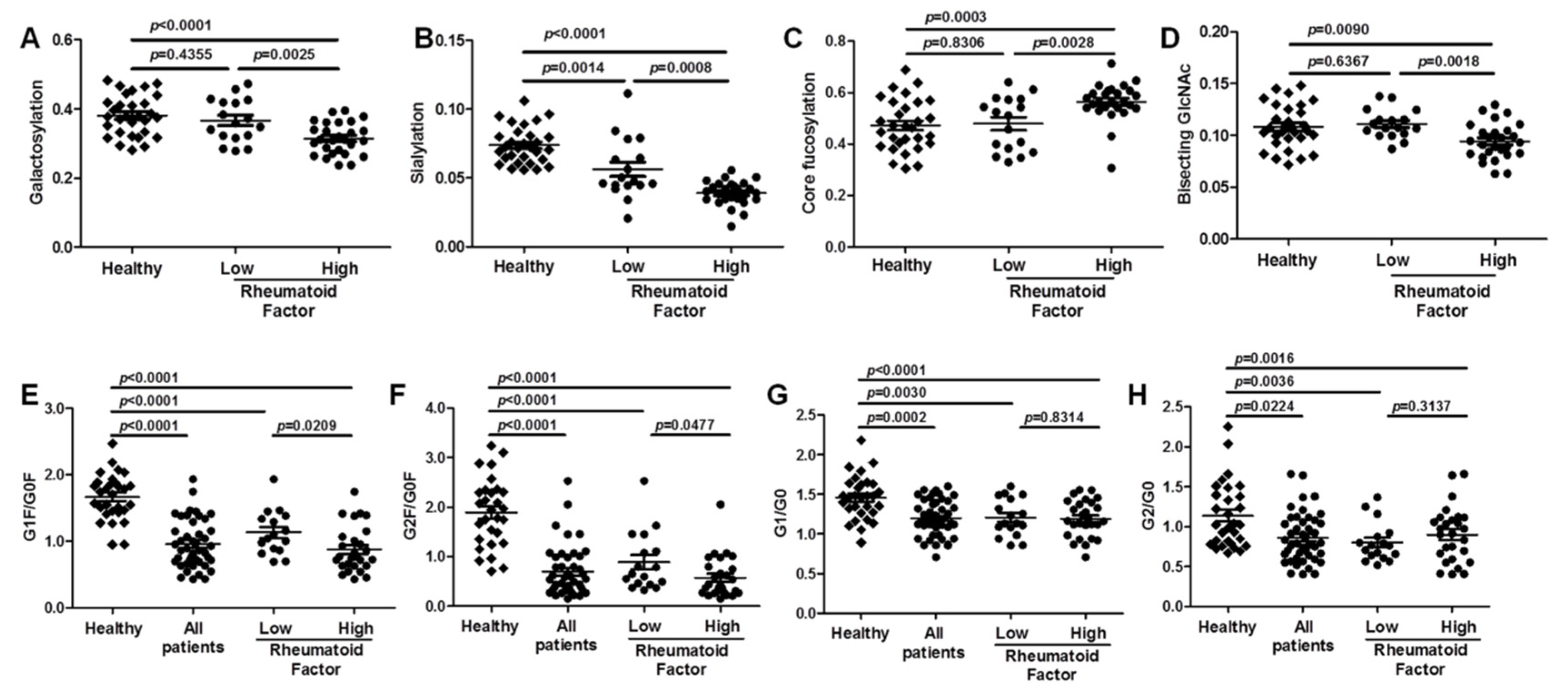
2.2. Aberrant IgG Glycosylation in RA Patients
2.3. Association between Aberrant IgG Glycosylation and RF Rheumatoid Factor Titer in RA
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Preparation of Total Immunoglobulin and IgG
4.3. Release and Solid-Phase Extraction of N-Glycans
4.4. Permethylation of N-Glycans
4.5. Analysis of N-Glycans Using Linear Ion-Trap Electrospray Ionisation Mass Spectrometry (LTQ-ESI-MS)
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cell cytotoxicity |
| LTQ-ESI-MS | linear ion-trap electrospray ionisation mass spectrometry |
| MS | mass spectrometry |
| RA | rheumatoid arthritis |
| RF | rheumatoid factor |
| SDS-PAGE | sodium dodecyl sulphate–polyacrylamide gel electrophoresis |
References
- Van Gaalen, F.; Ioan-Facsinay, A.; Huizinga, T.W.; Toes, R.E. The devil in the details: The emerging role of anticitrulline autoimmunity in rheumatoid arthritis. J. Immunol. 2005, 175, 5575–5580. [Google Scholar] [CrossRef] [Green Version]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Ann. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [Green Version]
- Burton, D.R.; Dwek, R.A. Immunology. Sugar determines antibody activity. Science 2006, 313, 627–628. [Google Scholar] [CrossRef] [Green Version]
- Parekh, R.B.; Dwek, R.A.; Sutton, B.J.; Fernandes, D.L.; Leung, A.; Stanworth, D.; Rademacher, T.W.; Mizuochi, T.; Taniguchi, T.; Matsuta, K.; et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985, 316, 452–457. [Google Scholar] [CrossRef]
- Ercan, A.; Cui, J.; Chatterton, D.E.; Deane, K.D.; Hazen, M.M.; Brintnell, W.; O’Donnell, C.I.; Derber, L.A.; Weinblatt, M.E.; Shadick, N.A.; et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Ohmi, Y.; Ise, W.; Harazono, A.; Takakura, D.; Fukuyama, H.; Baba, Y.; Narazaki, M.; Shoda, H.; Takahashi, N.; Ohkawa, Y.; et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun. 2016, 7, 11205. [Google Scholar] [CrossRef] [Green Version]
- Imafuku, Y.; Yoshida, H.; Yamada, Y. Reactivity of agalactosyl IgG with rheumatoid factor. Clin. Chim. Acta Int. J. Clin. Chem. 2003, 334, 217–223. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, E.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Iida, S.; Misaka, H.; Inoue, M.; Shibata, M.; Nakano, R.; Yamane-Ohnuki, N.; Wakitani, M.; Yano, K.; Shitara, K.; Satoh, M. Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin g on antibody-dependent cellular cytotoxicity through its high binding to Fc gamma RIIIa. Clin Cancer Res. 2006, 12, 2879–2887. [Google Scholar] [CrossRef] [Green Version]
