Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis
Abstract
:1. Introduction
2. Results
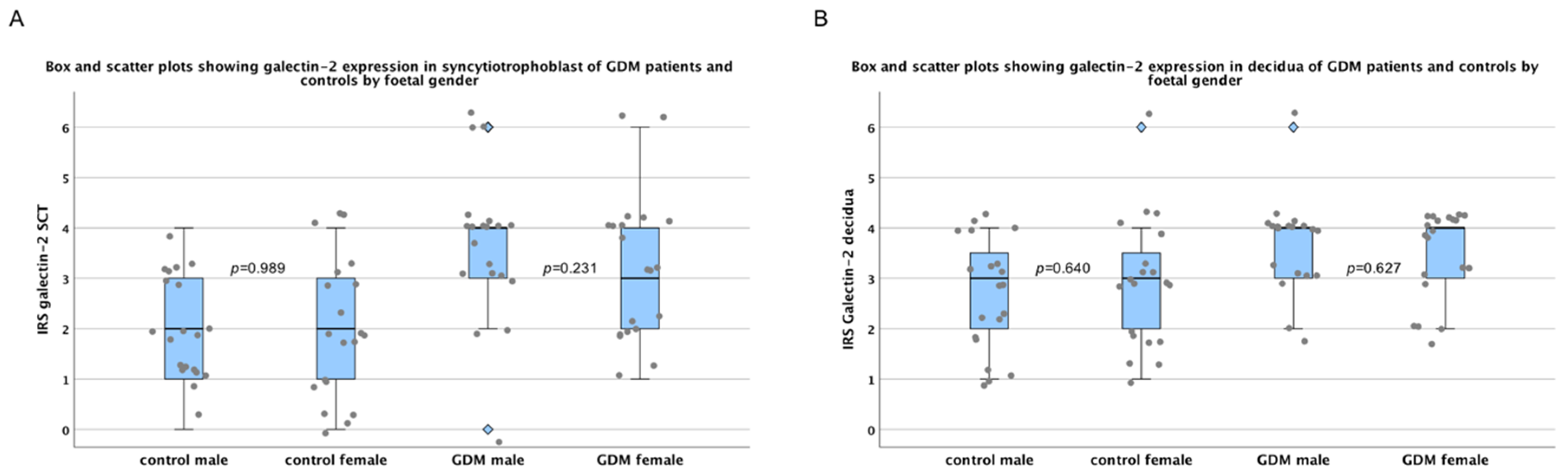
2.1. There Are No Sex-Specific Differences in Galectin-2 Expression
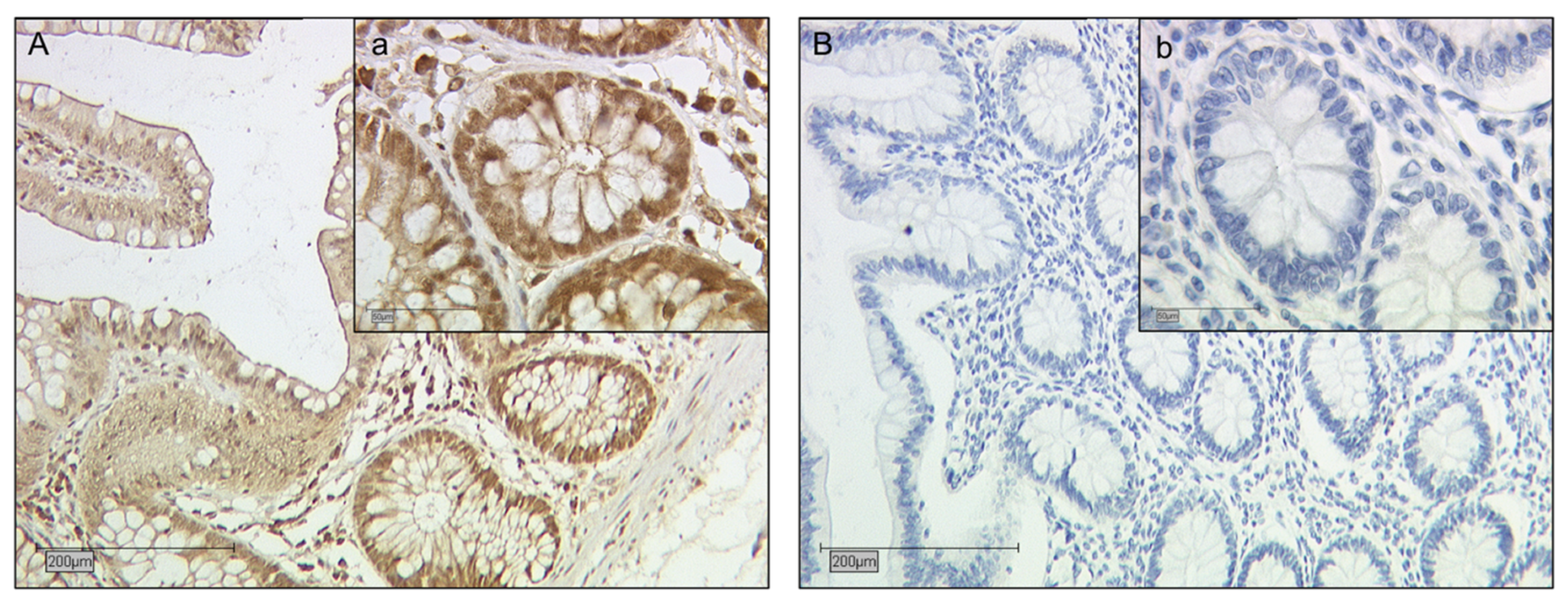
2.2. Galectin-2 Expression Is Upregulated in the Fetal Syncytiotrophoblast of GDM Placentas
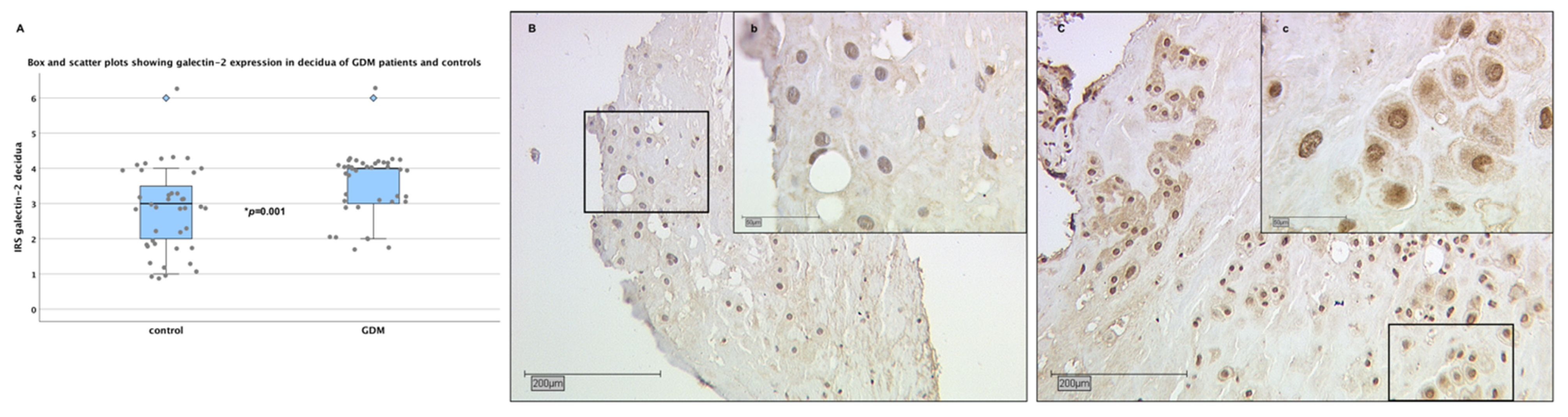
2.3. Galectin-2 Expression Is Upregulated in the Maternal Decidua of GDM Placentas
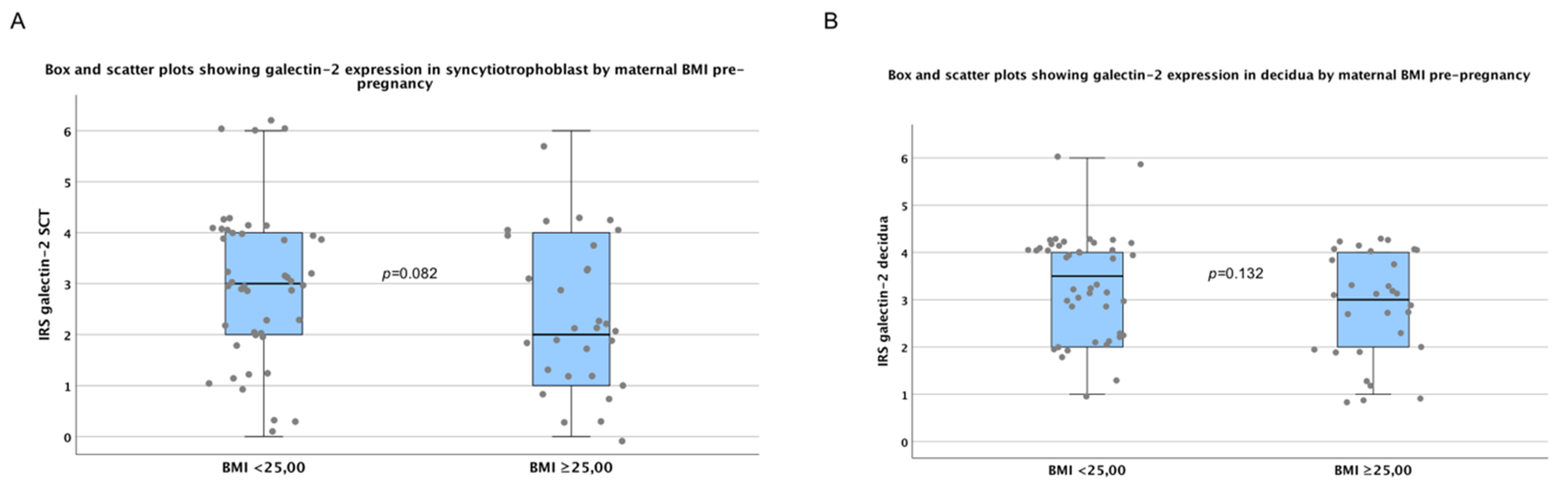
2.4. There are No Significant Differences in Galectin-2 Expression between Normal and Overweight Pregnancies
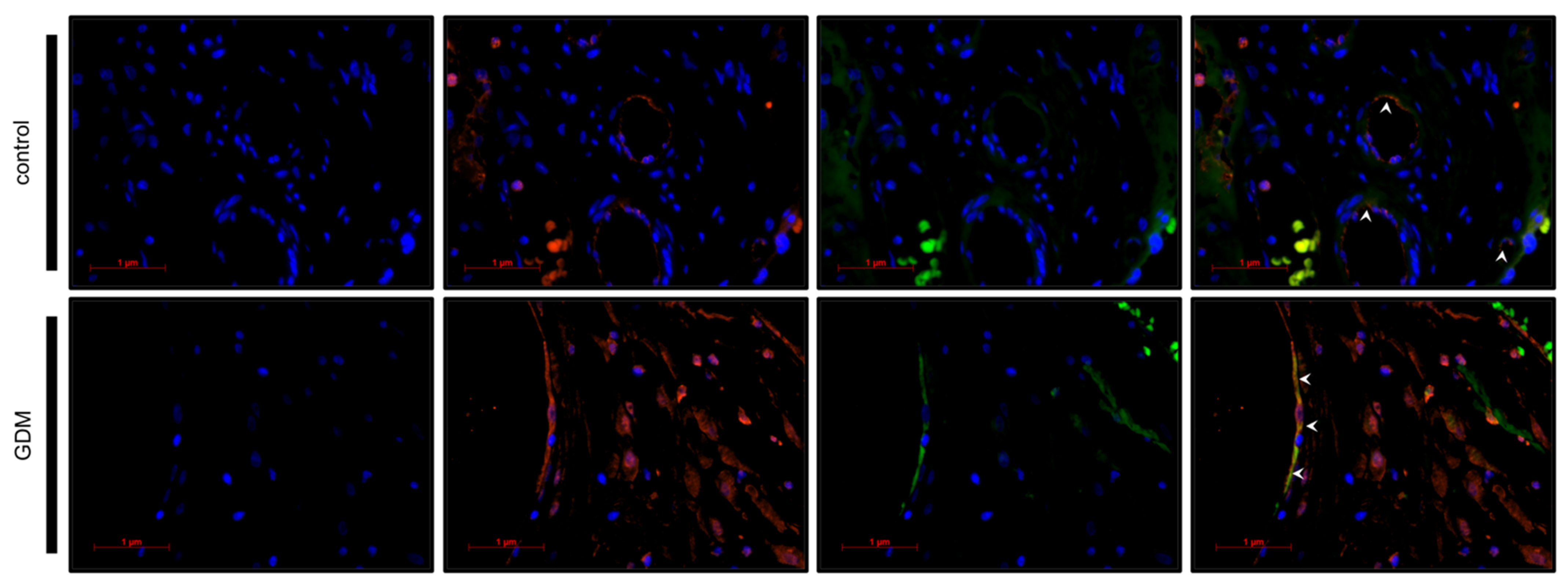
2.5. Identification of Galectin-2 Expressing Cells by Immunofluorescence Double Staining
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Immunohistochemistry
4.2.1. Staining
4.2.2. Evaluation
4.3. Double Immunofluorescence
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CD | Cluster of differentiation |
| CHD | Coronary heart disease |
| CK7 | Cytokeratin 7 |
| CRD | Carbohydrate recognition domain |
| DM2 | Diabetes Mellitus Type 2 |
| EVT | Extra-villous trophoblast cells |
| g | Gramm |
| GDM | Gestational Diabetes Mellitus |
| h | Hours |
| IL | Interleukin |
| INF | Interferon |
| IRS | Immunoreactivity score |
| IUGR | Intra uterine growth restriction |
| MMP | Matrix metalloproteinase |
| oGTT | Oral glucose tolerance test |
| PE | Preeclampsia |
| PP-13 | Placental protein 13 |
| SCT | Syncytiotrophoblast |
| Th | T-helper-cell |
| TIMP-1 | Tissue inhibitor of metalloprotease 1 |
| TNF | Tumor necrosis factor |
| VEGF-A | Vascular endothelial growth factor A |
References
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar]
- Cooper, D.N.; Barondes, S.H. God must love galectins; he made so many of them. Glycobiology 1999, 9, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent beta-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Bolgiano, B.; Liao, D.I.; Strecker, G.; Cantau, P.; Herzberg, O.; Feizi, T.; Cambillau, C. Crosslinking of mammalian lectin (galectin-1) by complex biantennary saccharides. Nat. Struct. Mol. Biol. 1994, 1, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Fred Brewer, C. Binding and cross-linking properties of galectins. Biochim. Biophys. Acta (BBA) Gen. Subj. 2002, 1572, 255–262. [Google Scholar] [CrossRef]
