Effects of Thryptophan Hydroxylase Blockade by P-Chlorophenylalanine on Contextual Memory Reconsolidation after Training of Different Intensity
Abstract
:1. Introduction
2. Results
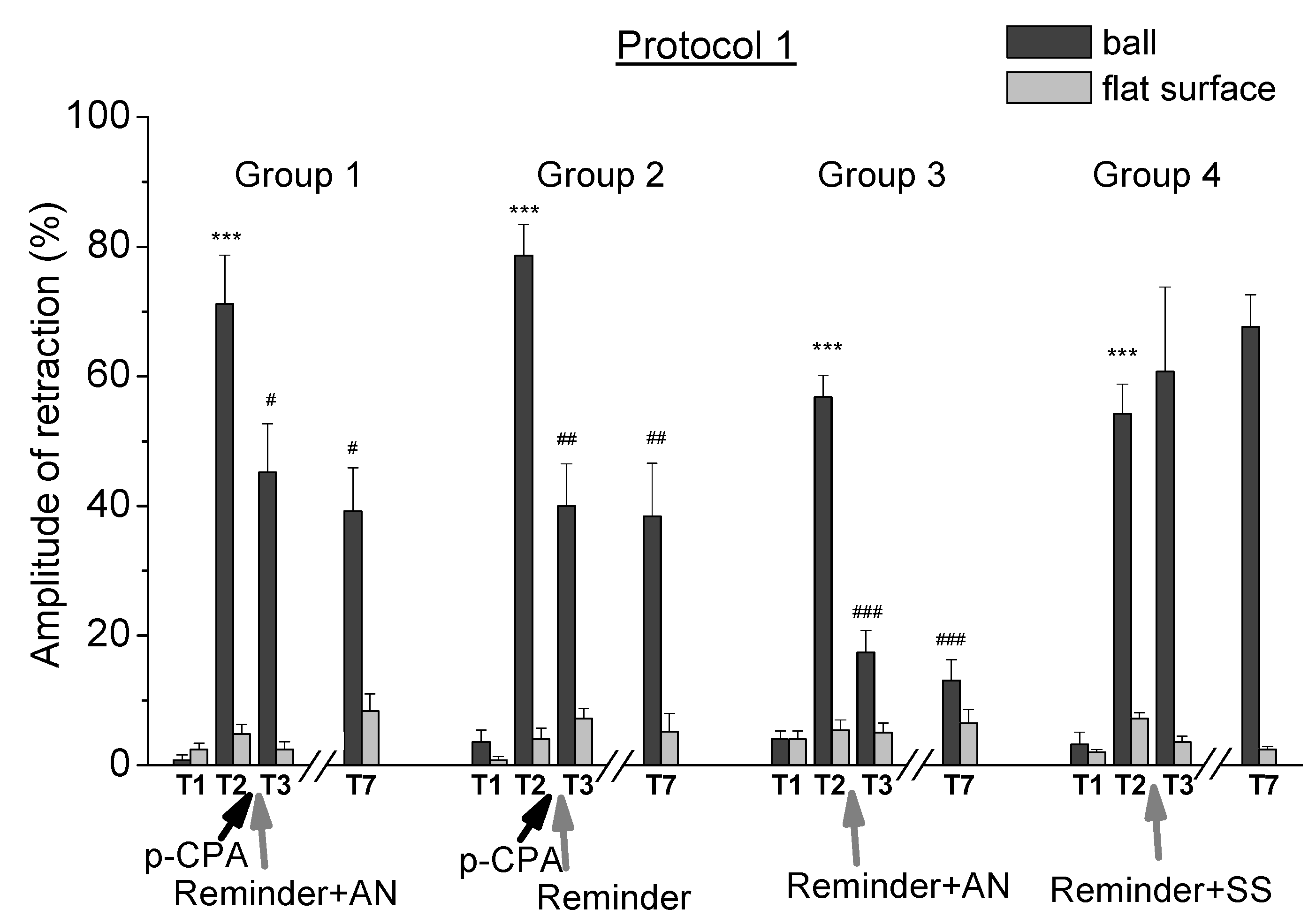
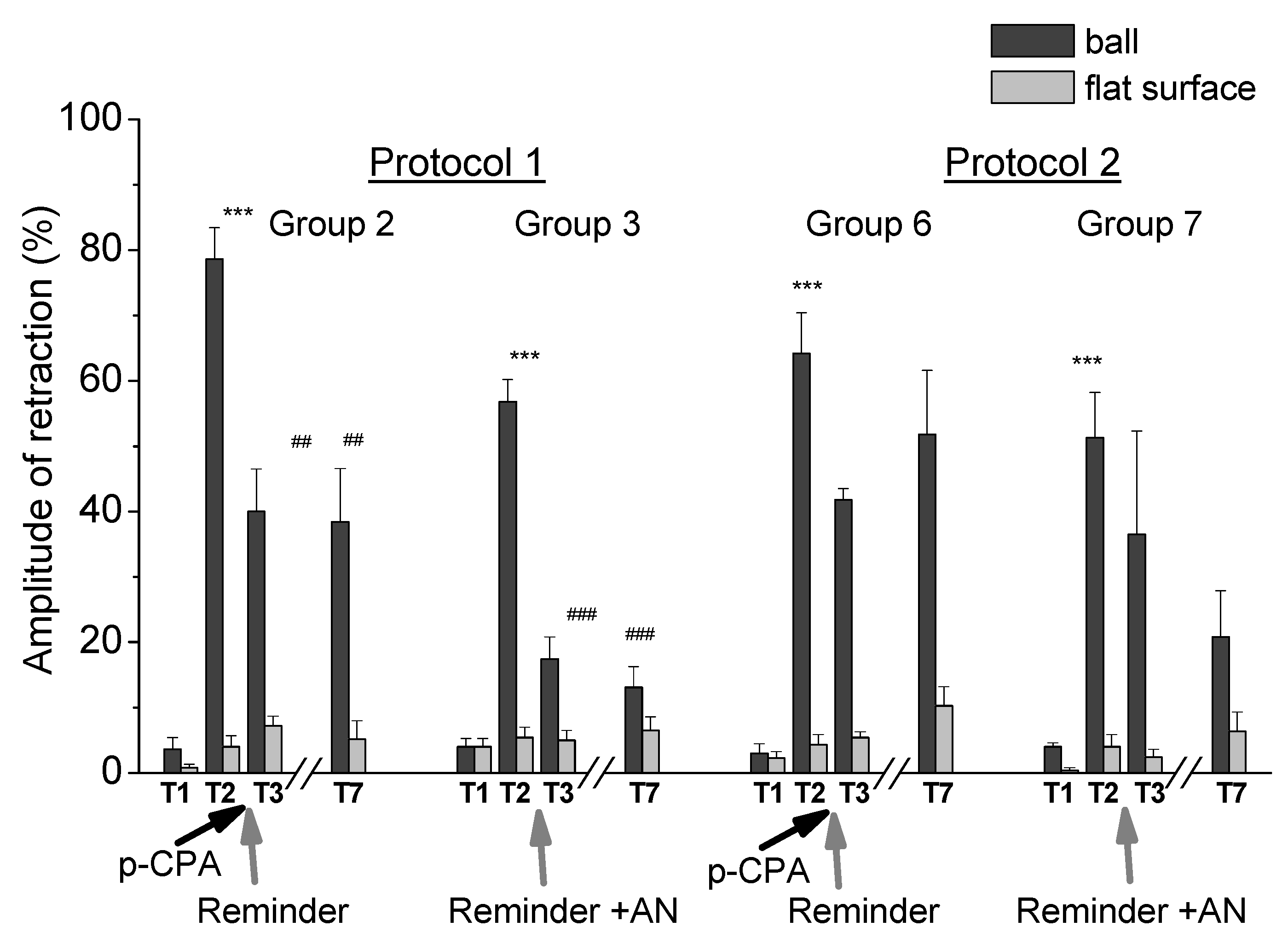
