Detection of Age-Related Changes in Tendon Molecular Composition by Raman Spectroscopy—Potential for Rapid, Non-Invasive Assessment of Susceptibility to Injury
Abstract
:1. Introduction
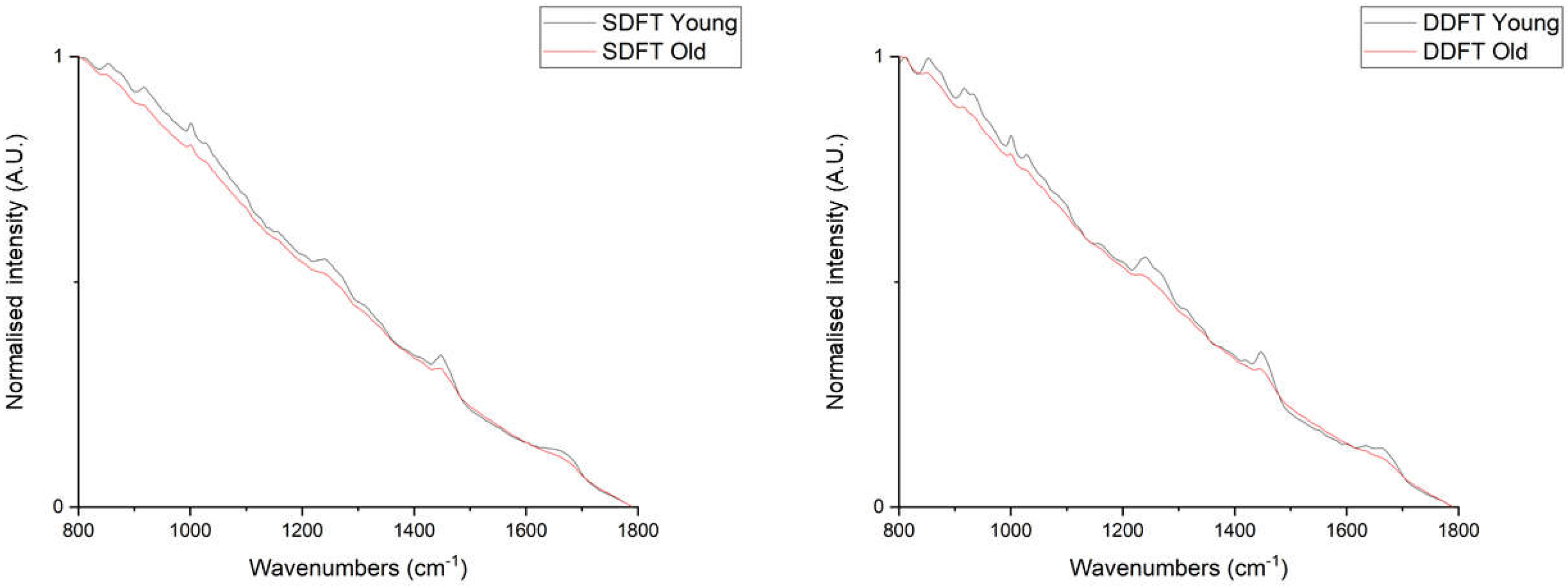
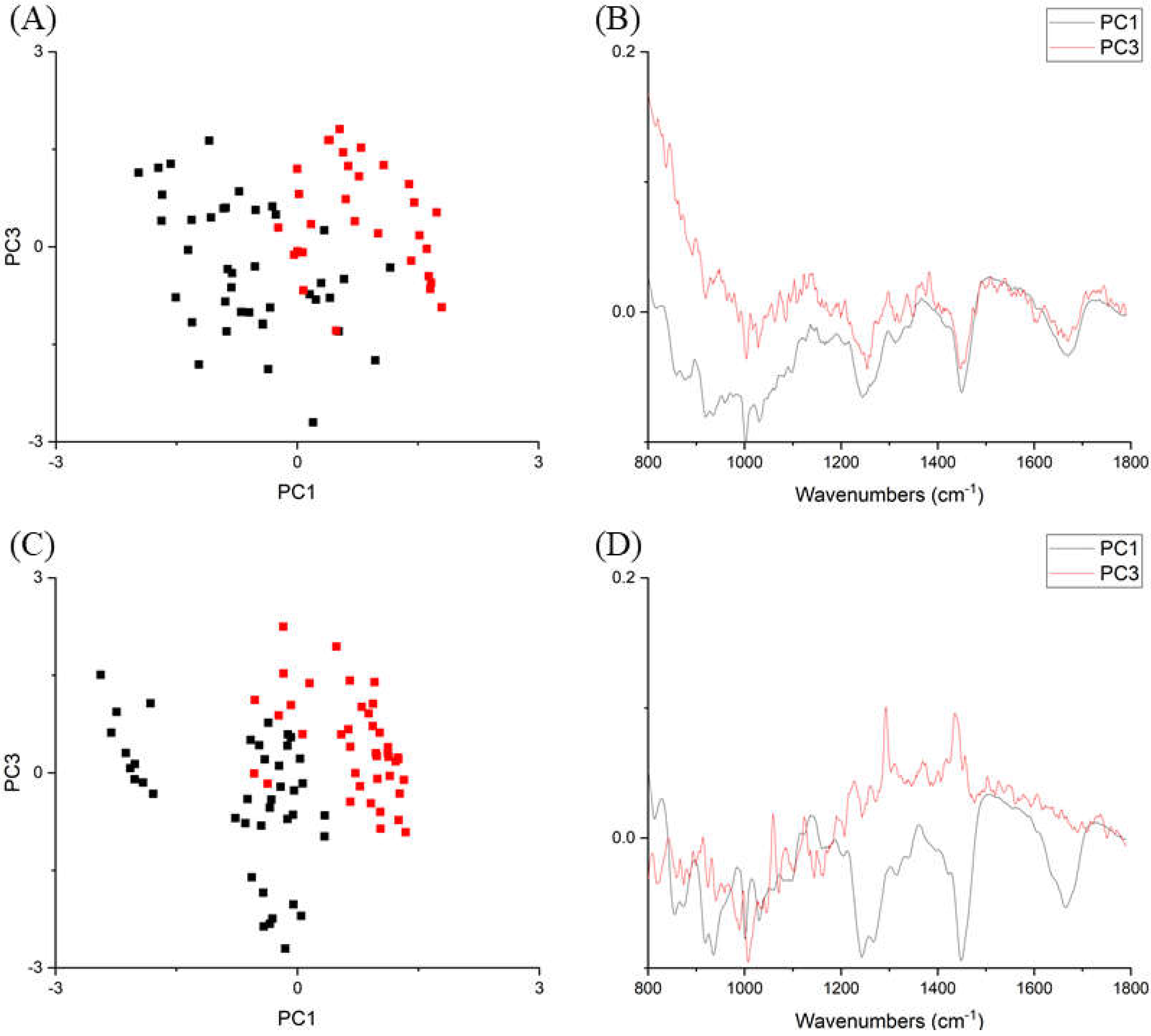
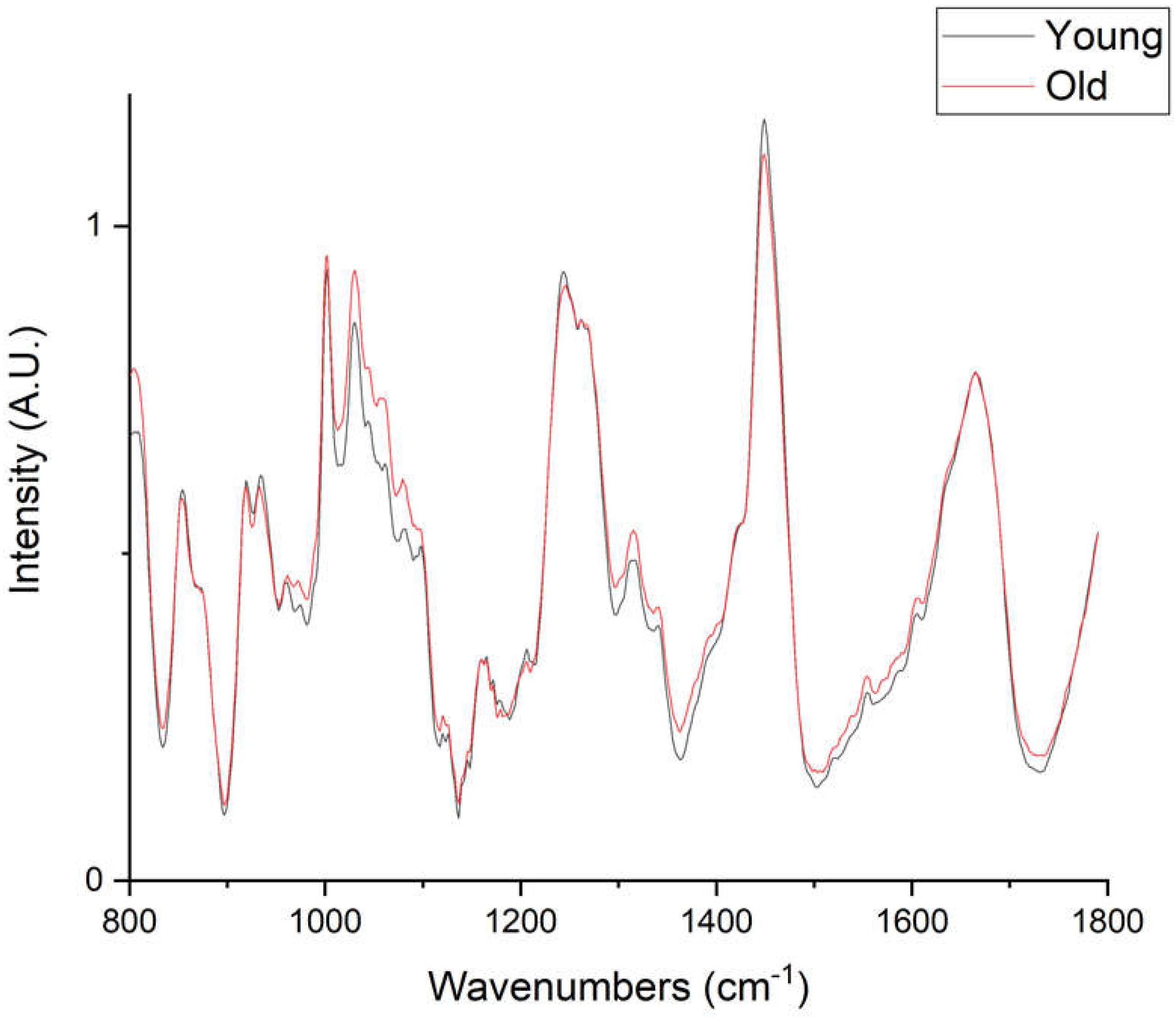
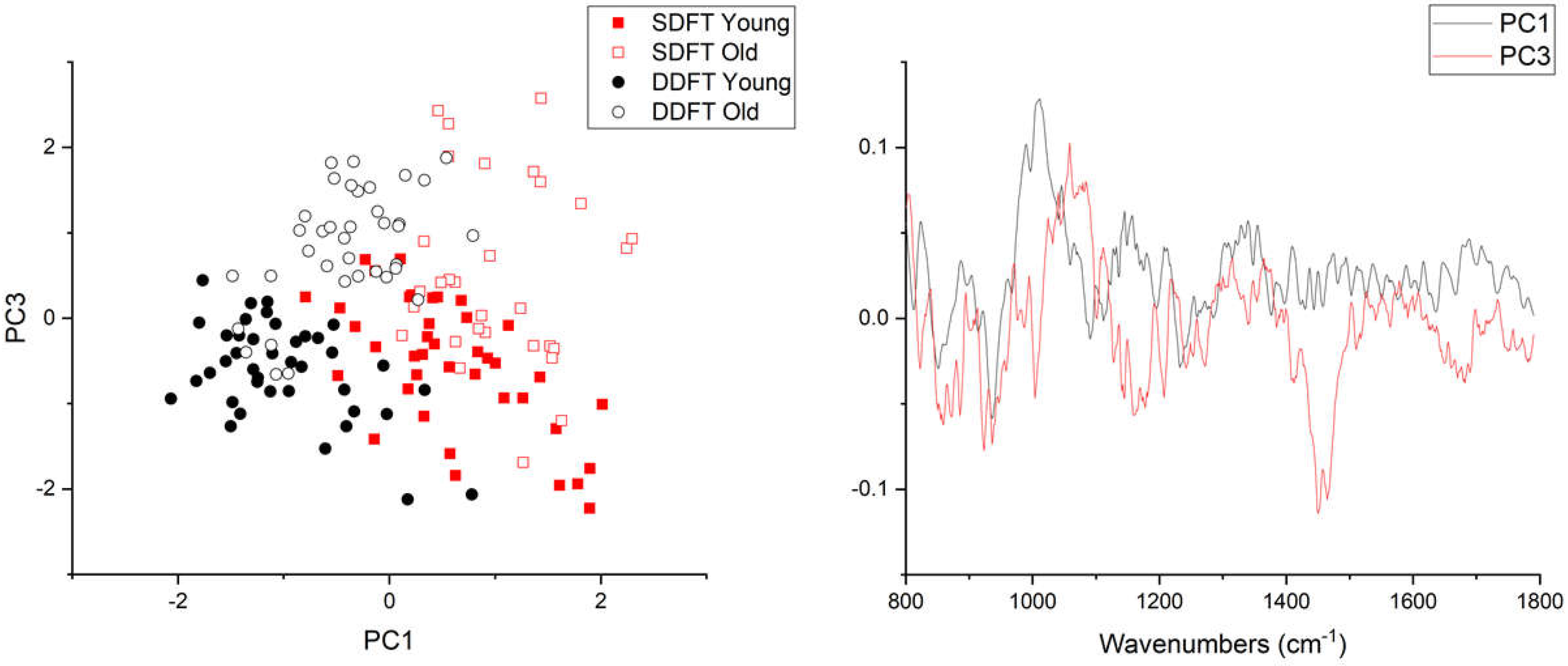
2. Results
3. Discussion
4. Materials and Methods
4.1. Tendon Sample Collection and Preparation
4.2. Raman Spectroscopy
4.3. Data analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end-product |
| DDFT | Deep digital flexor tendon |
| ECM | Extracellular matrix |
| PCA | Principal component analysis |
| SDFT | Superficial digital flexor tendon |
References
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Birch, H.L.; Thorpe, C.T.; Rumian, A.P. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. 2013, 3, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L. Tendon matrix composition and turnover in relation to functional requirements. Int. J. Exp. Pathol. 2007, 88, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Screen, H.R.C. Tendon structure and composition. In Metabolic Influences on Risk for Tendon Disorders; Ackermann, P.W., Hart, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–10. [Google Scholar]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013, 27, 2074–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, C.T.; Streeter, I.; Pinchbeck, G.L.; Goodship, A.E.; Clegg, P.D.; Birch, H.L. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J. Biol. Chem. 2010, 285, 15674–15681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birch, H.L. Extracellular matrix and ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science; Harris, J.R., Korolchuk, V.I., Eds.; Springer: Singapore, 2018; pp. 169–190. [Google