Hypergravity Activates a Pro-Angiogenic Homeostatic Response by Human Capillary Endothelial Cells
Abstract
:1. Introduction
2. Results
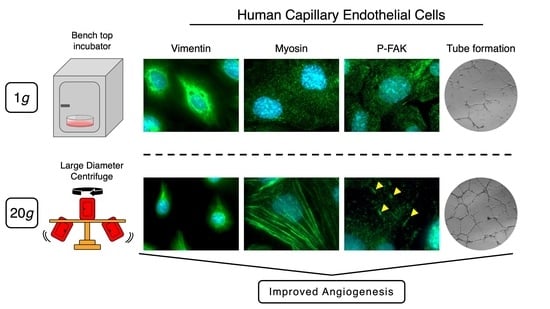
2.1. Effects of Short Hypergravity Treatments
2.2. Effect of Longer Hypergravity Treatments
3. Discussion
4. Materials and Methods
4.1. Cell line and Culture
4.2. Reagents
4.3. Hypergravity Protocols
4.4. Wound Healing (Scratch) Assay
4.5. Tube Formation Assay
4.6. Antibodies, Cell Staining and Fluorescence Analysis
4.7. Cell Fixation and RNA Extraction
4.8. RT2 Profiler PCR Arrays
4.9. Statistical Analysis
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Anova | Analysis of Variance |
| B2M | Beta-2-Microglobulin |
| BAEC | Bovine Aortic Endothelial Cells |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EC | Endothelial Cells |
| ESA | European Space Agency |
| ESTEC | European Space Research and Technology Centre |
| FAK | Focal Adhesion Kinase |
| HMEC-1 | human microvascular endothelial cells 1 |
| HPRT1 | Hypoxanthine Phosphoribosyltransferase 1 |
| HUVEC | human umbilical vein endothelial cells |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| IF | intermediate filaments |
| ISS | International Space Station |
| LDC | Large Diameter Centrifuge |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| PCR | Polymerase Chain Reaction |
| PROCR | Protein C Receptor |
| RPL0 | Ribosomal Protein Lateral Stalk Subunit P0 |
| RPM | Random Positioning Machines |
| RT | room temperature |
| SANS | space flight-associated neuro-ocular syndrome |
| SEM | standard error of the mean |
| SYT | Spin Your Thesis |
| YAP | Yes-Associated Protein 1 |
References
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Andreazzoli, M.; Angeloni, D.; Broccoli, V.; Demontis, G.C. Microgravity, Stem Cells, and Embryonic Development: Challenges and Opportunities for 3D Tissue Generation. Front. Astron. Sp. Sci. 2017, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Vernikos, J.; Schneider, V.S. Space, gravity and the physiology of aging: Parallel or convergent disciplines? A mini-review. Gerontology 2010, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Pavy-Le Traon, A.; Heer, M.; Narici, M.V.; Rittweger, J.; Vernikos, J. From space to Earth: Advances in human physiology from 20 years of bed rest studies (1986-2006). Eur. J. Appl. Physiol. 2007, 101, 143–194. [Google Scholar] [CrossRef]
- Arya, M.; Paloski, W.H.; Young, L.R. Centrifugation protocol for the NASA Artificial Gravity-Bed Rest Pilot Study. J. Gravit. Physiol. 2007, 14, P5-8. [Google Scholar]
- Clément, G.; Pavy-Le Traon, A. Centrifugation as a countermeasure during actual and simulated microgravity: A review. Eur. J. Appl. Physiol. 2004, 92, 235–248. [Google Scholar] [CrossRef]
- Stenger, M.B.; Evans, J.M.; Knapp, C.F.; Lee, S.M.C.; Phillips, T.R.; Perez, S.A.; Moore, A.D.; Paloski, W.H.; Platts, S.H. Artificial gravity training reduces bed rest-induced cardiovascular deconditioning. Eur. J. Appl. Physiol. 2012, 112, 605–616. [Google Scholar] [CrossRef]
- Caiozzo, V.J.; Haddad, F.; Lee, S.; Baker, M.; Paloski, W.; Baldwin, K.M. Artificial gravity as a countermeasure to microgravity: A pilot study examining the effects on knee extensor and plantar flexor muscle groups. J. Appl. Physiol. 2009, 107, 39–46. [Google Scholar] [CrossRef]
- Tominari, T.; Ichimaru, R.; Taniguchi, K.; Yumoto, A.; Shirakawa, M.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Itoh, Y.; Shiba, D.; et al. Hypergravity and microgravity exhibited reversal effects on the bone and muscle mass in mice. Sci. Rep. 2019, 9, 6614. [Google Scholar] [CrossRef]
- Jang, T.Y.; Jung, A.-Y.; Kim, Y.H. Hormetic Effect of Chronic Hypergravity in a Mouse Model of Allergic Asthma and Rhinitis. Sci. Rep. 2016, 6, 27260. [Google Scholar] [CrossRef] [Green Version]
- Morita, S.; Nakamura, H.; Kumei, Y.; Shimokawa, H.; Ohya, K.; Shinomiya, K. Hypergravity Stimulates Osteoblast Phenotype Expression: A Therapeutic Hint for Disuse Bone Atrophy. Ann. N. Y. Acad. Sci. 2004, 1030, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Li, J.; Liu, J.; Wang, Z.; Huo, T.; Sun, J.; Xiong, L. Preconditioning with +Gz acceleration (head-to-foot inertial load) produces neuroprotection against transient focal cerebral ischemia in rats. Neurosci. Lett. 2008, 445, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, J.J.W.A. A large human centrifuge for exploration and exploitation research. Available online: http://194.249.0.64/ojs/index.php/AK/article/view/67 (accessed on 6 February 2020).