- Bakovic, M.P.; Selman, M.H.; Hoffmann, M.; Rudan, I.; Campbell, H.; Deelder, A.M.; Lauc, G.; Wuhrer, M. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 2013, 12, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Vuckovic, F.; Kristic, J.; Gudelj, I.; Teruel, M.; Keser, T.; Pezer, M.; Pucic-Bakovic, M.; Stambuk, J.; Trbojevic-Akmacic, I.; Barrios, C.; et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 2015, 67, 2978–2989. [Google Scholar] [CrossRef]
- Trbojevic Akmacic, I.; Ventham, N.T.; Theodoratou, E.; Vuckovic, F.; Kennedy, N.A.; Kristic, J.; Nimmo, E.R.; Kalla, R.; Drummond, H.; Stambuk, J.; et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 2015, 21, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Watson, M.; Rudd, P.M.; Bland, M.; Dwek, R.A.; Axford, J.S. Sugar printing rheumatic diseases: A potential method for disease differentiation using immunoglobulin G oligosaccharides. Arthritis Rheum. 1999, 42, 1682–1690. [Google Scholar] [CrossRef]
- Wuhrer, M.; Selman, M.H.; McDonnell, L.A.; Kumpfel, T.; Derfuss, T.; Khademi, M.; Olsson, T.; Hohlfeld, R.; Meinl, E.; Krumbholz, M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflammation 2015, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Falkenburg, W.J.J.; Kempers, A.C.; Dekkers, G.; Ooijevaar-de Heer, P.; Bentlage, A.E.H.; Vidarsson, G.; van Schaardenburg, D.; Toes, R.E.M.; Scherer, H.U.; Rispens, T. Rheumatoid factors do not preferentially bind to ACPA-IgG or IgG with altered galactosylation. Rheumatology (Oxf.) 2017, 56, 2025–2030. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Shikata, K.; Takeuchi, F.; Kojima, N.; Mizuochi, T. Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J. Biochem. 2000, 128, 621–628. [Google Scholar] [CrossRef]
- Ju, L.L.; Wang, Y.P.; Xie, Q.; Xu, X.K.; Li, Y.; Chen, Z.J.; Li, Y.S. Elevated level of serum glycoprotein bifucosylation and prognostic value in Chinese breast cancer. Glycobiology 2016, 26, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, Y.; Ewing, E.; van de Stadt, L.A.; Selman, M.H.; Trouw, L.A.; Deelder, A.M.; Huizinga, T.W.; Wuhrer, M.; van Schaardenburg, D.; Toes, R.E.; et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 234–241. [Google Scholar] [CrossRef]