Scholar]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes—The good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014, 4, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Eekhoff, J.D.; Fang, F.; Lake, S.P. Multiscale mechanical effects of native collagen cross-linking in tendon. Connect. Tissue Res. 2018, 59, 410–422. [Google Scholar] [CrossRef]
- Nash, A.; Notou, M.; Lopez-Clavijo, A.F.; Bozec, L.; De Leeuw, N.H.; Birch, H.L. Glucosepane is associated with changes to structural and physical properties of collagen fibrils. Matrix Biol. Plus 2019, 4, 100013. [Google Scholar] [CrossRef]
- Thorpe, C.T.; McDermott, B.T.; Goodship, A.E.; Clegg, P.D.; Birch, H.L. Ageing does not result in a decline in cell synthetic activity in an injury prone tendon. Scand. J. Med. Sci. Sports 2016, 26, 684–693. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Harada, Y.; Takamatsu, T.; Tanaka, H. Label-free molecular imaging and analysis by Raman spectroscopy. Acta Histochem. Cytochem. 2018, 51, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Larkin, P. Chapter 1—Introduction: Infrared and Raman spectroscopy. In Infrared and Raman Spectroscopy; Larkin, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 1–5. [Google Scholar]
- Bonnier, F.; Byrne, H.J. Understanding the molecular information contained in principal component analysis of vibrational spectra of biological systems. Analyst 2012, 137, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman spectroscopy: Guiding light for the extracellular matrix. Front. Bioeng. Biotechnol. 2019, 1, 303. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.G.; Gikas, P.D.; Buckley, K.; Shepperd, A.; Birch, H.L.; McCarthy, I.; Miles, J.; Briggs, T.W.; Keen, R.; Parker, A.W.; et al. Evidence from Raman spectroscopy of a putative link between inherent bone matrix chemistry and degenerative joint disease. Arthritis Rheumatol. 