Vitamin D Attenuates Loss of Endothelial Biomarker Expression in Cardio-Endothelial Cells
Abstract
:1. Introduction
2. Results
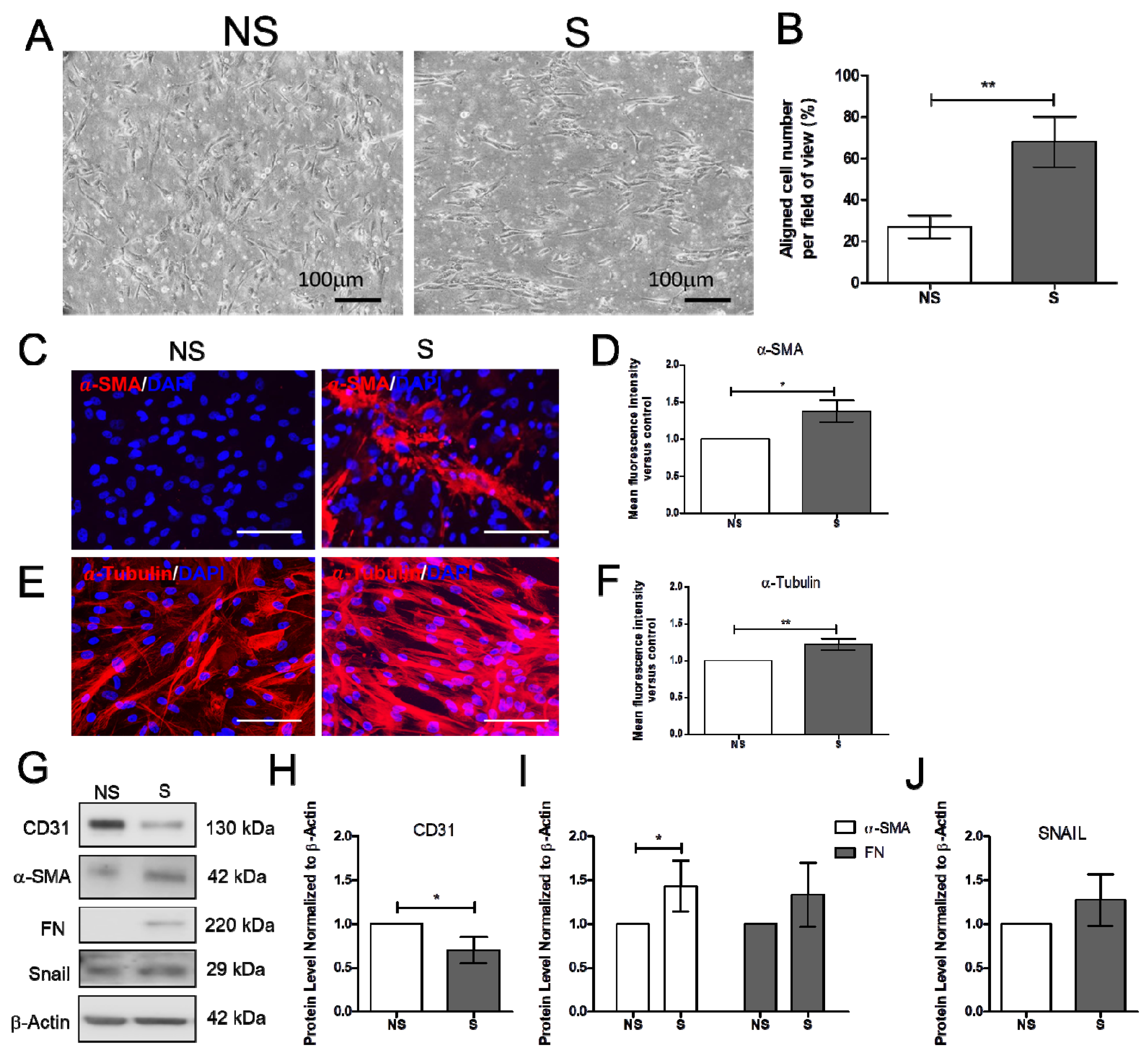
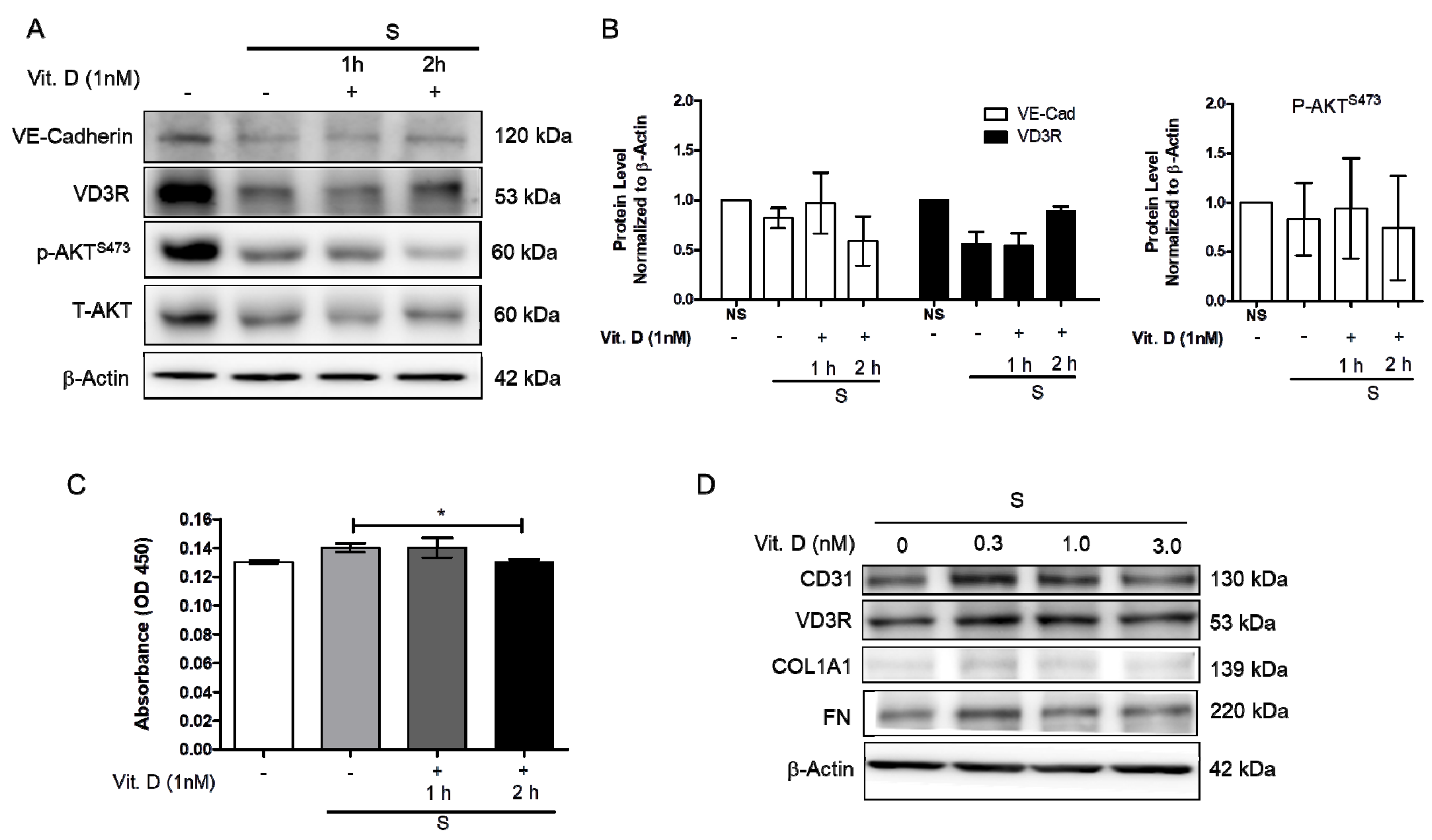
2.1. Effect of Vitamin D on the Mechanically Induced Fibrosis Model
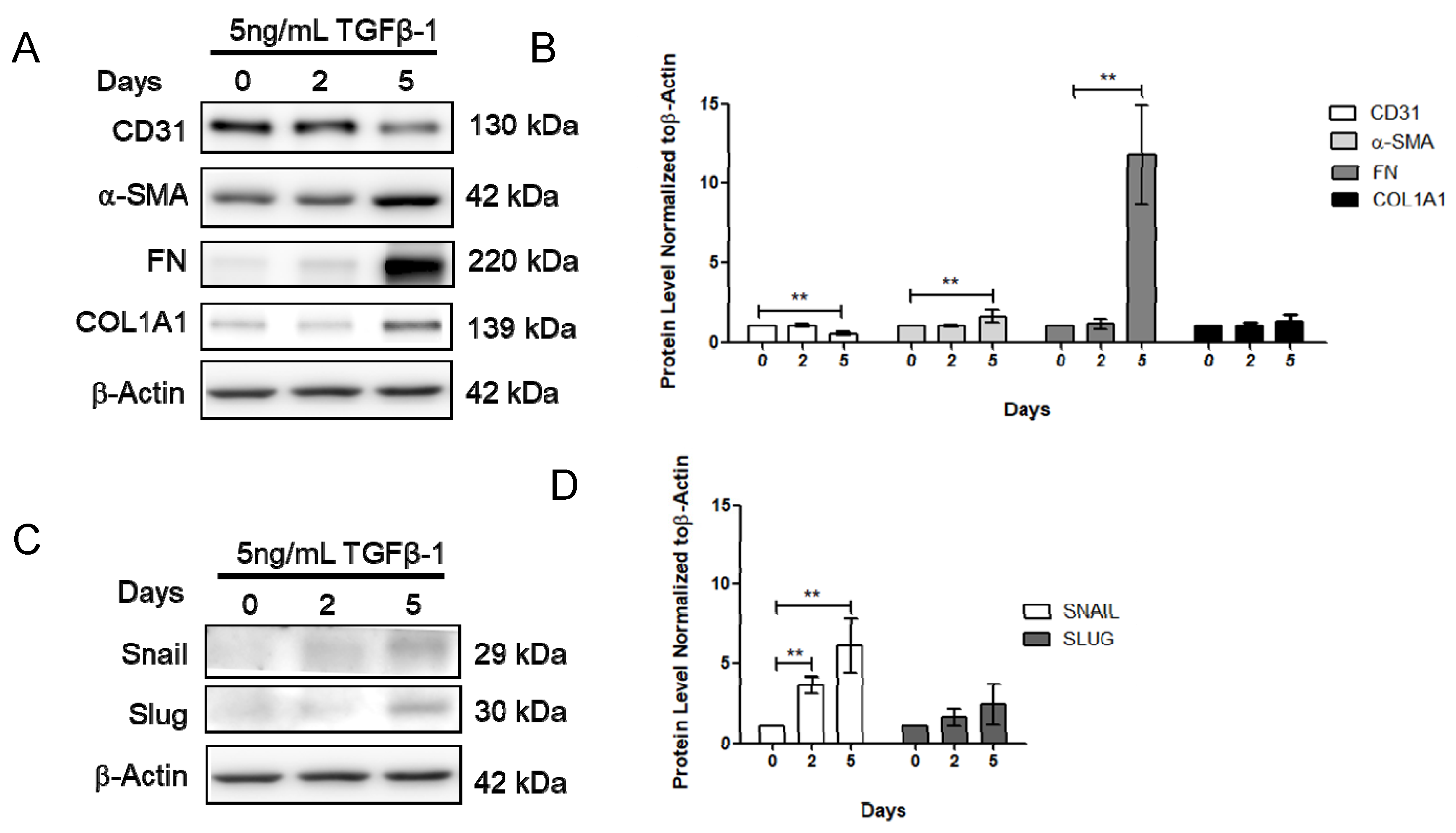
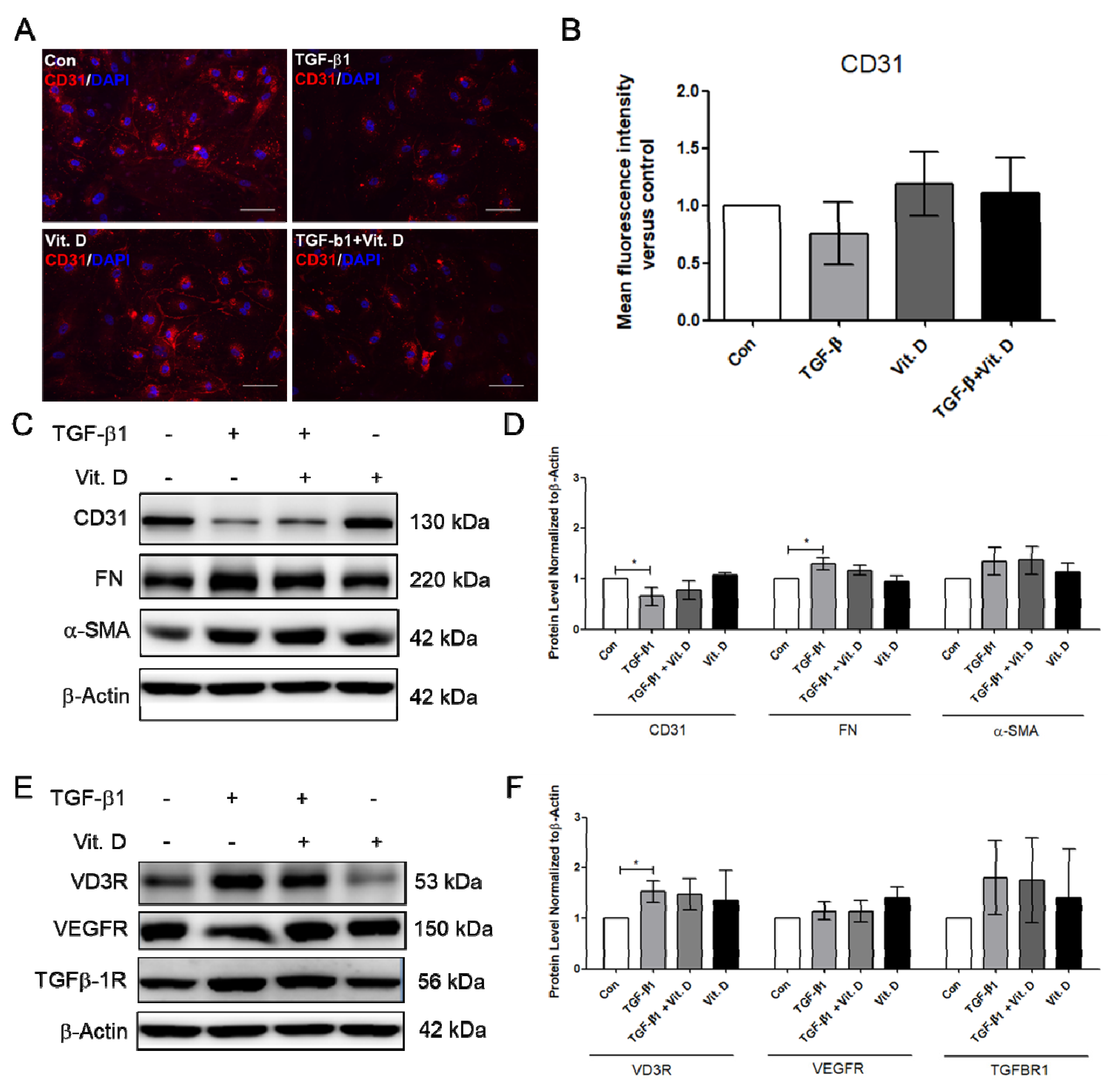
2.2. Effect of Vitamin D on the TGF-β1-Induced Fibrosis Model
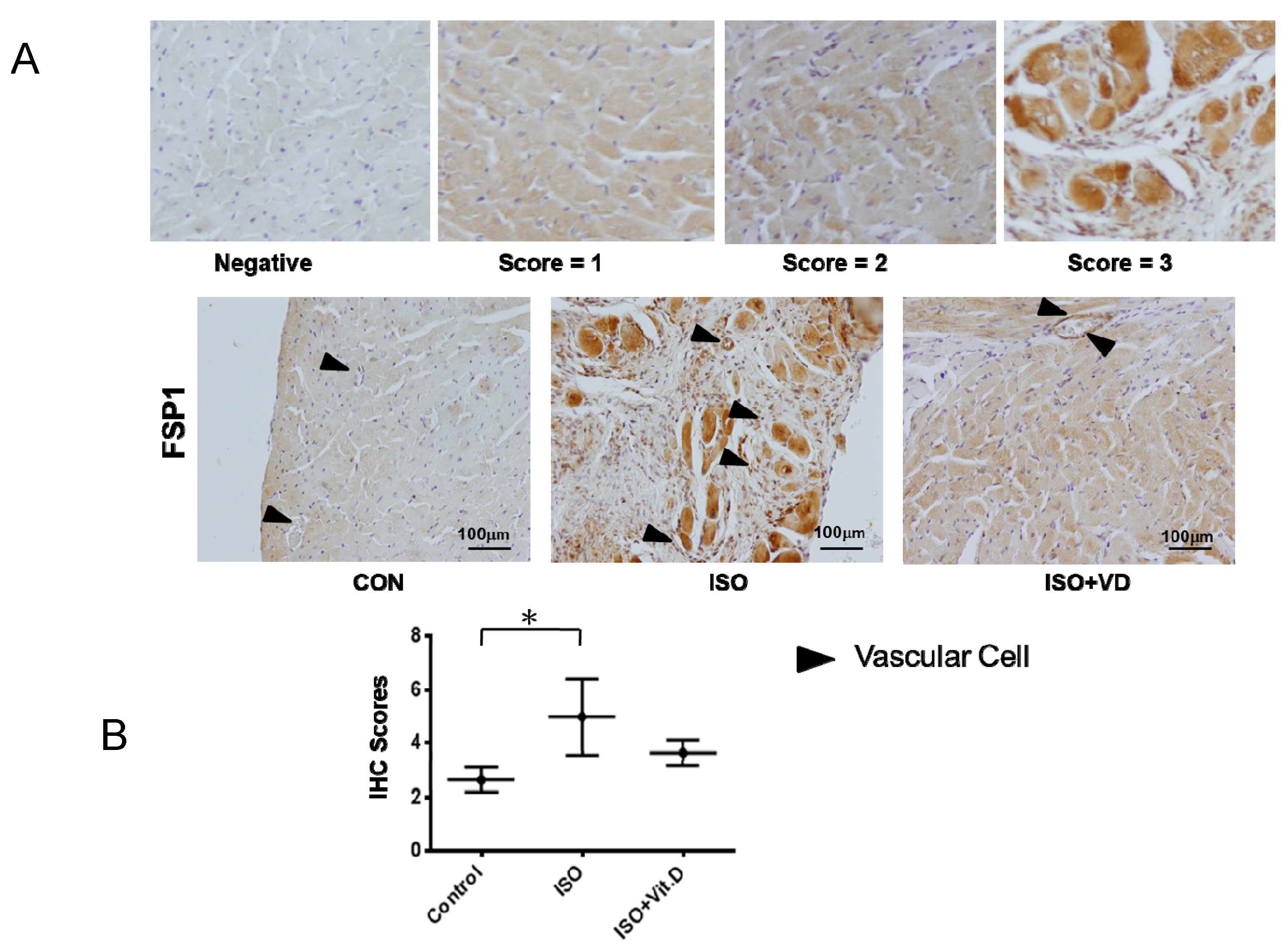
2.3. Vitamin D Slightly Attenuated Fibrosis Biomarker in an ISO-Induced Fibrosis Model
3. Discussions
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. In Vitro Stretching Device
4.4. Immunofluorescence Microscopy
4.5. Western Blot
4.6. Immunohistochemistry Assay
4.7. ELISA
4.8. Animal
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ng, L.L.; Sandhu, J.K.; Squire, I.B.; Davies, J.E.; Jones, D.J. Vitamin D and prognosis in acute myocardial infarction. Int. J. Cardiol. 2013, 168, 2341–2346. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; McCarthy, C.M.; Bhan, I.; Camargo, C.A., Jr. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit. Care. Med. 2014, 42, 1365–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamez, H.; Zoccali, C.; Packham, D.; Wenger, J.; Bhan, I.; Appelbaum, E.; Pritchett, Y.; Chang, Y.; Agarwal, R.; Wanner, C.; et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am. Heart