- Belardi, B.; O’Donoghue, G.P.; Smith, A.W.; Groves, J.T.; Bertozzi, C.R. Investigating cell surface galectin-mediated cross-linking on glycoengineered cells. J. Am. Chem. Soc. 2012, 134, 9549–9552. [Google Scholar] [CrossRef] [PubMed]
- Leffler, H.; Carlsson, S.; Hedlund, M.; Qian, Y.; Poirier, F. Introduction to galectins. Glycoconj. J. 2002, 19, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Hutter, S.; Heublein, S.; Vrekoussis, T.; Andergassen, U.; Unverdorben, L.; Papadakis, G.; Makrigiannakis, A. Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta 2013, 34, 863–872. [Google Scholar] [CrossRef]
- Block, M.; Molne, J.; Leffler, H.; Borjesson, L.; Breimer, M.E. Immunohistochemical studies on galectin expression in colectomised patients with ulcerative colitis. Biomed. Res. Int. 2016, 2016, 5989128. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, N.; Ilarregui, J.M.; Toscano, M.A.; Rabinovich, G.A. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 2004, 64, 1–12. [Google Scholar] [CrossRef]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T cells mediated by galectin-1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.G.; Andrews, N.; Zhao, Q.; McKean, D.; Williams, J.F.; Connor, L.J.; Gerasimenko, O.V.; Hilkens, J.; Hirabayashi, J.; Kasai, K.; et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 2007, 282, 773–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.Y.; Havel, P.J.; Liu, F.T. Galectin-12: A protein associated with lipid droplets that regulates lipid metabolism and energy balance. Adipocyte 2012, 1, 96–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, S.M.; Conrad, M.L.; Freitag, N.; Barrientos, G. Galectins in angiogenesis: Consequences for gestation. J. Reprod. Immunol. 2015, 108, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Barrow, H.; Guo, X.; Wandall, H.H.; Pedersen, J.W.; Fu, B.; Zhao, Q.; Chen, C.; Rhodes, J.M.; Yu, L.G. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 2011, 17, 7035–7046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santucci, L.; Fiorucci, S.; Rubinstein, N.; Mencarelli, A.; Palazzetti, B.; Federici, B.; Rabinovich, G.A.; Morelli, A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology 2003, 124, 1381–1394. [Google Scholar] [CrossRef]