2014, 66, 1237–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerns, J.G.; Buckley, K.; Churchwell, J.; Parker, A.W.; Matousek, P.; Goodship, A.E. Is the collagen primed for mineralization in specific regions of the Turkey tendon? An investigation of the protein-mineral interface using Raman spectroscopy. Anal. Chem. 2016, 88, 1559–1563. [Google Scholar] [CrossRef] [Green Version]
- Matousek, P.; Draper, E.R.; Goodship, A.E.; Clark, I.P.; Ronayne, K.L.; Parker, A.W. Noninvasive Raman spectroscopy of human tissue in vivo. Appl. Spectrosc. 2006, 60, 758–763. [Google Scholar] [CrossRef]
- Stone, N.; Baker, R.; Rogers, K.; Parker, A.W.; Matousek, P. Subsurface probing of calcifications with spatially offset Raman spectroscopy (SORS): Future possibilities for the diagnosis of breast cancer. Analyst 2007, 132, 899–905. [Google Scholar] [CrossRef]
- Esmonde-White, K. Raman spectroscopy of soft musculoskeletal tissues. Appl. Spectrosc. 2014, 68, 1203–1218. [Google Scholar] [CrossRef] [Green Version]
- Moura Junior Mde, J.; Maia Filho, A.L.; Pessoa, D.R.; Alves, M.D.; Justino Jde, S.; Andrade Mdos, S.; Rebelo, A.M.; De Lima, C.J.; Pinheiro, A.L.; Silveira, L., Jr. Assessing the biochemical changes of tendons of rats in an experimental model of tenotomy under therapeutic ultrasound and LEDs (625 and 945 nm) by near-infrared Raman spectroscopy. Lasers Med. Sci. 2015, 30, 1729–1738. [Google Scholar] [CrossRef]
- Penteado, S.C.; Fogazza, B.P.; Carvalho Cda, S.; Arisawa, E.A.; Martins, M.A.; Martin, A.A.; Martinho Hda, S. Diagnosis of degenerative lesions of supraspinatus rotator cuff tendons by Fourier transform-Raman spectroscopy. J. Biomed. Opt. 2008, 13, 014018. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, P.K.; Silveira, L., Jr.; Barbosa, D.; Munin, E.; Salgado, M.A.; Villaverde, A.B. Analysis of experimental tendinitis in rats treated with laser and platelet-rich plasma therapies by Raman spectroscopy and histometry. Lasers Med. Sci. 2016, 31, 19–26. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Verdelis, K.; Doty, S.B.; Boskey, A.L.; Mendelsohn, R.; Yamauchi, M. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Dehring, K.A.; Smukler, A.R.; Roessler, B.J.; Morris, M.D. Correlating changes in collagen secondary structure with aging and defective type II collagen by Raman spectroscopy. Appl. Spectrosc. 2006, 60, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L.; Bailey, J.V.; Bailey, A.J.; Goodship, A.E. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet. J. 1999, 31, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T. Extracellular Matrix Synthesis and Degradation in Functionally Distinct Tendons; University College London: London, UK, 2010. [Google Scholar]
- Denoix, J.M. Functional anatomy of tendons and ligaments in the distal limbs (manus and pes). Vet. Clin. N. Am. Equine Pract. 1994, 10, 273–322. [Google Scholar] [CrossRef]
- Larkin, P.J. Chapter 3—Instrumentation and sampling methods. In Infrared and Raman Spectroscopy, 2nd ed.; Larkin, P.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–61. [Google Scholar]
- Wei, D.; Chen, S.; Liu, Q. Review of fluorescence suppression techniques in Raman spectroscopy. Appl. Spectrosc. Rev. 2015, 50, 387–406. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Lingelbach, L.B.; Mitchell, A.E.; Rucker, R.B.; McDonald, R.B. Accumulation of advanced glycation endproducts in aging male Fischer 344 rats during long-term feeding of various dietary carbohydrates. J. Nutr. 2000, 130, 1247–1255. [Google Scholar] [CrossRef]