2.1. Reconsolidation after Training According to Protocol 1
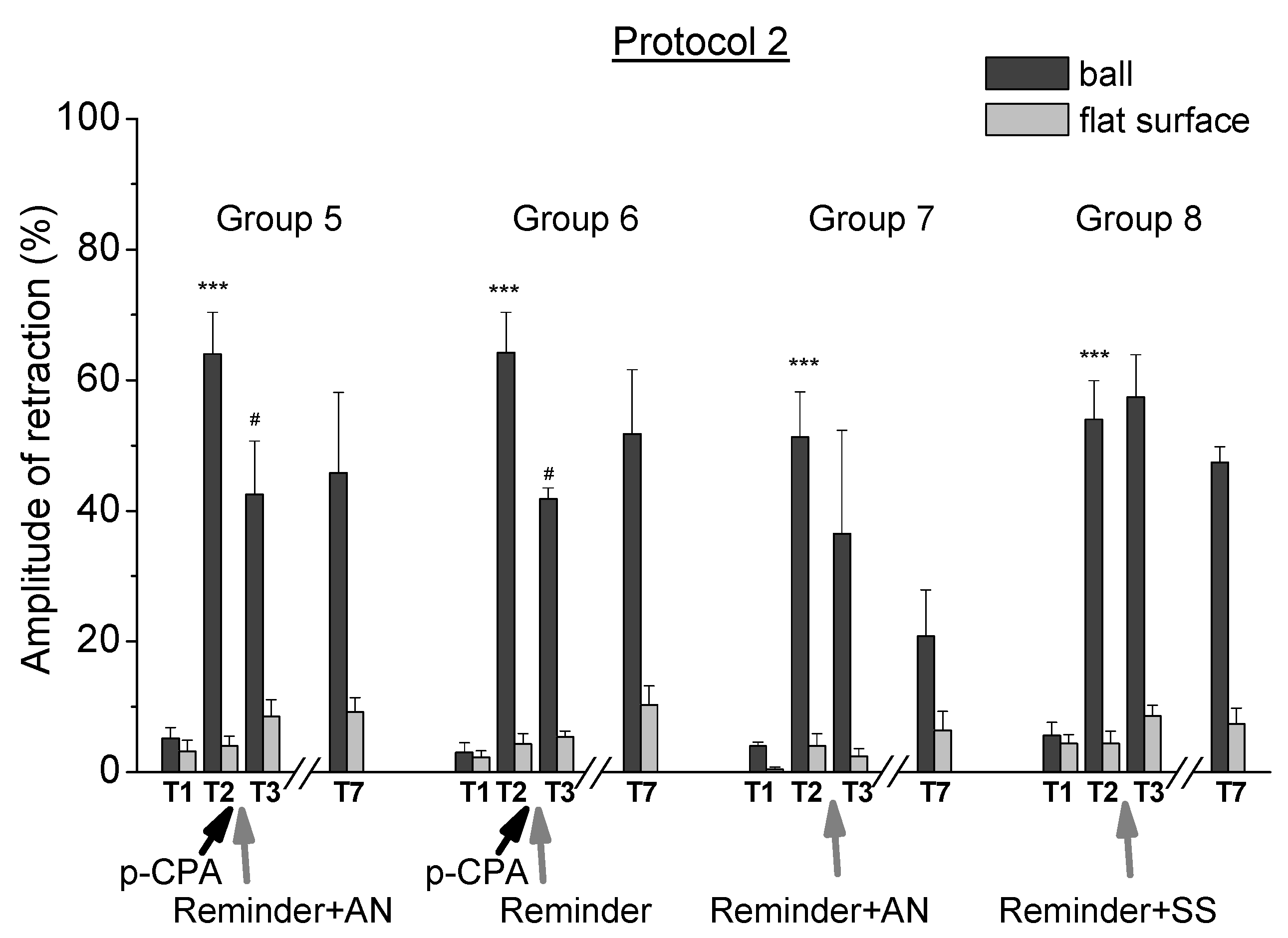
2.2. Reconsolidation after Training According to Protocol 2
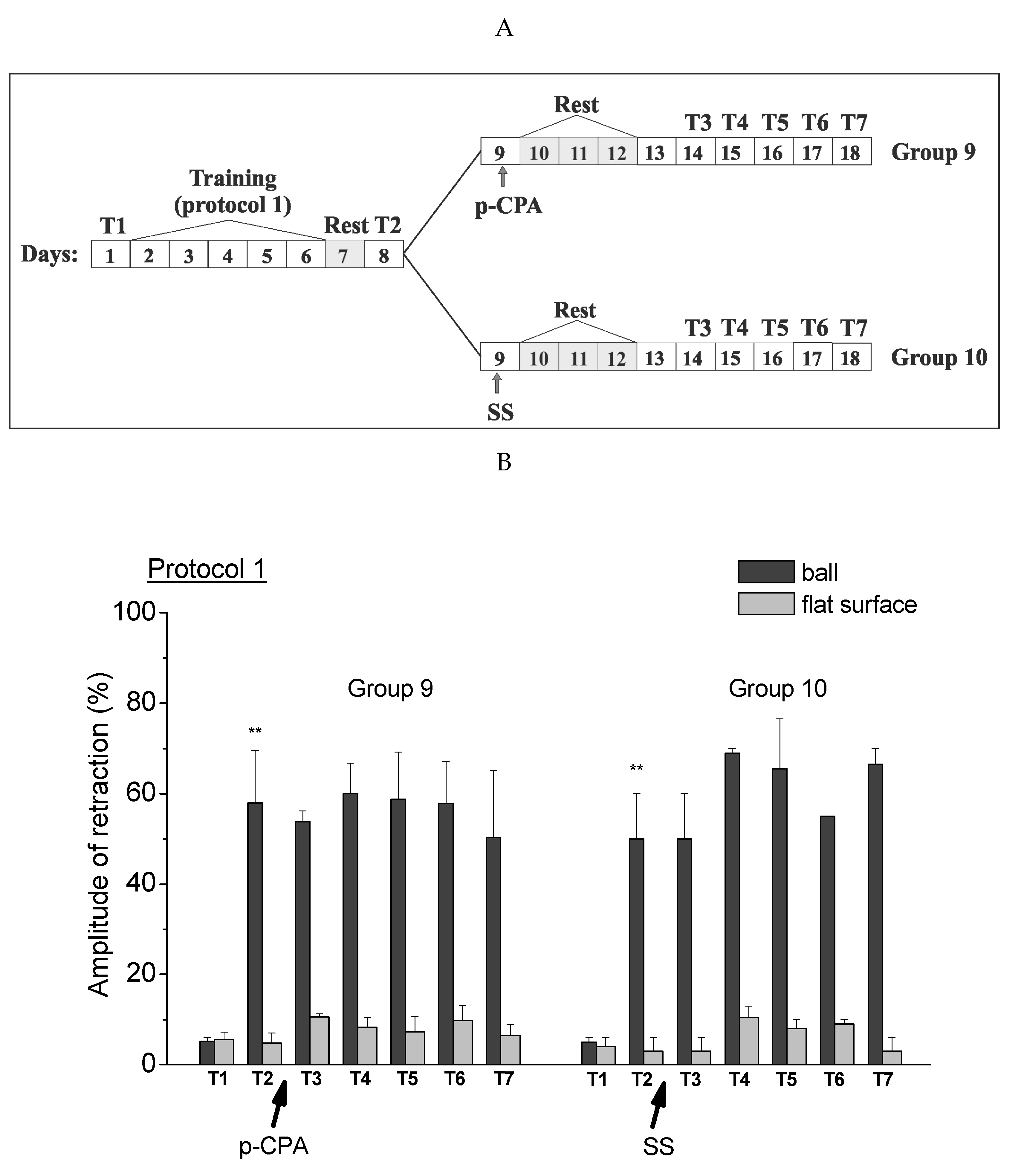
2.3. Testing Procedures in the Context of “on the Ball” after Inhibition of Serotonin Synthesis
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Drugs and Injections
4.3. Contextual Learning
4.4. Testing
4.5. Experimental Groups
4.6. Statistics
Highlights
- A blockade of tryptophan hydroxylase by p-chlorophenylalanine led to a weakening of contextual memory after reminding the situation of trained animals.