- Biermann, M.H.; Griffante, G.; Podolska, M.J.; Boeltz, S.; Sturmer, J.; Munoz, L.E.; Bilyy, R.; Herrmann, M. Sweet but dangerous - the role of immunoglobulin G glycosylation in autoimmunity and inflammation. Lupus 2016, 25, 934–942. [Google Scholar] [CrossRef]
- Dorner, T.; Egerer, K.; Feist, E.; Burmester, G.R. Rheumatoid factor revisited. Curr. Opin. Rheumatol. 2004, 16, 246–253. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Castelli, R.; Gualtierotti, R. Rheumatoid factors: Clinical applications. Dis. Markers 2013, 35, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Gao, J.; Xie, Q.; Wang, Y.; Li, Y. Possible role of beta-galactosidase in rheumatoid arthritis. Mod. Rheumatol. 2019, 1–10. [Google Scholar] [CrossRef]
- Dekkers, G.; Rispens, T.; Vidarsson, G. Novel Concepts of Altered Immunoglobulin G Galactosylation in Autoimmune Diseases. Front. Immunol. 2018, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Rook, G.A.; Steele, J.; Brealey, R.; Whyte, A.; Isenberg, D.; Sumar, N.; Nelson, J.L.; Bodman, K.B.; Young, A.; Roitt, I.M.; et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 1991, 4, 779–794. [Google Scholar] [CrossRef]
- Rademacher, T.W.; Williams, P.; Dwek, R.A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 6123–6127. [Google Scholar] [CrossRef] [Green Version]
- Forger, F.; Ostensen, M. Is IgG galactosylation the relevant factor for pregnancy-induced remission of rheumatoid arthritis? Arthritis Res. Ther. 2010, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Anthony, R.M.; Nimmerjahn, F.; Ashline, D.J.; Reinhold, V.N.; Paulson, J.C.; Ravetch, J.V. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008, 320, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Washburn, N.; Schwab, I.; Ortiz, D.; Bhatnagar, N.; Lansing, J.C.; Medeiros, A.; Tyler, S.; Mekala, D.; Cochran, E.; Sarvaiya, H.; et al. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc. Natl. Acad. Sci. USA 2015, 112, E1297–E1306. [Google Scholar] [CrossRef] [Green Version]
- Pagan, J.D.; Kitaoka, M.; Anthony, R.M. Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease. Cell 2018, 172, 564–577 e513. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Mishra, S.K.; Tokoro, Y.; Sato, K.; Nakajima, K.; Yamaguchi, Y.; Taniguchi, N.; Kizuka, Y. Bisecting GlcNAc is a general suppressor of terminal modification of N-glycan. Mol Cell Proteom. 2019. [Google Scholar] [CrossRef] [Green Version]