- Couppe, C.; Hansen, P.; Kongsgaard, M.; Kovanen, V.; Suetta, C.; Aagaard, P.; Kjaer, M.; Magnusson, S.P. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J. Appl. Physiol. 1985 2009, 107, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.H.; Yue, F.; Saw, S.K.; Foguth, R.; Cannon, J.R.; Shannahan, J.H.; Kuang, S.; Sabbaghi, A.; Carroll, C.C. Advanced glycation end-products suppress mitochondrial function and proliferative capacity of achilles tendon-derived fibroblasts. Sci. Rep. 2019, 9, 12614. [Google Scholar] [CrossRef] [Green Version]
- Konova, E.; Baydanoff, S.; Atanasova, M.; Velkova, A. Age-related changes in the glycation of human aortic elastin. Exp. Gerontol. 2004, 39, 249–254. [Google Scholar] [CrossRef]
- Kikugawa, K.; Beppu, M. Involvement of lipid oxidation products in the formation of fluorescent and cross-linked proteins. Chem. Phys. Lipids 1987, 44, 277–296. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Piccolini, V.M.; Tarantola, E.; Boncompagni, E.; Bertone, V.; Milanesi, G.; Freitas, I.; Vairetti, M.; Bottiroli, G. Integrated autofluorescence characterization of a modified-diet liver model with accumulation of lipids and oxidative stress. BioMed Res. Int. 2014, 2014, 803491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couppe, C.; Svensson, R.B.; Grosset, J.F.; Kovanen, V.; Nielsen, R.H.; Olsen, M.R.; Larsen, J.O.; Praet, S.F.; Skovgaard, D.; Hansen, M.; et al. Life-long endurance running is associated with reduced glycation and mechanical stress in connective tissue. Age (Dordr.) 2014, 36, 9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjerrild, J.N.; Wobbe, A.; Stausholm, M.B.; Larsen, A.E.; Josefsen, C.O.; Malmgaard-Clausen, N.M.; Dela, F.; Kjaer, M.; Magnusson, S.P.; Hansen, M.; et al. Effects of long-term physical activity and diet on skin glycation and achilles tendon structure. Nutrients 2019, 11, 1409. [Google Scholar] [CrossRef] [Green Version]