- Harjacek, M.; Diaz-Cano, S.; De Miguel, M.; Wolfe, H.; Maldonado, C.A.; Rabinovich, G.A. Expression of galectins-1 and -3 correlates with defective mononuclear cell apoptosis in patients with juvenile idiopathic arthritis. J. Rheumatol. 2001, 28, 1914–1922. [Google Scholar]
- Van der Hoeven, N.W.; Hollander, M.R.; Yıldırım, C.; Jansen, M.F.; Teunissen, P.F.; Horrevoets, A.J.; van der Pouw Kraan, T.C.T.M.; van Royen, N. The emerging role of galectins in cardiovascular disease. Vasc. Pharmacol. 2016, 81, 31–41. [Google Scholar] [CrossRef]
- Blois, S.M.; Ilarregui, J.M.; Tometten, M.; Garcia, M.; Orsal, A.S.; Cordo-Russo, R.; Toscano, M.A.; Bianco, G.A.; Kobelt, P.; Handjiski, B.; et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 2007, 13, 1450–1457. [Google Scholar] [CrossRef]
- Tirado-Gonzalez, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lei, C.; Zhang, W. Expression of galectin-3 in mouse endometrium and its effect during embryo implantation. Reprod. Biomed. Online 2012, 24, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, U.; Mayr, D.; Schiessl, B.; Mylonas, I.; Schulze, S.; Kuhn, C.; Friese, K.; Walzel, H. Expression of galectin-1, -3 (gal-1, gal-3) and the Thomsen-Friedenreich (TF) antigen in normal, IUGR, preeclamptic and HELLP placentas. Placenta 2007, 28, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Hutter, S.; Knabl, J.; Andergassen, U.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Placental expression patterns of Galectin-1, Galectin-2, Galectin-3 and Galectin-13 in cases of intrauterine growth restriction (IUGR). Int. J. Mol. Sci. 2016, 17, 523. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, K.; Goscik, J.; Raba, G.; Laudanski, P. Syndecan 4, galectin 2, and death receptor 3 (DR3) as novel proteins in pathophysiology of preeclampsia. J. Matern. Fetal Neonatal Med. 2019, 1–6. [Google Scholar] [CrossRef]
- Khalil, A.; Cowans, N.J.; Spencer, K.; Goichman, S.; Meiri, H.; Harrington, K. First-trimester markers for the prediction of pre-eclampsia in women with a-priori high risk. Ultrasound. Obstet. Gynecol. 2010, 35, 671–679. [Google Scholar] [CrossRef]