- Pucic, M.; Knezevic, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzic, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteom. 2011, 10, M111 010090. [Google Scholar] [CrossRef] [Green Version]
- Bondt, A.; Selman, M.H.; Deelder, A.M.; Hazes, J.M.; Willemsen, S.P.; Wuhrer, M.; Dolhain, R.J. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J. Proteome Res. 2013, 12, 4522–4531. [Google Scholar] [CrossRef]
- Bondt, A.; Rombouts, Y.; Selman, M.H.; Hensbergen, P.J.; Reiding, K.R.; Hazes, J.M.; Dolhain, R.J.; Wuhrer, M. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell. Proteom. 2014, 13, 3029–3039. [Google Scholar] [CrossRef] [Green Version]
- Kemna, M.J.; Plomp, R.; van Paassen, P.; Koeleman, C.A.M.; Jansen, B.C.; Damoiseaux, J.; Cohen Tervaert, J.W.; Wuhrer, M. Galactosylation and Sialylation Levels of IgG Predict Relapse in Patients With PR3-ANCA Associated Vasculitis. EBioMedicine 2017, 17, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Sonneveld, M.E.; de Haas, M.; Koeleman, C.; de Haan, N.; Zeerleder, S.S.; Ligthart, P.C.; Wuhrer, M.; van der Schoot, C.E.; Vidarsson, G. Patients with IgG1-anti-red blood cell autoantibodies show aberrant Fc-glycosylation. Sci. Rep. 2017, 7, 8187. [Google Scholar] [CrossRef] [Green Version]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [Green Version]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, C.; Grau, S.; Jager, C.; Sondermann, P.; Brunker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, C.; Stuart, F.; Sondermann, P.; Brunker, P.; Umana, P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 2006, 281, 5032–5036. [Google Scholar] [CrossRef] [Green Version]
- Mahan, A.E.; Jennewein, M.F.; Suscovich, T.; Dionne, K.; Tedesco, J.; Chung, A.W.; Streeck, H.; Pau, M.; Schuitemaker, H.; Francis, D.; et al. Antigen-Specific Antibody Glycosylation Is Regulated via Vaccination. Plos Pathog. 2016, 12, e1005456. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Xie, Q.; Wang, Q.; Wang, Y.; Miao, J.; Li, L.; Zhang, T.; Cao, X.; Li, Y. Mass spectrometry analysis reveals aberrant N-glycans in colorectal cancer tissues. Glycobiology 2019, 29, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Maamary, J.; Tan, G.S.; Bournazos, S.; Davis, C.W.; Krammer, F.; Schlesinger, S.J.; Palese, P.; Ahmed, R.; Ravetch, J.V. Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell 2015, 162, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A Functional Role for Antibodies in Tuberculosis. Cell 2016, 167, 433–443 e414. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.B.; Nasirikenari, M.; Lugade, A.A.; Thanavala, Y.; Lau, J.T. Anti-inflammatory IgG production requires functional P1 promoter in beta-galactoside alpha2,6-sialyltransferase 1 (ST6Gal-1) gene. J. Biol. Chem. 2012, 287, 15365–15370. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.M.; Nasirikenari, M.; Manhardt, C.T.; Ashline, D.J.; Hanneman, A.J.; Reinhold, V.N.; Lau, J.T. Platelets support extracellular sialylation by supplying the sugar donor substrate. J. Biol. Chem. 2014, 289, 8742–8748. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.B.; Oswald, D.M.; Joshi, S.; Whiteheart, S.W.; Orlando, R.; Cobb, B.A. B-cell-independent sialylation of IgG. Proc. Natl. Acad. Sci. USA 2016, 113, 7207–7212. [Google Scholar] [CrossRef] [Green Version]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Axford, J.S.; Lydyard, P.M.; Isenberg, D.A.; Mackenzie, L.; Hay, F.C.; Roitt, I.M. Reduced B-Cell Galactosyltransferase Activity In Rheumatoid-Arthritis. Lancet 1987, 2, 1486–1488. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xu, D.W.; Tao, R.; Wang, H.R.; Wang, Q.H.; Shen, A.G. beta 1,4-Galactosyltransferase-I contributes to the inflammatory processes in synovial tissue of patients with rheumatoid arthritis. Inflamm Res. 2010, 59, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Katoh, T.; Saldova, R.; O’Flaherty, R.; Izumi, T.; Mimura-Kimura, Y.; Utsunomiya, T.; Mizukami, Y.; Yamamoto, K.; Matsumoto, T.; et al. Glycosylation engineering of therapeutic IgG antibodies: Challenges for the safety, functionality and efficacy. Protein Cell 2018, 9, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Porath, J. Salting-out Adsorption Techniques for Protein-Purification. Biopolymers 1987, 26, S193–S204. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Costello, C.E. Elimination of oxidative degradation during the per-O-methylation of carbohydrates. J. Am. Chem. Soc. 2003, 125, 16213–16219. [Google Scholar] [CrossRef]
- Hong, Q.; Ruhaak, L.R.; Stroble, C.; Parker, E.; Huang, J.; Maverakis, E.; Lebrilla, C.B. A Method for Comprehensive Glycosite-Mapping and Direct Quantitation of Serum Glycoproteins. J. Proteome Res. 2015, 14, 5179–5192. [Google Scholar] [CrossRef]

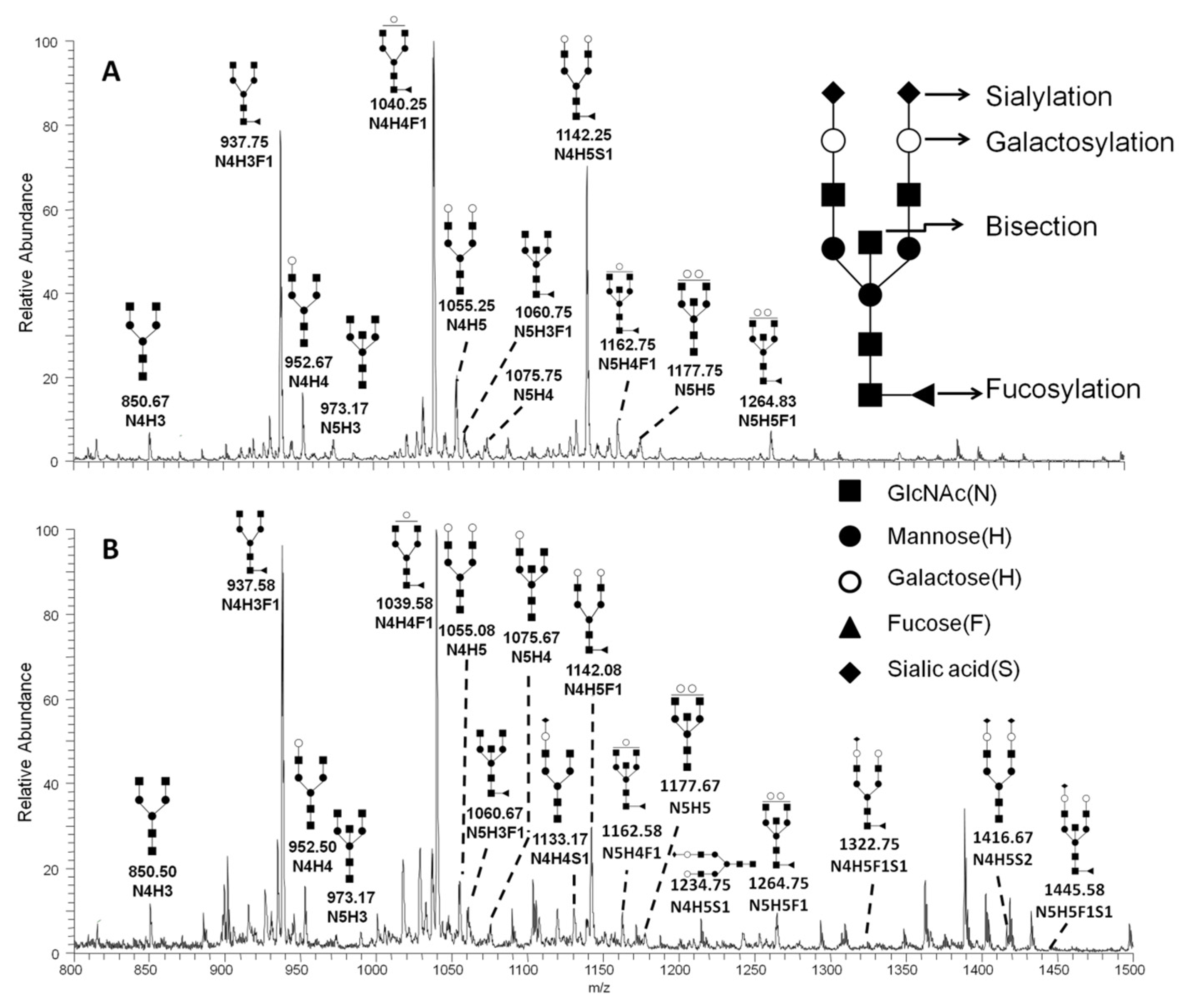