- Fokkens, B.T.; Smit, A.J. Skin fluorescence as a clinical tool for non-invasive assessment of advanced glycation and long-term complications of diabetes. Glycoconj. J. 2016, 33, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Churchwell, J.H.; Sowoidnich, K.; Chan, O.; Goodship, A.E.; Parker, A.W.; Matousek, P. Adaptive band target entropy minimization: Optimization for the decomposition of spatially offset Raman spectra of bone. J. Raman Spectrosc. 2019, 51, 66–78. [Google Scholar] [CrossRef]
- Sanders, K. Proteomic Analysis of Tendons at Different Risk of Injury; University of Liverpool: Liverpool, UK, 2016. [Google Scholar]
- Thorpe, C.T.; Peffers, M.J.; Simpson, D.; Halliwell, E.; Screen, H.R.C.; Clegg, P.D. Anatomical heterogeneity of tendon: Fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci. Rep. 2016, 6, 20455. [Google Scholar] [CrossRef]
- Dukor, R.K.; Griffiths, P.R. Vibrational spectroscopy in the detection of cancer. In Handbook of Vibrational Spectroscopy; John Wiley and Sons Ltd: Chichester, UK, 2006. [Google Scholar]
- Bolboac, S.; Jäntschi, L. Amino acids sequence analysis on collagen. Bull. USAMV CN 2007, 64. [Google Scholar] [CrossRef]
- Jastrzebska, M.; Wrzalik, R.; Kocot, A.; Zalewska-Rejdak, J.; Cwalina, B. Raman spectroscopic study of glutaraldehyde-stabilized collagen and pericardium tissue. J. Biomater. Sci. Polym. Ed. 2003, 14, 185–197. [Google Scholar] [CrossRef]
- Guilbert, M.; Said, G.; Happillon, T.; Untereiner, V.; Garnotel, R.; Jeannesson, P.; Sockalingum, G.D. Probing non-enzymatic glycation of type I collagen: A novel approach using Raman and infrared biophotonic methods. Biochim. Biophys. Acta 2013, 1830, 3525–3531. [Google Scholar] [CrossRef]