- International Diabetes Federation; Han Cho, N.; Whiting, D.; Forouhi, N.; Guariguata, L.; Hambleton, I.; Li, R.; Majeed, A.; Mbanya, J.C.; Aschner Montoya, P.; et al. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussel, Belgium, 2015. [Google Scholar]
- Albrecht, S.S.; Kuklina, E.V.; Bansil, P.; Jamieson, D.J.; Whiteman, M.K.; Kourtis, A.P.; Posner, S.F.; Callaghan, W.M. Diabetes trends among delivery hospitalizations in the U.S. 1994–2004. Diabetes Care 2010, 33, 768–773. [Google Scholar] [CrossRef] [Green Version]
- Schäfer-Graf, U.; Laubner, K.; Hummel, S.; Gembruch, U.; Groten, T.; Kainer, F.; Grieshop, M.; Bancher-Todesca, D.; Cerva-Zivakovic, M.; Hoesli, I.; et al. S3 Leitlinie Gestationsdiabetes Mellitus (GDM), Diagnostik, Therapie u. Nachsorge; Deutschen Diabetes-Gesellschaft (DDG) & Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG): Berlin, Germany, 2018. [Google Scholar]
- Fadl, H.E.; Ostlund, I.K.; Magnuson, A.F.; Hanson, U.S. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet. Med. 2010, 27, 436–441. [Google Scholar] [CrossRef]
- Hapo Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Tam, W.H.; Ma, R.C.; Yang, X.; Ko, G.T.; Tong, P.C.; Cockram, C.S.; Sahota, D.S.; Rogers, M.S.; Chan, J.C. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics 2008, 122, 1229–1234. [Google Scholar] [CrossRef]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaarasmaki, M.; Pouta, A.; Elliot, P.; Tapanainen, P.; Sovio, U.; Ruokonen, A.; Hartikainen, A.L.; McCarthy, M.; Jarvelin, M.R. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am. J. Epidemiol. 2009, 169, 1209–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunderson, E.P.; Chiang, V.; Pletcher, M.J.; Jacobs, D.R.; Quesenberry, C.P.; Sidney, S.; Lewis, C.E. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: The coronary artery risk development in young adults study. J. Am. Heart Assoc. 2014, 3, e000490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, T.; Grab, D.; Grubert, T.; Hantschmann, P.; Kainer, F.; Kästner, R.; Kentenich, C.; Klockenbusch, W.; Lammert, F.; Louwen, F.; et al. 17—Maternale Erkrankungen in der Schwangerschaft. In Facharztwissen Geburtsmedizin (Dritte Ausgabe); Kainer, F., Ed.; Urban & Fischer: Munich, Germany, 2016; pp. 347–618. [Google Scholar]