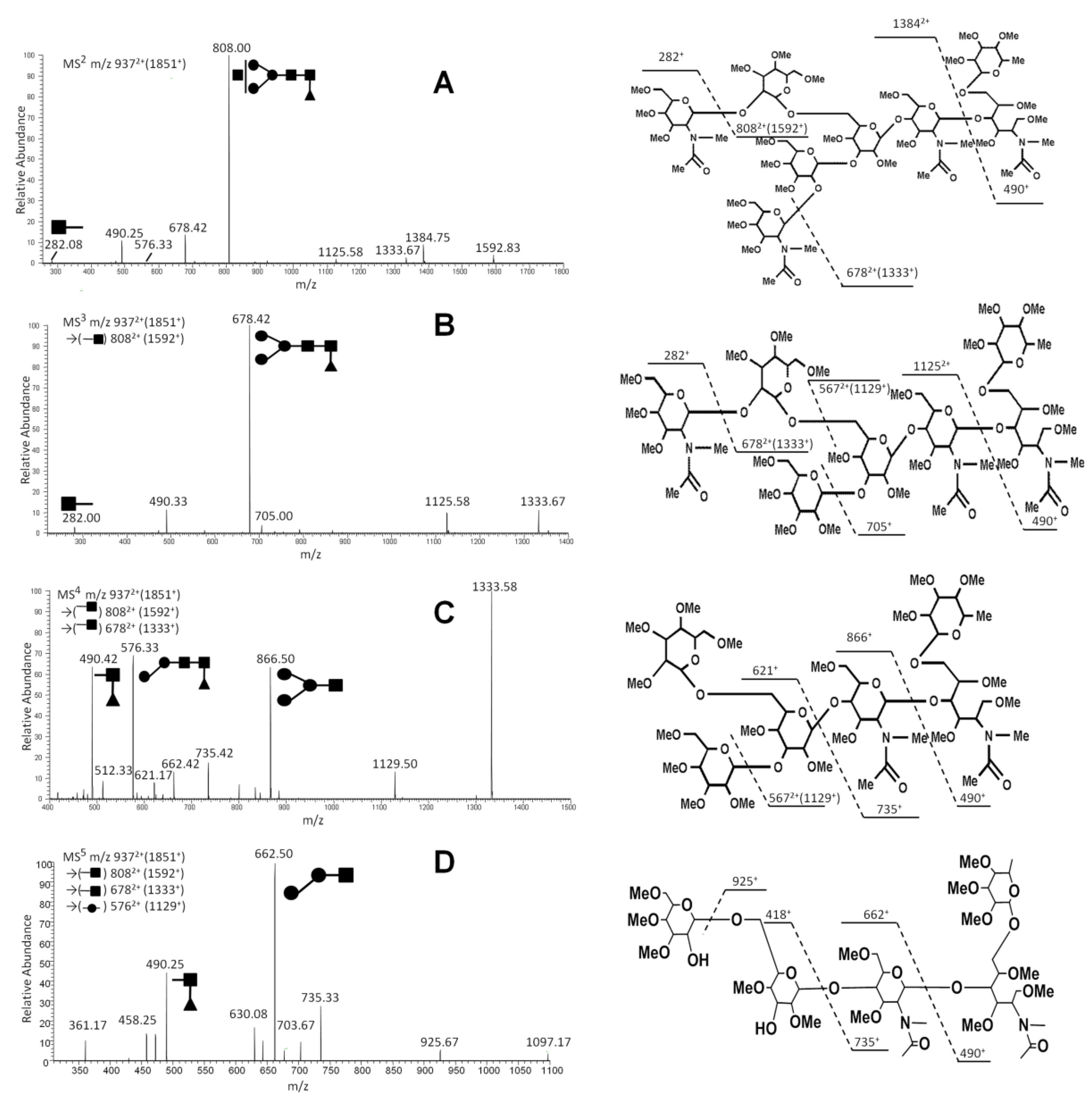

| m/z | Charge | Composition | Main Structure | MSn Pathway |
|---|---|---|---|---|
| 850 | 2+ | N4H3 |  | 8502+→7212+ (loss of  )→5912+ (loss of )→5912+ (loss of  )→866+ (loss of )→866+ (loss of 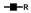 )→662+ (loss of )→662+ (loss of  ) ) |
| 937 | 2+ | N4H3F1 | 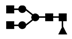 | 9372+→1592+ (loss of  )→ 1333+ (loss of )→ 1333+ (loss of  )→1129+ (loss of )→1129+ (loss of  )→ 662+ (loss of )→ 662+ (loss of 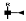 ) ) |
| 953 | 2+ | N4H4 | 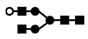 | 9532+→8232+ (loss of  )→ 1159+ (loss of )→ 1159+ (loss of  )→866+ (loss of )→866+ (loss of 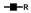 )→ 662+ (loss of )→ 662+ (loss of  ) ) |
| 973 | 2+ | N5H3 |  | 9732+→8432+ (loss of  )→ 7142+ (loss of )→ 7142+ (loss of  )→5842+ (loss of )→5842+ (loss of  )→ 852+ (loss of )→ 852+ (loss of 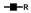 ) ) |
| 1039 | 2+ | N4H4F1 |  | 10392+→9102+ (loss of  )→ 1333+ (loss of )→ 1333+ (loss of  )→866+ (loss of )→866+ (loss of 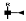 ) ) |
| 1055 | 2+ | N4H4 | 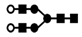 | 10552+→1623+ (loss of  )→ 1329+ (loss of )→ 1329+ (loss of 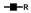 )→866+ (loss of )→866+ (loss of  ) →662+ (loss of ) →662+ (loss of  ) ) |
| 1060 | 2+ | N5H3F1 | 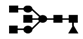 | 10602+→9312+ (loss of  )→ 8012+ (loss of )→ 8012+ (loss of  )→6722+ (loss of )→6722+ (loss of  )→852+ (loss of )→852+ (loss of 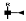 )→648+ (loss of )→648+ (loss of  )→444+ (loss of )→444+ (loss of  ) ) |
| 1075 | 2+ | N5H4 |  | 10752+→9462+ (loss of  )→ 8162+ (loss of )→ 8162+ (loss of  )→1146+ (loss of )→1146+ (loss of  )→853+ (loss of )→853+ (loss of 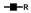 )→648+ (loss of )→648+ (loss of  )→444+ (loss of )→444+ (loss of  ) ) |
| 1127 | 2+ | N4H4F2 | 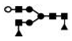 | 11272+→1768+ (loss of  )→ 1301+ (loss of )→ 1301+ (loss of  )→867+ (loss of )→867+ (loss of  ) ) |
| 1133 | 2+ | N4H4S1 |  | 11332+→9462+ (loss of  )→ 1419+ (loss of )→ 1419+ (loss of  )→1159+ (loss of )→1159+ (loss of  )→866+ (loss of )→866+ (loss of  )→662+ (loss of )→662+ (loss of  ) ) |