- Ingber, D.E. Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najrana, T.; Sanchez-Esteban, J. Mechanotransduction as an Adaptation to Gravity. Front. Pediatr. 2016, 4, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, M.T.; Duckles, H.; Feng, S.; Hsiao, S.T.; Kim, H.R.; Serbanovic-Canic, J.; Evans, P.C. Mechanoresponsive networks controlling vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2199–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Balsamo, M.; Barravecchia, I.; Mariotti, S.; Merenda, A.; De Cesari, C.; Vukich, M.; Angeloni, D. Molecular and Cellular Characterization of Space Flight Effects on Microvascular Endothelial Cell Function – PreparatoryWork for the SFEF Project. Microgravity Sci. Technol. 2014, 26, 351–363. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Cialdai, F.; Monici, M.; Morbidelli, L. The impact of microgravity and hypergravity on endothelial cells. Biomed Res. Int. 2015, 2015, 434803. [Google Scholar] [CrossRef]
- Van Loon, J.J.W.A. Centrifuges for Microgravity Simulation. The Reduced Gravity Paradigm. Front. Astron. Sp. Sci. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Szulcek, R.; Van Bezu, J.; Boonstra, J.; Van Loon, J.J.W.; Van Nieuw Amerongen, G.P. Transient intervals of hyper-gravity enhance endothelial barrier integrity: Impact of mechanical and gravitational forces measured electrically. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Invest. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barravecchia, I.; De Cesari, C.; Pyankova, O.; Scebba, F.; Pè, M.; Forcato, M.; Bicciato, S.; Foster, H.; Bridger, J.; Angeloni, D. A comprehensive molecular and morphological study of the effects of space flight on human capillary endothelial cells: Sample quality assessment and preliminary results. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Porazinski, S.; Wang, H.; Asaoka, Y.; Behrndt, M.; Miyamoto, T.; Morita, H.; Hata, S.; Sasaki, T.; Krens, S.F.G.; Osada, Y.; et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 2015, 521, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zharikov, S.I.; Sigova, A.A.; Chen, S.; Bubb, M.R.; Block, E.R. Cytoskeletal regulation of the L-arginine/no pathway in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280. [Google Scholar] [CrossRef] [Green Version]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.L.; Van Loon, J.J.W.A. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014, 28, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Morbidelli, L.; Marziliano, N.; Basile, V.; Pezzatini, S.; Romano, G.; Conti, A.; Monici, M. Effect of Hypergravity on Endothelial Cell Function and Gene Expression. Microgravity Sci. Technol. 2009, 21, 135–140. [Google Scholar] [CrossRef]
- Koyama, T.; Kimura, C.; Hayashi, M.; Watanabe, M.; Karashima, Y.; Oike, M. Hypergravity induces ATP release and actin reorganization via tyrosine phosphorylation and RhoA activation in bovine endothelial cells. Pflügers Arch. - Eur. J. Physiol. 2009, 457, 711–719. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Lin, M.I.; Murata, T.; Landskroner-Eiger, S.; Schleicher, M.; Kothiya, M.; Iwakiri, Y.; Yu, J.; Huang, P.L.; Sessa, W.C. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J. Cell Sci. 2013, 126, 5541–5552. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide Biol. Chem. 2013, 35, 175–185. [Google Scholar] [CrossRef]
- Spisni, E.; Bianco, M.C.; Griffoni, C.; Toni, M.; D’Angelo, R.; Santi, S.; Riccio, M.; Tomasi, V. Mechanosensing role of caveolae and caveolar constituents in human endothelial cells. J. Cell. Physiol. 2003, 197, 198–204. [Google Scholar] [CrossRef]