- A blockade of tryptophan hydroxylase by p-chlorophenylalanine did not lead to a significant change in contextual memory after reminding of the situation to animals learned by lesser intensity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balaban, P.M.; Vehovzsky, A.; Maksimova, O.A.; Zakharov, I.S. Effect of 5,7-dihydroxytryptamine on the food-aversive conditioning in the snail Helix lucorum L. Brain Res. 1987, 404, 201–210. [Google Scholar] [CrossRef]
- Glanzman, D.L.; Mackey, S.L.; Hawkins, R.D.; Dyke, A.M.; Lloyd, P.E.; Kandel, E.R. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J. Neurosci. 1989, 12, 4200–4213. [Google Scholar] [CrossRef] [Green Version]
- Gainutdinov, K.L.; Andrianov, V.V.; Gainutdinova, T.K. The action of the neurotoxins 5,6-dihydroxytryptamine and p-chlorphenylalanine on the electrical activity parameters of the command neurons during long-term sensitization and learning in the snail. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 1999, 49, 48–58. [Google Scholar]
- Dyakonova, V.E. Behavioral functions of serotonin and octopamin: Some paradoxes of comparative physiology. Uspekhi Physiol. Nauk (Russ.) 2007, 38, 3–20. [Google Scholar]
- Il-Han, J.; Janes, T.; Lukowiak, K. The role of serotonin in the enhancement of long-term memory resulting from predator detection in Lymnaea. J. Exp. Biol. 2010, 213, 3603–3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, K.; Moroz, L.L.; Nathan, G.; Hatcher, N.G.; Gillette, R. Neuromodulatory control of a goal-directed decision. PLoS ONE 2014, 9, 102240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrianov, V.V.; Bogodvid, T.K.; Deryabina, I.B.; Golovchenko, A.N.; Muranova, L.N.; Tagirova, R.R.; Vinarskaya, A.K.; Gainutdinov, K.L. Modulation of withdrawal reflex conditioning in snails by serotonin. Front. Behav. Neurosci. 2015, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Sakharov, D.A. Integrative function of serotonin in primitive Metazoa. Z. Obch. Biol. (Russ.) 1990, 51, 437–449. [Google Scholar]
- Liao, X.; Brou, C.G.; Walters, E.T. Limited contributions of serotonin to long-term hyperexcitability of Aplysia sensory neurons. J. Neurophysiol. 1999, 82, 3223–3235. [Google Scholar] [CrossRef]
- Marinesco, S.; Wickremasinghe, N.; Kolkman, K.E.; Carew, T.J. Serotonergic modulation in Aplysia. II. Cellular and behavioral consequences of increased serotonergic tone. J. Neurophysiol. 2004, 92, 2487–2496. [Google Scholar] [CrossRef] [Green Version]
- Ierusalimskii, V.N.; Balaban, P.M. Serotonergic system of neurons in the CNS of terrestrial snail: Morphology, ontogenesis, control of behavior. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 2010, 60, 515–524. [Google Scholar]
- Timoshenko, A.K.; Shevelkin, A.V.; Nikitin, V.P.; Sherstnev, V.V. Live-cell imaging microscopy and quantitative analysis of Ca2+-dependent effects of neurotransmitters on DNA in snail neurons. Biophysics 2014, 59, 91–97. [Google Scholar] [CrossRef]
- Clark, G.A.; Kandel, E.R. Induction of long-term facilitation in Aplysia sensory neurones by local application of serotonin to remote synapses. Proc. Natl. Acad. Sci. USA 1993, 90, 11411–11415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malyshev, A.Y.; Bravarenko, N.I.; Pivovarov, A.S.; Balaban, P.M. Effects of serotonin levels on postsynaptically induced potentiation of snail neuron responses. Neurosci. Behav. Physiol. 1998, 28, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Shevelkin, A.V.; Nikitin, V.P.; Kozyrev, S.A.; Samoilov, M.O.; Sherstnev, V.V. Serotonin imitates several of the neuronal effects of nociceptive sensitization in the common snail. Neurosci. Behav. Physiol. 1998, 28, 547–555. [Google Scholar] [CrossRef]
- Mauelshagen, J.; Sherff, C.M.; Carew, T.J. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn. Mem. 1998, 5, 246–256. [Google Scholar]
- Marinesco, S.; Wickremasinghe, N.; Carew, T.J. Regulation of behavioral and synaptic plasticity by serotonin release within local modulatory fields in the CNS of Aplysia. J. Neurosci. 2006, 92, 2487–2496. [Google Scholar] [CrossRef]
- Hernadi, L.; Karpati, L.; Gyori, J.; Vehovszky, A.; Hiripi, L. Humoral serotonin and dopamine modulate the feeding in the snail, Helix pomatia L. Acta Biol. Hung. 2008, 59, 39–46. [Google Scholar] [CrossRef]
- Lin, A.H.; Cohen, J.E.; Wan, Q.; Niu, K.; Shrestha, P.; Bernstein, S.L.; Abrams, T.W. Serotonin stimulation of cAMP-dependent plasticity in Aplysia sensory neurons is mediated by calmodulin-sensitive adenylyl cyclase. Proc. Natl. Acad. Sci. USA 2010, 107, 15607–15612. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.K.; Fioravante, D.; Liu, R.-Y.; Phares, G.A.; Cleary, L.J.; Byrne, J.H. Serotonin-mediated synapsin expression is necessary for long-term facilitation of the Aplysia sensorimotor synapse. J. Neurosci. 2011, 31, 18401–18411. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.V.; Jensen, C.S.; Crépe, V.; Falkerslev, M.; Perrier, J.-F. Serotonin regulates the firing of principal cells of the subiculum by inhibiting a T-type Ca2+ current. Front. Cell. Neurosci. 2017, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadotti, D.; Bauce, L.G.; Lukowiak, K.; Bulloch, A.G.M. Transient depletion of serotonin in the nervous system of Helisoma. J. Neurobiol. 1986, 17, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Glanzman, D.L.; Krasne, F.B. 5,7-Dihydroxytryptamine lesions of crayfish serotonin-containing neurons: Effect on the lateral giant escape reaction. J. Neurosci. 1986, 6, 1560–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernadi, L.; Hiripi, L.; Vehovszky, A.; Kemenes, G.; Rozsa, K. Ultrastructural, biochemical and electrophysiological changes induced by 5,6-dihydroxytryptamine in the CNS of the snail Helix pomatia L. Brain Res. 1992, 578, 221–234. [Google Scholar] [CrossRef]