J. 2012, 164, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Meems, L.M.; Cannon, M.V.; Mahmud, H.; Voors, A.A.; van Gilst, W.H.; Sillje, H.H.; Ruifrok, W.P.; de Boer, R.A. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J. Steroid. Biochem. Mol. Biol. 2012, 132, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Yalamarti, B.; Ke, Q.; Choudhury, S.; Yu, H.; Karumanchi, S.A.; Kroeger, P.; Thadhani, R.; Kang, P.M. Preventing progression of cardiac hypertrophy and development of heart failure by paricalcitol therapy in rats. Cardiovasc. Res. 2011, 91, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Wolf, M.; Lowrie, E.; Ofsthun, N.; Lazarus, J.M.; Thadhani, R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N. Engl. J. Med. 2003, 349, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Appelbaum, E.; Chang, Y.; Pritchett, Y.; Bhan, I.; Agarwal, R.; Zoccali, C.; Wanner, C.; Lloyd-Jones, D.; Cannata, J.; et al. Vitamin D receptor activation and left ventricular hypertrophy in advanced kidney disease. Am. J. Nephrol. 2011, 33, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Krone, W.; Berthold, H.K. Vitamin D and cardiovascular disease. Current. Vascular. Pharmacol. 2009, 7, 414–422. [Google Scholar] [CrossRef]