- Kautzky-Willer, A.; Prager, R.; Waldhausl, W.; Pacini, G.; Thomaseth, K.; Wagner, O.F.; Ulm, M.; Streli, C.; Ludvik, B. Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care 1997, 20, 1717–1723. [Google Scholar] [CrossRef]
- Abell, S.K.; De Courten, B.; Boyle, J.A.; Teede, H.J. Inflammatory and other biomarkers: Role in pathophysiology and prediction of gestational diabetes mellitus. Int. J. Mol. Sci. 2015, 16, 13442–13473. [Google Scholar] [CrossRef]
- Kuzmicki, M.; Telejko, B.; Szamatowicz, J.; Zonenberg, A.; Nikolajuk, A.; Kretowski, A.; Gorska, M. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol. Endocrinol. 2009, 25, 258–263. [Google Scholar] [CrossRef]
- Lorenzo-Almoros, A.; Hang, T.; Peiro, C.; Soriano-Guillen, L.; Egido, J.; Tunon, J.; Lorenzo, O. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc. Diabetol. 2019, 18, 140. [Google Scholar] [CrossRef]
- Patterson, C.C.; Smith, A.E.; Yarnell, J.W.; Rumley, A.; Ben-Shlomo, Y.; Lowe, G.D. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: The Caerphilly Study. Atherosclerosis 2010, 209, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Vilmi-Kerala, T.; Lauhio, A.; Tervahartiala, T.; Palomaki, O.; Uotila, J.; Sorsa, T.; Palomaki, A. Subclinical inflammation associated with prolonged TIMP-1 upregulation and arterial stiffness after gestational diabetes mellitus: A hospital-based cohort study. Cardiovasc. Diabetol. 2017, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Han, X.; Meng, Q.; Luo, Q. Early second trimester maternal serum markers in the prediction of gestational diabetes mellitus. J. Diabetes Investig. 2018, 9, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Blois, S.M.; Gueuvoghlanian-Silva, B.Y.; Tirado-Gonzalez, I.; Torloni, M.R.; Freitag, N.; Mattar, R.; Conrad, M.L.; Unverdorben, L.; Barrientos, G.; Knabl, J.; et al. Getting too sweet: Galectin-1 dysregulation in gestational diabetes mellitus. Mol. Hum. Reprod. 