- Sahley, С.L. Serotonin depletion impairs but does not eliminate classical conditioning in the leech Hirudo medicinalis. Behav. Neurosci. 1994, 108, 1043–1052. [Google Scholar] [CrossRef]
- Shirahata, T.; Tsunoda, M.; Santa, T.; Kirino, Y.; Watanabe, S. Depletion of serotonin selectively impairs short-term memory without affecting long-term memory in odor learning in the terrestrial slug Limax valentianus. Learn. Mem. 2006, 13, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Tagirova, R.R.; Deryabina, I.B.; Gainutdinova, T.K.; Andrianov, V.V.; Gainutdinov, K.L. Effects of the serotonin precursor 5-hydroxytryptophan and the neurotoxic analog 5,7-dihydroxytryptamine on the formation of a conditioned defensive reflex in the common snail. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 2010, 60, 239–243. [Google Scholar] [CrossRef]
- Balaban, P.M.; Vinarskaya, A.K.; Zuzina, A.B.; Ierusalimsky, V.N.; Malyshev, A.Y. Impairment of the serotonergic neurons underlying reinforcement elicits extinction of the repeatedly reactivated context memory. Sci. Rep. 2016, 6, 36933. [Google Scholar] [CrossRef] [Green Version]
- Reader, T.A.; Gauthier, P. Catecholamines and serotonin in the rat central nervous system after 6-OHDA, 5-7-DHT and p-CPA. J. Neural Transm. 1984, 59, 207–227. [Google Scholar] [CrossRef]
- Park, D.H.; Stone, D.M.; Baker, H.; Kim, K.S.; Joh, T.H. Early induction of rat brain tryptophan hydroxylase (TPH) mRNA following parachlorophenylalanine (PCPA) treatment. Brain Res. Mol. Brain Res. 1994, 22, 20–28. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Bechtholt, A.J.; Crowley, J.J.; Hill, T.E.; Page, M.E.; Lucki, I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berlin) 2007, 192, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Balaban, P.V.; Bravarenko, N.I. Long-term sensitization and environmental conditioning in terrestrial snails. Exp. Brain Res. 1993, 96, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, V.V.; Gainutdinova, T.H.; Gainutdinov, K.L. The investigation of serotonin role in long-term sensitization in snail: Changes of electrical characteristics of withdrawal interneurons after influence of 5,6-dihydroxytryptamine. Acta Neurobiol. Exp. 2003, 63, 254. [Google Scholar]
- Deryabina, I.; Bogodvid, T.; Muranova, L.; Andrianov, V.; Gainutdinov, K. Effects of serotonin depletion by p-chlorophenylalanine on reconsolidation of contextual memory. Eur. J. Clin. Investig. 2017, 47, 123. [Google Scholar]
- Deryabina, I.; Muranova, L.; Gainutdinov, K. Reconsolidation of contextual memory after reminder in terrestrial snail depend from serotonin. Neurochem. J. 2018, 12, 27. [Google Scholar] [CrossRef]
- Goelet, P.; Castellucci, V.F.; Goelet, P.; Schacher, S.; Kandel, E.R. The long and the short of long-term memory—A molecular framework. Nature 1986, 322, 419–422. [Google Scholar] [CrossRef]
- McGaugh, J.L. Memory: A century of consolidation. Science 2000, 287, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Sara, S.J.; Hars, B. In memory of consolidation. Learn. Mem. 2006, 13, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Grinkevich, L.N.; Lisachev, P.D.; Grinkevich, L.N.; Kharchenko, O.A.; Vasil’ev, G.V. Expression of MAP/ERK kinase cascade corresponds to the ability to develop food aversion in terrestrial snail at different stages of ontogeneses. Brain Res. 2008, 1187, 12–19. [Google Scholar] [CrossRef]
- Balaban, P.M. Molecular mechanisms of memory modifications. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 2017, 67, 131–140. [Google Scholar] [CrossRef]
- Anokhin, K.V.; Tiunova, A.A.; Rose, S.P.R. Reminder effects—Reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur. J. Neurosci. 2002, 15, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004, 55, 51–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberini, C.M. Mechanisms of memory stabilisation: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005, 28, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Nader, K.; Schafe, G.E.; LeDoux, J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000, 406, 722–726. [Google Scholar] [CrossRef]
- Milekic, M.H.; Alberini, C.M. Temporally graded requirement for protein synthesis following memory reactivation. Neuron 2002, 36, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, M.; Kobilo, T.; Berman, D.E.; Dudai, Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science 2003, 301, 1102–1104. [Google Scholar] [CrossRef] [Green Version]