- Danik, J.S.; Manson, J.E. Vitamin d and cardiovascular disease. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Lan, T.H.; Huang, X.Q.; Tan, H.M. Vascular fibrosis in atherosclerosis. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2013, 22, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Najjar, S.S.; Cornish, T.C.; Halushka, M.K. A comprehensive histopathological evaluation of vascular medial fibrosis: Insights into the pathophysiology of arterial stiffening. Atherosclerosis 2010, 208, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Liu, T.; Yao, L.; Xing, Y.; Zhao, X.; Fu, J.; Xue, X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci. Rep. 2017, 7, 3312. [Google Scholar] [CrossRef] [PubMed]
- Repo, J.M.; Rantala, I.S.; Honkanen, T.T.; Mustonen, J.T.; Koobi, P.; Tahvanainen, A.M.; Niemela, O.J.; Tikkanen, I.; Rysa, J.M.; Ruskoaho, H.J.; et al. Paricalcitol aggravates perivascular fibrosis in rats with renal insufficiency and low calcitriol. Kidney Int. 2007, 72, 977–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Li, Y.; Liu, Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J. Am. Soc. Nephrol. JASN 2006, 17, 3382–3393. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Bae, E.H.; Kim, I.J.; Ma, S.K.; Choi, C.; Lee, J.; Kim, S.W. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am. J. Physiol. -Ren. Physiol. 2009, 298, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Pincikova, T.; Paquin-Proulx, D.; Sandberg, J.K.; Flodström-Tullberg, M.; Hjelte, L. Vitamin D treatment modulates immune activation in cystic fibrosis. Clin. Exp. Immunol. 2017, 189, 359–371. [Google Scholar] [CrossRef] [Green Version]
- González-Mateo, G.T.; Fernández-Míllara, V.; Bellón, T.; Liappas, G.; Ruiz-Ortega, M.; López-Cabrera, M.; Selgas, R.; Aroeira, L.S. Paricalcitol Reduces Peritoneal Fibrosis in Mice through the Activation of Regulatory T Cells and Reduction in IL-17 Production. PLoS ONE 2014, 9, e108477. [Google Scholar] [CrossRef]
- Abramovitch, S.; Sharvit, E.; Weisman, Y.; Bentov, A.; Brazowski, E.; Cohen, G.; Volovelsky, O.; Reif, S. Vitamin D inhibits development of liver fibrosis in an animal model but cannot ameliorate established cirrhosis. Am. J. Physiol. -Gastrointest. Liver Physiol. 2015, 308, 112–120. [Google Scholar] [CrossRef]
- Lai, C.C.; Liu, C.P.; Cheng, P.W.; Lu, P.J.; Hsiao, M.; Lu, W.H.; Sun, G.C.; Liou, J.C.; Tseng, C.J. Paricalcitol Attenuates Cardiac Fibrosis and Expression of Endothelial Cell Transition Markers in Isoproterenol-Induced Cardiomyopathic Rats. Crit. Care Med. 2016, 44, 866–874. [Google Scholar] [CrossRef]