2014, 20, 644–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unverdorben, L.; Huttenbrenner, R.; Knabl, J.; Jeschke, U.; Hutter, S. Galectin-13/PP-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta 2015, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Boutsikou, T.; Giotaki, M.; Boutsikou, M.; Briana, D.D.; Baka, S.; Piatopoulou, D.; Hassiakos, D.; Gourgiotis, D.; Malamitsi-Puchner, A. Cord blood galectin-1 and -3 concentrations in term pregnancies with normal restricted and increased fetal growth. J. Perinat. Med. 2015, 43, 305–309. [Google Scholar] [CrossRef]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Paclik, D.; Berndt, U.; Guzy, C.; Dankof, A.; Danese, S.; Holzloehner, P.; Rosewicz, S.; Wiedenmann, B.; Wittig, B.M.; Dignass, A.U.; et al. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J. Mol. Med. (Berl.) 2008, 86, 1395–1406. [Google Scholar] [CrossRef]
- Yildirim, C.; Vogel, D.Y.; Hollander, M.R.; Baggen, J.M.; Fontijn, R.D.; Nieuwenhuis, S.; Haverkamp, A.; de Vries, M.R.; Quax, P.H.; Garcia-Vallejo, J.J.; et al. Galectin-2 induces a proinflammatory, anti-arteriogenic phenotype in monocytes and macrophages. PLoS ONE 2015, 10, e0124347. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, L.; Haufe, T.; Santoso, L.; Hofmann, S.; Jeschke, U.; Hutter, S. Prototype and chimera-type galectins in placentas with spontaneous and recurrent miscarriages. Int. J. Mol. Sci. 2016, 17, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutter, S.; Martin, N.; von Schonfeldt, V.; Messner, J.; Kuhn, C.; Hofmann, S.; Andergassen, U.; Knabl, J.; Jeschke, U. Galectin 2 (gal-2) expression is downregulated on protein and mRNA level in placentas of preeclamptic (PE) patients. Placenta 2015, 36, 438–445. [Google Scholar] [CrossRef]
- Christensen, M.B.; Lawlor, D.A.; Gaunt, T.R.; Howell, M.W.; Davey Smith, G.; Ebrahim, S.; Day, I.N.M. Genotype of galectin 2 (LGALS2) is associated with insulin-glucose profile in the British Women’s Heart and Health Study. Diabetologia 2006, 49, 673–677. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, K.; Inoue, K.; Sato, H.; Iida, A.; Ohnishi, Y.; Sekine, A.; Sato, H.; Odashiro, K.; Nobuyoshi, M.; Hori, M.; et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature 2004, 429, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Hu, S.; Wang, F.; Yang, X.; Li, Y.; Huang, C. PPARG, AGTR1, CXCL16 and LGALS2 polymorphisms are correlated with the risk for coronary heart disease. Int. J. Clin. Exp. Pathol. 2015, 8, 3138–3143. [Google Scholar] [PubMed]