- Gainutdinova, T.H.; Tagirova, R.R.; Ismailova, A.I.; Muranova, L.N.; Samarova, E.I.; Gainutdinov, K.L.; Balaban, P.M. Reconsolidation of a context long-term memory in the terrestrial snail requires protein synthesis. Learn. Mem. 2005, 12, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Alberini, C.M. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front. Behav. Neurosci. 2011, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Pearce, K.; Chen, S.; Glanzman, D.L. Reconsolidation of long-term memory in Aplysia. Curr. Biol. 2012, 22, 1783–1788. [Google Scholar] [CrossRef] [Green Version]
- Lattal, K.M.; Wood, M.A. Epigenetics and persistent memory: Implications for reconsolidation and silent extinction beyond the zero. Nat. Neurosci. 2013, 16, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.L.C.; Nader, K.; Schiller, D. An update on memory reconsolidation updating. Trends Cognit. Sci. 2017, 1677, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem. 2000, 7, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Nader, K.; Hardt, O. A single standard for memory: The case for reconsolidation. Nat. Rev. Neurosci. 2009, 10, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, N.; Fukushima, H.; Suzuki, A.; Matsuyama, Z.; Homma, S.; Frankland, P.W.; Kida, S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J. Neurosci. 2009, 29, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Soeter, M.; Kindt, M. High trait anxiety: A challenge for disrupting fear memory reconsolidation. PLoS ONE 2013, 8, e75239. [Google Scholar] [CrossRef] [Green Version]
- Child, F.M.; Epstein, H.T.; Kuzirian, A.M.; Alkon, D.L. Memory reconsolidation in Hermissenda. Biol. Bull. 2003, 205, 218–219. [Google Scholar] [CrossRef]
- Gainutdinova, T.H.; Tagirova, R.R.; Ismailova, A.I.; Muranova, L.N.; Gainutdinov, K.L.; Balaban, P.M. Dependent from protein syntheses reactivation of situational defensive reflex in terrestrial snail. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 2004, 54, 795–800. [Google Scholar]
- Kemenes, G.; Kemenes, I.; Michel, M.; Papp, A.; Mueller, U. Phase-dependent molecular requirements for memory reconsolidation: Differential roles for protein synthesis and protein kinase A activity. J. Neurosci. 2006, 26, 6298–6302. [Google Scholar] [CrossRef]
- Lukowiak, K.; Fras, M.; Smyth, K.; Wong, C.; Hittel, K. Reconsolidation and memory infidelity in Lymnaea. Neurobiol. Learn. Mem. 2007, 87, 547–560. [Google Scholar] [CrossRef]
- Balaban, P.M.; Roshchin, M.V.; Timoshenko, A.K.; Gainutdinov, K.L.; Bogodvid, T.K.; Muranova, L.N.; Zuzina, A.B.; Korshunova, T.A. Nitric oxide is necessary for labilization of a consolidated context memory during reconsolidation in terrestrial snails. Eur. J. Neurosci. 2014, 40, 2963–2970. [Google Scholar] [CrossRef]
- Nikitin, V.P.; Solntseva, S.V.; Kozyrev, S.A.; Nikitin, P.V.; Shevelkin, A.V. NMDA or 5-HT receptor antagonists impair memory reconsolidation and induce various types of amnesia. Behav. Brain Res. 2018, 345, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Cleary, L.J.; Lee, W.L.; Byrne, J.H. Cellular correlates of long-term sensitization in Aplysia. J. Neurosci. 1998, 18, 5988–5998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gainutdinov, K.L.; Chekmarev, L.Y.; Gainutdinova, T.H. Excitability increase in withdrawal interneurons after conditioning in snail. NeuroReport 1998, 9, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Gainutdinov, K.L.; Andrianov, V.V.; Gainutdinova, T.K. Changes of the neuronal membrane excitability as cellular mechanisms of learning and memory. Uspekhi Physiol. Nauk (Russ.) 2011, 42, 33–52. [Google Scholar]
- Balaban, P.М.; Bravarenko, N.I.; Maksimova, O.A.; Nikitin, E.; Ierusalimsky, V.N.; Zakharov, I.S. A single serotoninergic modulatory cell can mediate reinforcement in the withdrawal network of the terrestrial snail. Neurobiol. Learn. Mem. 2001, 75, 30–50. [Google Scholar] [CrossRef] [Green Version]
- Barbas, D.; DesGroseillers, L.; Castellucci, V.F.; Carew, T.J.; Marinesco, S. Multiple serotonergic mechanisms contributing to sensitization in evidence of diverse serotonin receptor subtypes Aplysia. Learn. Mem. 2003, 10, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Gainutdinova, T.K.; Andrianov, V.V.; Gainutdinov, K.L.; Mukhamedshina, D.I.; Tagirova, R.R. Duration in electrical characteristics of command neurons after withdrawal conditioning in snail. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 2003, 53, 379–382. [Google Scholar]
- Abramova, M.S.; Nistratova, V.L.; Moskvitin, A.A.; Pivovarov, A.S. Methiothepin-sensitive serotonin receptors are involved in the postsynaptic mechanism of sensitization of the withdrawal response of the common snail. Neurosci. Behav. Physiol. 2006, 36, 589–596. [Google Scholar] [CrossRef]
- Mozzachiodi, R.; Lorenzetti, F.D.; Baxter, D.A.; Byrne, J.H. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nat. Neurosci. 2008, 11, 1146–1148. [Google Scholar] [CrossRef] [Green Version]