- Norman, P.E.; Powell, J.T. Vitamin D and Cardiovascular Disease. Circ. Res. 2014, 114, 379. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Watts, S.W.; Ng, M.; Chen, S.C.; Glenn, D.J.; Gardner, D.G. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension 2014, 64, 1290–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panizo, S.; Barrio-Vazquez, S.; Naves-Diaz, M.; Carrillo-Lopez, N.; Rodriguez, I.; Fernandez-Vazquez, A.; Valdivielso, J.M.; Thadhani, R.; Cannata-Andia, J.B. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol. Dial. Transplant.: Off. Publ. Eur. Dial. Transpl. Assoc. - Eur. Ren. Assoc. 2013, 28, 2735–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, A.; Boroomand, S.; Carthy, J.; Luo, Z.; McManus, B. 1,25 Dihydroxyvitamin D3 Inhibits TGFbeta1-Mediated Primary Human Cardiac Myofibroblast Activation. PLoS ONE 2015, 10, e0128655. [Google Scholar] [CrossRef]
- Kasabova, M.; Joulin-Giet, A.; Lecaille, F.; Gilmore, B.F.; Marchand-Adam, S.; Saidi, A.; Lalmanach, G. Regulation of TGF-beta1-driven differentiation of human lung fibroblasts: Emerging roles of cathepsin B and cystatin C. J. Biol. Chem. 2014, 289, 16239–16251. [Google Scholar] [CrossRef] [Green Version]