- Grosse, J.; Wehland, M.; Pietsch, J.; Ma, X.; Ulbrich, C.; Schulz, H.; Saar, K.; Hübner, N.; Hauslage, J.; Hemmersbach, R.; et al. Short-term weightlessness produced by parabolic flight maneuvers altered gene expression patterns in human endothelial cells. FASEB J. 2012, 26, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.; Weinreich, D.M.; Farma, J.M.; Carroll, N.M.; Turner, E.M.; Alexander, H.R. Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J. Transl. Med. 2005, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenkranz-Weiss, P.; Sessa, W.C.; Milstien, S.; Kaufman, S.; Watson, C.A.; Pober, J.S. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells. Elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J. Clin. Invest. 1994, 93, 2236–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, K.; Hirata, Y.; Imai, T.; Iwashina, M.; Marumo, F. Regulation of inducible nitric oxide synthase gene by interleukin-1 beta in rat vascular endothelial cells. Am. J. Physiol. 1994, 267, H2318–H2324. [Google Scholar] [CrossRef]
- Yue, L.; Bian, J.T.; Grizelj, I.; Cavka, A.; Phillips, S.A.; Makino, A.; Mazzone, T. Apolipoprotein e enhances endothelial-NO production by modulating caveolin 1 interaction with endothelial no synthase. Hypertension 2012, 60, 1040–1046. [Google Scholar] [CrossRef] [Green Version]
- Nagata, S.; Golstein, P. The Fas death factor. Science 1995, 267, 1449–1456. [Google Scholar] [CrossRef]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Shi, M.; Jie, F.; Bai, Y.; Shen, P.; Yu, Z.; Wang, X.; Huang, C.; Tao, M.; Wang, Z.; et al. Phase III study of dulanermin (recombinant human tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand) combined with vinorelbine and cisplatin in patients with advanced non-small-cell lung cancer. Invest. New Drugs 2018, 36, 315–322. [Google Scholar] [CrossRef]
- Herbst, R.S.; Eckhardt, S.G.; Kurzrock, R.; Ebbinghaus, S.; O’Dwyer, P.J.; Gordon, M.S.; Novotny, W.; Goldwasser, M.A.; Tohnya, T.M.; Lum, B.L.; et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J. Clin. Oncol. 2010, 28, 2839–2846. [Google Scholar] [CrossRef]
- Kuhn, M. Endothelial actions of atrial and B-type natriuretic peptides. Br. J. Pharmacol. 2012, 166, 522–531. [Google Scholar] [CrossRef]
- Schnittler, H.J.; Wilke, A.; Gress, T.; Suttorp, N.; Drenckhahn, D. Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J. Physiol. 1990, 431, 379–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, G.M. Actin, Myosin, and Cell Movement; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Guan, J.L.; Shalloway, D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature 1992, 358, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Romer, L.H.; Mclean, N.; Turner, C.E.; Burridge, K. Tyrosine Kinase Activity, Cytoskeletal Organization, and Motility in Human Vascular Endothelial Cells. Mol. Biol. Cell. 1994, 5, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Ueda, H.; Zhou, H.; Stokol, T.; Shen, T.-L.; Alcaraz, A.; Nagy, T.; Vassalli, J.-D.; Guan, J.-L. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc. Res. 2004, 64, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Bauer, J.; Kossmehl-Zorn, S.; Cogoli, A.; Curcio, F.; Oksche, A.; Wehland, M.; Kreutz, R.; et al. Simulated weightlessness changes the cytoskeleton and extracellular matrix proteins in papillary thyroid carcinoma cells. Cell Tissue Res. 2006, 324, 267–277. [Google Scholar] [CrossRef]
- Aleshcheva, G.; Sahana, J.; Ma, X.; Hauslage, J.; Hemmersbach, R.; Egli, M.; Infanger, M.; Bauer, J.; Grimm, D. Changes in morphology, gene expression and protein content in chondrocytes cultured on a random positioning machine. PLoS ONE 2013, 8, e79057. [Google Scholar] [CrossRef] [Green Version]
- Masiello, M.G.; Cucina, A.; Proietti, S.; Palombo, A.; Coluccia, P.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Morini, V.; Bizzarri, M. Phenotypic switch induced by simulated microgravity on MDA-MB-231 breast cancer cells. Biomed Res. Int. 2014, 2014, 652434. [Google Scholar] [CrossRef]