- Glanzman, D.L. Common mechanisms of synaptic plasticity in vertebrates and invertebrates. Curr. Biol. 2010, 20, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Sakharov, D.A. Biological substrate for generation of behavioral acts. Zurnal Obch. Biol. (Russ.) 2012, 73, 324–348. [Google Scholar]
- Bogodvid, T.K.; Andrianov, V.V.; Deryabina, I.B.; Muranova, L.N.; Silantyeva, D.I.; Vinarskaya, A.K.; Balaban, P.M.; Gainutdinov, K.L. Responses of premotor interneurons to serotonin application in naïve and learned snails are different. Front. Cell. Neurosci. 2017, 11, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidorov, A.V. Neuromodulation effects of hydrogen peroxide on central neurons within feeding network in the mollusc Lymnaea stagnalis. J. Evol. Biochem. Physiol. 2017, 53, 493–500. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [Green Version]
- Dyakonova, T.L.; Sultanakhmetov, G.S.; Mezheritskiy, M.I.; Sakharov, D.A.; Dyakonova, V.E. Storage and erausure of behavioral experiences at the single neuron level. Sci. Rep. 2019, 9, 14733. [Google Scholar] [CrossRef]
- Suzuki, A.; Josselyn, S.A.; Frankland, P.W.; Masushige, S.; Silva, A.J.; Kida, S. Memory reconsolidation and extinction. Have distinct temporal and biochemical signatures. J. Neurosci. 2004, 24, 4787–4795. [Google Scholar] [CrossRef]
- Sorg, B.A. Reconsolidation of drug memories. Neurosci. Biobehav. Rev. 2012, 36, 1400–1417, Epub 10 February 2012. [Google Scholar] [CrossRef] [Green Version]
- Deryabina, I.B.; Muranova, L.N.; Andrianov, V.V.; Gainutdinov, K.L. Impairing of serotonin synthesis by p-clorphenylalanine prevents the forgetting of contextual memory after reminder and the protein synthesis inhibition. Front. Pharmacol. 2018, 9, 607. [Google Scholar] [CrossRef]
- Pappius, H.M.; Dadoun, R.; McHugh, M. The effect of p-chlorophenylalanine on cerebral metabolism and biogenic amine content of traumatized brain. J. Cereb. Blood Flow Metab. 1988, 8, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Popova, N.K.; Naumenko, E.V.; Kolpakov, V.G. Serotonin and behavior (Russian). Novosib. Nauka (Sib. Dep.) 1978. [Google Scholar]
- Gillette, R. Evolution and function in serotonergic systems. Integr. Comp. Biol. 2006, 46, 838–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyakonova, V.E. Neurotransmitter mechanisms of context-dependent behavior. Neurosci. Behav. Physiol. 2014, 44, 256–267. [Google Scholar] [CrossRef]
- Grinkevich, L.N.; Vorobiova, O.V. The role of the modulator transmitter serotonin in induction epigenetic processts at the formation of long-term memory in Helix. Vavil. J. Genet. Sel. (Russ.) 2014, 18, 298–307. [Google Scholar]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N. Engl. J. Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef] [Green Version]
- Curran, K.P.; Chalasani, S.H. Serotonin circuits and anxiety: What can invertebrates teach us? Invertebr. Neurosci. 2012, 12, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Haberzettl, R.; Berta, B.; Fink, H.; Fox, M.A. Animal models of the serotonin syndrome: A systematic review. Behav. Brain Res. 2013, 256, 328–345. [Google Scholar] [CrossRef]
- Francescangeli, J.; Karamchandani, K.; Powell, M.; Bonavia, A. The serotonin syndrome: From molecular mechanisms to clinical practice. Int. J. Mol. Sci. 2019, 20, 2288. [Google Scholar] [CrossRef] [Green Version]
- Owens, M.J.; Nemeroff, C.B. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clin. Chem. 1994, 40, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Meneses, A. Serotonin, neural markers, and memory. Front. Pharmacol. 2015, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, A.; Perez-Garcia, G.; Ponce-Lopez, T.; Tellez, R.; Castillo, C. Serotonin transporter and memory. Neuropharmacology 2011, 61, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Kumar, A.; Malhotra, P.; Maher, D.; Singh, V.; Dudeja, P.K.; Alrefai, W.; Saksena, S. Regulation of intestinal serotonin transporter expression via epigenetic mechanisms: Role of HDAC2. Am. J. Physiol. Cell Physiol. 2013, 304, C334–C341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.W.; Lim, B.V.; Baek, D.; Ryu, D.-S.; Seo, J.H. Stress-induced depression is alleviated by aerobic exercise through up-regulation of 5-hydroxytryptamine 1A receptors in rats. Int. Neurourol. J. 2015, 19, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Petrassi, M.; Barber, R.; Be, C.; Beach, S.; Cox, B.; D’Souza, A.-M.; Duggan, N.; Hussey, M.; Fox, R.; Hunt, P.; et al. Identification of a novel allosteric inhibitory site on tryptophan hydroxylase 1 enabling unprecedented selectivity over all related hydroxylases. Front. Pharmacol. 2017, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Bloom, F.E.; German, N.J. Physiologic and pharmacologic considerations of biogenic amines in the nervous system. Ann. Rev. Pharmacol. Toxicol. 1968, 8, 225–229. [Google Scholar] [CrossRef]
- Dringenberg, H.C.; Hargreaves, E.L.; Baker, G.B.; Cooley, R.K.; Vanderwolf, C.H. p-Chlorophenylalanine-induced serotonin depletion: Reduction in exploratory locomotion but no obvious sensory-motor deficits. Behav. Brain Res. 1995, 68, 229–237. [Google Scholar] [CrossRef]