| 1142 | 2+ | N4H5F1 | 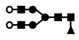 | 11422+→1797+ (loss of  )→ 1330+ (loss of )→ 1330+ (loss of 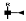 )→867+ (loss of )→867+ (loss of  )→ 662+ (loss of )→ 662+ (loss of  ) ) |
| 1162 | 2+ | N5H4F1 | 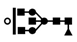 | 11622+→10332+ (loss of  )→ 9032+ (loss of )→ 9032+ (loss of  )→1320+ (loss of )→1320+ (loss of  )→852+ (loss of )→852+ (loss of 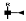 )→648+ (loss of )→648+ (loss of  )→444+ (loss of )→444+ (loss of  ) ) |
| 1177 | 2+ | N5H5 |  | 11772+→9462+ (loss of  )→ 8162+ (loss of )→ 8162+ (loss of  )→1146+ (loss of )→1146+ (loss of  )→852+ (loss of )→852+ (loss of 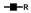 )→648+ (loss of )→648+ (loss of  )→444+ (loss of )→444+ (loss of  ) ) |
| 1235 | 2+ | N4H5S1 | 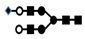 | 12352+→10472+ (loss of  )→ 8232+ (loss of )→ 8232+ (loss of  )→1329+ (loss of )→1329+ (loss of 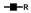 )→867+ (loss of )→867+ (loss of  )→ 662+ (loss of )→ 662+ (loss of  ) ) |
| 1264 | 2+ | N5H5F1 |  | 12642+→11352+ (loss of  )→ 9032+ (loss of )→ 9032+ (loss of  )→1319+ (loss of )→1319+ (loss of  )→852+ (loss of )→852+ (loss of 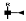 )→ 662+ (loss of )→ 662+ (loss of 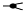 )→458+ (loss of )→458+ (loss of  ) ) |
| 1320 | 2+ | N2H10 |  | 13202+→11732+ (loss of 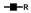 )→ 1696+ (loss of )→ 1696+ (loss of  )→1492+ (loss of )→1492+ (loss of  )→1274+ (loss of )→1274+ (loss of 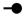 ) ) |
| 1322 | 2+ | N4H5F1S1 | 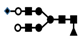 | 13222+→11352+ (loss of  )→ 9102+ (loss of )→ 9102+ (loss of  )→1333+ (loss of )→1333+ (loss of  )→866+ (loss of )→866+ (loss of 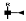 )→662+ (loss of )→662+ (loss of  ) ) |
| 1409 | 2+ | N4H5F2S1 | 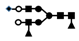 | 14092+→12212+ (loss of  )→ 9962+ (loss of )→ 9962+ (loss of  )→1333+ (loss of )→1333+ (loss of 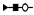 ) ) |
| 1416 | 2+ | N4H5S2 | 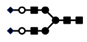 | 14162+→12282+ (loss of  )→ 10412+ (loss of )→ 10412+ (loss of  )→1609+ (loss of )→1609+ (loss of  )→1315+ (loss of )→1315+ (loss of 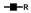 )→866+ (loss of )→866+ (loss of  )→662+ (loss of )→662+ (loss of  ) ) |