- Piera-Velazquez, S.; Li, Z.; Jimenez, S.A. Role of Endothelial-Mesenchymal Transition (EndoMT) in the Pathogenesis of Fibrotic Disorders. Am. J. Pathol. 2011, 179, 1074–1080. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Wipff, P.J.; Rifkin, D.B.; Meister, J.J.; Hinz, B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007, 179, 1311–1323. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hagood, J.S.; Lu, B.; Merryman, W.D.; Murphy-Ullrich, J.E. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J. Biol. Chem. 2010, 285, 22382–22393. [Google Scholar] [CrossRef] [Green Version]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-dependent cell mechanosensitivity. Ann. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingber, D.E. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008, 97, 163–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-Y.; Hong, J.; Gannon, J.; Kakkar, R.; Lee, R.T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. USA 2015, 112, 7249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranade, S.S.; Qiu, Z.; Woo, S.-H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Widyantoro, B.; Emoto, N.; Nakayama, K.; Anggrahini, D.W.; Adiarto, S.; Iwasa, N.; Yagi, K.; Miyagawa, K.; Rikitake, Y.; Suzuki, T.; et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 2010, 121, 2407–2418. [Google Scholar] [CrossRef] [Green Version]
| Approaches | +S | + TGFβ-1 | +ISO |
|---|---|---|---|
| Dynamics | Cyclic stretching | Static | Rat, in vivo |
| Duration for induction | 6 Hours | 5 Days | 4 Weeks |
| Fibrosis markers | FN, α-SMA | FN, α-SMA | FSP-1 |
| Vitamin D effect | VE-Cad decrease attenuated | CD31 decrease attenuated | FSP-1 decreased |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-C.; Juang, W.-C.; Sun, G.-C.; Tseng, Y.-K.; Jhong, R.-C.; Tseng, C.-J.; Wong, T.-Y.; Cheng, P.-W. Vitamin D Attenuates Loss of Endothelial Biomarker Expression in Cardio-Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 2196. https://doi.org/10.3390/ijms21062196
Lai C-C, Juang W-C, Sun G-C, Tseng Y-K, Jhong R-C, Tseng C-J, Wong T-Y, Cheng P-W. Vitamin D Attenuates Loss of Endothelial Biomarker Expression in Cardio-Endothelial Cells. International Journal of Molecular Sciences. 2020; 21(6):2196. https://doi.org/10.3390/ijms21062196
Chicago/Turabian StyleLai, Chi-Cheng, Wang-Chuan Juang, Gwo-Ching Sun, Yu-Kai Tseng, Rong-Chang Jhong, Ching-Jiunn Tseng, Tzyy-Yue Wong, and Pei-Wen Cheng. 2020. "Vitamin D Attenuates Loss of Endothelial Biomarker Expression in Cardio-Endothelial Cells" International Journal of Molecular Sciences 21, no. 6: 2196. https://doi.org/10.3390/ijms21062196