- Koprowska, M.; Krotewicz, M.; Romaniuk, A.; Strzelczuk, M.; Wieczorek, M. Behavioral and neurochemical alterations evoked by p-Chlorophenylalanine application in rats examined in the light-dark crossing test. Acta Neurobiol. Exp. 1999, 59, 15–22. [Google Scholar]
- Sodhi, M.S.K.; Sanders-Bush, E. A tryptophan and serotonin depletion studies. Int. Rev. Neurobiol. 2004, 59, 111–174. [Google Scholar] [PubMed]
- Кoе, B.K.; Weissman, A. P-chlorphenylalanine: A specific depletory of brain serotonin. J. Pharmacol. Exp. Ther. 1966, 154, 499–516. [Google Scholar]
- Keller, H.H. Depletion of cerebral monoamines by p-chlorophenylalanine in the cat. Experientia 1972, 28, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Tenen, S.S. The effects of p-chlorphenylalanine, a serotonin depletory, on avoidance acquisiytion, pain sensitivity and related behavior in the rat. Psychopharmalogia 1967, 10, 204–219. [Google Scholar]
- Reader, T.A.; Briere, R.; Grondin, L.; Ferron, A. Effects of p-clorphenylalanine on cortical monoamines and on the activity of noradrenergic neurons. Neurochem. Res. 1986, 11, 1025–1035. [Google Scholar] [CrossRef]
- Baker, M.W.; Croll, R.P. Modulation of in vivo neuronal sprouting by serotonin in the adult CNS of the snail. Cell. Mol. Neurobiol. 1996, 16, 561–576. [Google Scholar] [CrossRef]
- Hernadi, L.; Elekes, K.; Katalin, S. Distribution of serotonin-containing neurons in the central nervous system of the snail, Helix pomatia. Cell Tissue Res. 1989, 257, 313–323. [Google Scholar] [CrossRef]
- Fossat, P.; Bacqué-Cazenave, J.; De Deurwaerdère, P.; Delbecque, J.-P.; Cattaert, D. Anxiety-like behavior in crayfish is controlled by serotonin. Science 2014, 6189, 1293–1297. [Google Scholar] [CrossRef]
- Arkhipova, S.S.; Gainutdinova, T.K.; Ismailova, A.I.; Gainutdinov, K.L. Comparative studies of the effects of chlorpromazine and 5,6-dihydroxytryptamine on locomotion, defensive reactions in the snail Helix Lucorum, and command neuron excitability in long-term sensitization. Neurosci. Behav. Physiol. 2006, 36, 759–766. [Google Scholar] [CrossRef]
- Gainutdinova, T.K.; Andrianov, V.V.; Gainutdinov, K.L. Effect of 5,6-dihydroxytryptamine on the membrane and threshold potentials of command neurons upon long-term sensitization in the garden snail. Dokl. Biol. Sci. (Physiol.) 1998, 361, 307–309. [Google Scholar]
- Levenson, J.; Byrne, J.H.; Eskin, A. Levels of serotonin in the hemolymph of Aplysia are modulated by light/dark cycles and sensitization training. J. Neurosci. 1999, 19, 8094–8103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghirardi, M.; Benfenati, F.; Giovedi, S.; Fiumara, F.; Milanese, C.; Montarolo, P.G. Inhibition of neurotransmitter release by a nonphysiological target requires protein synthesis and involves cAMP-dependent and mitogen-activated protein kinases. J. Neurosci. 2004, 24, 5054–5062. [Google Scholar] [CrossRef] [PubMed]
- Quadros, I.M.; Takahashi, A.; Miczek, K.A. Tryptophan hydroxylase (Serotonin) and aggression. In Handbook of the Behavioral Neurobiology of Serotonin; Academic Press: New York, NY, USA, 2010; Volume 21, pp. 687–713. [Google Scholar]
- Nazar, M.; Siemiatkowski, M.; Bidziński, A.; Członkowska, A.; Sienkiewicz-Jarosz, H.; Płaźnik, A. The influence of serotonin depletion on rat behavior in the Vogel test and brain 3H-zolpidem binding. J. Neural Transm. (Vienna) 1999, 106, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.L.; Molosh, A.; Fitz, S.D.; Arendt, D.; Deehan, G.A.; Federici, L.M.; Bernabe, C.; Engleman, E.A.; Rodd, Z.A.; Lowry, C.A.; et al. Pharmacological depletion of serotonin in the basolateral amygdala complex reduces anxiety and disrupts fear conditioning. Pharmacol. Biochem. Behav. 2015, 138, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Grechenko, T.N. The action of electroshocks on behavioural and neuronal reactions of snail. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 1977, 27, 203–206. [Google Scholar]
- Gainutdinov, K.L.; Beregovoi, N.A. Long-term sensitization in snail: Electrophysiological correlations in command neurons of avoidance behavior. Zhurnal. Vyss. Nervn. Deiat. Im. I P Pavlov. (Russ.) 1994, 44, 307–315. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryabina, I.B.; Andrianov, V.V.; Muranova, L.N.; Bogodvid, T.K.; Gainutdinov, K.L. Effects of Thryptophan Hydroxylase Blockade by P-Chlorophenylalanine on Contextual Memory Reconsolidation after Training of Different Intensity. Int. J. Mol. Sci. 2020, 21, 2087. https://doi.org/10.3390/ijms21062087
Deryabina IB, Andrianov VV, Muranova LN, Bogodvid TK, Gainutdinov KL. Effects of Thryptophan Hydroxylase Blockade by P-Chlorophenylalanine on Contextual Memory Reconsolidation after Training of Different Intensity. International Journal of Molecular Sciences. 2020; 21(6):2087. https://doi.org/10.3390/ijms21062087
Chicago/Turabian StyleDeryabina, Irina B., Viatcheslav V. Andrianov, Lyudmila N. Muranova, Tatiana K. Bogodvid, and Khalil L. Gainutdinov. 2020. "Effects of Thryptophan Hydroxylase Blockade by P-Chlorophenylalanine on Contextual Memory Reconsolidation after Training of Different Intensity" International Journal of Molecular Sciences 21, no. 6: 2087. https://doi.org/10.3390/ijms21062087