| 1445 | 2+ | N5H5F1S1 | 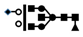 | 14452+→12572+ (loss of  )→ 10332+ (loss of )→ 10332+ (loss of  )→9032+ (loss of )→9032+ (loss of  )→1316+ (loss of )→1316+ (loss of 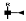 )→852+ (loss of )→852+ (loss of  ) ) |
| 1460 | 2+ | N5H6S1 |  | 14602+→12722+ (loss of  )→ 10402+ (loss of )→ 10402+ (loss of  )→8942+ (loss of )→8942+ (loss of 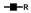 )→6692+(loss of )→6692+(loss of  )→852+ (loss of )→852+ (loss of  ) ) |
| Fragment | Neutral Loss | [M + Na]+ | Symbol |
|---|---|---|---|
| Terminal Hex | 218 | 241 |  |
| Internal Hex | 204 | 227 |  |
| Branched Hex | 190 | 213 |  |
| Terminal HexNAc | 259 | 282 |  |
| Internal HexNAc | 245 | 268 |  |
| Terminal NeuAc | 375 | 398 |  |
| Terminal Fuc | 188 | 211 |  |
| Reducing-end GlcNAc | 293 | 316 |  |
| GlcNAc-Fuc | 467 | 490 | 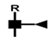 |
| Terminal Hex-HexNac | 463 | 486 |  |
| Internal Hex-HexNac | 449 | 472 |  |
| Core Man-GlcNAc | - | 444 |  |
| N-Glycosylation | Description | m/z of N-Glycans | |
|---|---|---|---|
| Galactosylation | galactoses per antenna on N-glycans | monogalactosylated | 952, 1039, 1075, 1127, 1133, 1162 |
| Fully galactosylated | 1054, 1142, 1177, 1235, 1264, 1322, 1409, 1416, 1445 | ||
| Sialylation | N-acetylneuraminic (sialic) acids per antenna on N-glycans | 1133, 1235, 1322, 1409, 1416, 1445, 1460 | |
| Fucosylation | N-glycans which carry a core fucose | 937, 1039, 1142, 1060, 1162, 1264, 1322, 1445, 1127, 1409 | |
| Bisection | N-glycans which carry a bisecting N-acetylglucosamine | 973, 1060, 1075, 1162, 1177, 1264, 1445, 1460 | |
| Characteristics | Patient | Normal |
|---|---|---|
| Number a | 44 | 30 |
| Female (%) b | 66.67 | 76.67 |
| Age (year) (median, range) | 61 (30–87) | 36 (21–44) |
| RF positivity (%) c | 100 | - |
| RF (median, U/mL) d | 759.75 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Xie, Q.; Wang, Y.; Li, Y. Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS. Int. J. Mol. Sci. 2020, 21, 2045. https://doi.org/10.3390/ijms21062045
Su Z, Xie Q, Wang Y, Li Y. Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS. International Journal of Molecular Sciences. 2020; 21(6):2045. https://doi.org/10.3390/ijms21062045
Chicago/Turabian StyleSu, Zhipeng, Qing Xie, Yanping Wang, and Yunsen Li. 2020. "Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS" International Journal of Molecular Sciences 21, no. 6: 2045. https://doi.org/10.3390/ijms21062045