- Keeling, M.C.; Flores, L.R.; Dodhy, A.H.; Murray, E.R.; Gavara, N. Actomyosin and vimentin cytoskeletal networks regulate nuclear shape, mechanics and chromatin organization. Sci. Rep. 2017, 7, 5219. [Google Scholar] [CrossRef] [Green Version]
- Cogoli-Greuter, M.; Lovis, P.; Vadrucci, S. Signal transduction in T cells: An overview. J. Gravit. Physiol. 2004, 11, P53-6. [Google Scholar]
- Sarelius, I.H.; Glading, A.J. Control of vascular permeability by adhesion molecules. Tissue Barriers 2015, 3, e985954. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Foote, H.P.; Lechler, T. beta-Catenin protects the epidermis from mechanical stresses. J. Cell Biol. 2013, 202, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heisenberg, C.-P.; Bellaïche, Y. Forces in Tissue Morphogenesis and Patterning. Cell 2013, 153, 948–962. [Google Scholar] [CrossRef] [Green Version]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef] [PubMed]
- Benham-Pyle, B.W.; Pruitt, B.L.; Nelson, W.J. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 2015, 348, 1024–1027. [Google Scholar] [CrossRef] [Green Version]
- Sansores-Garcia, L.; Bossuyt, W.; Wada, K.-I.; Yonemura, S.; Tao, C.; Sasaki, H.; Halder, G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011, 30, 2325–2335. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, E.M.; Girvan, P.; Eckert, J.; Lopez-Duarte, I.; Kubánková, M.; van Loon, J.J.W.A.; Brooks, N.J.; Kuimova, M.K. Measuring Intracellular Viscosity in Conditions of Hypergravity. Biophys. J. 2019, 116, 1984–1993. [Google Scholar] [CrossRef]
- Mao, X.; Byrum, S.; Nishiyama, N.; Pecaut, M.; Sridharan, V.; Boerma, M.; Tackett, A.; Shiba, D.; Shirakawa, M.; Takahashi, S.; et al. Impact of Spaceflight and Artificial Gravity on the Mouse Retina: Biochemical and Proteomic Analysis. Int. J. Mol. Sci. 2018, 19, 2546. [Google Scholar] [CrossRef] [Green Version]
- Tascher, G.; Brioche, T.; Maes, P.; Chopard, A.; O’Gorman, D.; Gauquelin-Koch, G.; Blanc, S.; Bertile, F. Proteome-wide Adaptations of Mouse Skeletal Muscles during a Full Month in Space. J. Proteome Res. 2017, 16, 2623–2638. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Bauer, J.; Kossmehl, P.; Shakibaei, M.; Schönberger, J.; Pickenhahn, H.; Schulze-Tanzil, G.; Vetter, R.; Eilles, C.; Paul, M.; et al. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002, 16, 604–606. [Google Scholar] [CrossRef]
- Dang, B.; Yang, Y.; Zhang, E.; Li, W.; Mi, X.; Meng, Y.; Yan, S.; Wang, Z.; Wei, W.; Shao, C.; et al. Simulated microgravity increases heavy ion radiation-induced apoptosis in human B lymphoblasts. Life Sci. 2014, 97, 123–128. [Google Scholar] [CrossRef]
- He, W.; Wang, Q.; Xu, J.; Xu, X.; Padilla, M.T.; Ren, G.; Gou, X.; Lin, Y. Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2- and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy 2012, 8, 1811–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehland, M.; Ma, X.; Braun, M.; Hauslage, J.; Hemmersbach, R.; Bauer, J.; Grosse, J.; Infanger, M.; Grimm, D. The impact of altered gravity and vibration on endothelial cells during a parabolic flight. Cell. Physiol. Biochem. 2013, 31, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Monici, M.; Marziliano, N.; Basile, V.; Pezzatini, S.; Romano, G.; Conti, A.; Morbidelli, L. Hypergravity affects morphology and function in microvascular endothelial cells. Microgravity Sci. Technol. 2006, 18, 234–238. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Orci, L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell 1985, 42, 469–477. [Google Scholar] [CrossRef]