- Collier, T.A.; Nash, A.; Birch, H.L.; De Leeuw, N.H. Effect on the mechanical properties of type I collagen of intra-molecular lysine-arginine derived advanced glycation end-product cross-linking. J. Biomech. 2018, 67, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Passini, F.S.; Silván, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2011, 59, 95–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masic, A.; Bertinetti, L.; Schuetz, R.; Chang, S.W.; Metzger, T.H.; Buehler, M.J.; Fratzl, P. Osmotic pressure induced tensile forces in tendon collagen. Nat. Commun. 2015, 6, 5942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Andrew Chan, K.L.; Zhang, G.; Gillece, T.; Senak, L.; Moore, D.J.; Mendelsohn, R.; Flach, C.R. Raman microspectroscopic and dynamic vapor sorption characterization of hydration in collagen and dermal tissue. Biopolymers 2011, 95, 607–615. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, N.-H.; Parker, A.W.; Matousek, P.; Birch, H.L. Detection of Age-Related Changes in Tendon Molecular Composition by Raman Spectroscopy—Potential for Rapid, Non-Invasive Assessment of Susceptibility to Injury. Int. J. Mol. Sci. 2020, 21, 2150. https://doi.org/10.3390/ijms21062150
Yin N-H, Parker AW, Matousek P, Birch HL. Detection of Age-Related Changes in Tendon Molecular Composition by Raman Spectroscopy—Potential for Rapid, Non-Invasive Assessment of Susceptibility to Injury. International Journal of Molecular Sciences. 2020; 21(6):2150. https://doi.org/10.3390/ijms21062150
Chicago/Turabian StyleYin, Nai-Hao, Anthony W. Parker, Pavel Matousek, and Helen L. Birch. 2020. "Detection of Age-Related Changes in Tendon Molecular Composition by Raman Spectroscopy—Potential for Rapid, Non-Invasive Assessment of Susceptibility to Injury" International Journal of Molecular Sciences 21, no. 6: 2150. https://doi.org/10.3390/ijms21062150