- Schnaper, H.W.; Grant, D.S.; Stetler-Stevenson, W.G.; Fridman, R.; D’Orazi, G.; Murphy, A.N.; Bird, R.E.; Hoythya, M.; Fuerst, T.R.; French, D.L.; et al. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J. Cell. Physiol. 1993, 156, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Aĭsina, R.B.; Mukhametova, L.I. Structure and functions of plasminogen/plasmin system. Bioorg. Khim. 2014, 40, 642–657. [Google Scholar] [CrossRef]
- Kulkarni, S.; Dopheide, S.M.; Yap, C.L.; Ravanat, C.; Freund, M.; Mangin, P.; Heel, K.A.; Street, A.; Harper, I.S.; Lanza, F.; et al. A revised model of platelet aggregation. J. Clin. Invest. 2000, 105, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Dayananda, K.M.; Singh, I.; Mondal, N.; Neelamegham, S. von Willebrand factor self-association on platelet GpIbalpha under hydrodynamic shear: Effect on shear-induced platelet activation. Blood 2010, 116, 3990–3998. [Google Scholar] [CrossRef] [Green Version]
- Crawley, J.T.B.; de Groot, R.; Xiang, Y.; Luken, B.M.; Lane, D.A. Unraveling the scissile bond: How ADAMTS13 recognizes and cleaves von Willebrand factor. Blood 2011, 118, 3212–3221. [Google Scholar] [CrossRef] [Green Version]
- Spiel, A.O.; Gilbert, J.C.; Jilma, B. Von Willebrand Factor in Cardiovascular Disease. Circulation 2008, 117, 1449–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Meyer, S.F.; Stoll, G.; Wagner, D.D.; Kleinschnitz, C. von Willebrand Factor. Stroke 2012, 43, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Gombos, T.; Makó, V.; Cervenak, L.; Papassotiriou, J.; Kunde, J.; Hársfalvi, J.; Förhécz, Z.; Pozsonyi, Z.; Borgulya, G.; Jánoskuti, L.; et al. Levels of von Willebrand factor antigen and von Willebrand factor cleaving protease (ADAMTS13) activity predict clinical events in chronic heart failure. Thromb. Haemost. 2009, 102, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Y.; Hwa, J. Regulation of VWF expression, and secretion in health and disease. Curr. Opin. Hematol. 2016, 23, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, J.J.W.A.; Krause, J.; Cunha, H.; Goncalves, J.; Almeida, H.; Schiller, P. The Large Diameter Centrifuge, Ldc, for Life and Physical Sciences and Technology; Life in Space for Life on Earth: Angers, France, 2008. [Google Scholar]
- Van Loon, J.J.W.A.; Folgering, E.H.T.E.; Bouten, C.V.C.; Veldhuijzen, J.P.; Smit, T.H. Inertial shear forces and the use of centrifuges in gravity research. What is the proper control? J. Biomech. Eng. 2003, 125, 342–346. [Google Scholar] [CrossRef]




| 4g Vs. Ctr | 20g Vs. Ctr | 20g Vs. 4g | |
|---|---|---|---|
| Up | FASLG, NPPB, PLG. | TNFSF10, IL1β, ICAM1, TGFB1, MMP2, CFLAR, APOE, COL18A1, FN1, NOS3, PLAU, VWF. | ENG, MMP1, PLAT, PROCR, SERPINE1, TEK, TFPI, TGFB1, VWF, ACTB, GAPDH, NOS3. |
| Down | VWF | THBD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cesari, C.; Barravecchia, I.; Pyankova, O.V.; Vezza, M.; Germani, M.M.; Scebba, F.; van Loon, J.J.W.A.; Angeloni, D. Hypergravity Activates a Pro-Angiogenic Homeostatic Response by Human Capillary Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 2354. https://doi.org/10.3390/ijms21072354
De Cesari C, Barravecchia I, Pyankova OV, Vezza M, Germani MM, Scebba F, van Loon JJWA, Angeloni D. Hypergravity Activates a Pro-Angiogenic Homeostatic Response by Human Capillary Endothelial Cells. International Journal of Molecular Sciences. 2020; 21(7):2354. https://doi.org/10.3390/ijms21072354
Chicago/Turabian StyleDe Cesari, Chiara, Ivana Barravecchia, Olga V. Pyankova, Matteo Vezza, Marco M. Germani, Francesca Scebba, Jack J. W. A. van Loon, and Debora Angeloni. 2020. "Hypergravity Activates a Pro-Angiogenic Homeostatic Response by Human Capillary Endothelial Cells" International Journal of Molecular Sciences 21, no. 7: 2354. https://doi.org/10.3390/ijms21072354