- Yamada, Y.; Kato, K.; Oguri, M.; Yoshida, T.; Yokoi, K.; Watanabe, S.; Metoki, N.; Yoshida, H.; Satoh, K.; Ichihara, S.; et al. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int. J. Mol. Med. 2008, 21, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedlacek, K.; Neureuther, K.; Mueller, J.C.; Stark, K.; Fischer, M.; Baessler, A.; Reinhard, W.; Broeckel, U.; Lieb, W.; Erdmann, J.; et al. Lymphotoxin-alpha and galectin-2 SNPs are not associated with myocardial infarction in two different German populations. J. Mol. Med. (Berl.) 2007, 85, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Mangino, M.; Braund, P.; Singh, R.; Steeds, R.; Thompson, J.R.; Channer, K.; Samani, N.J. LGALS2 functional variant rs7291467 is not associated with susceptibility to myocardial infarction in Caucasians. Atherosclerosis 2007, 194, 112–115. [Google Scholar] [CrossRef]
- Maldonado-Estrada, J.; Menu, E.; Roques, P.; Barre-Sinoussi, F.; Chaouat, G. Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J. Immunol. Methods 2004, 286, 21–34. [Google Scholar] [CrossRef]
- Favaloro, E.J. Differential expression of surface antigens on activated endothelium. Immunol. Cell Biol. 1993, 71, 571–581. [Google Scholar] [CrossRef]
- Sturm, A.; Lensch, M.; Andre, S.; Kaltner, H.; Wiedenmann, B.; Rosewicz, S.; Dignass, A.U.; Gabius, H.J. Human galectin-2: Novel inducer of T cell apoptosis with distinct profile of caspase activation. J. Immunol. 2004, 173, 3825–3837. [Google Scholar] [CrossRef] [Green Version]
- Loser, K.; Sturm, A.; Voskort, M.; Kupas, V.; Balkow, S.; Auriemma, M.; Sternemann, C.; Dignass, A.U.; Luger, T.A.; Beissert, S. Galectin-2 suppresses contact allergy by inducing apoptosis in activated CD8+ T cells. J. Immunol. 2009, 182, 5419–5429. [Google Scholar] [CrossRef] [Green Version]
- Stowell, S.R.; Karmakar, S.; Stowell, C.J.; Dias-Baruffi, M.; McEver, R.P.; Cummings, R.D. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood 2007, 109, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Tasaki, M.; Ando, Y.; Ohtsubo, K. Galectin-lattice sustains function of cationic amino acid transporter and insulin secretion of pancreatic β cells. J. Biochem. 2020. [Google Scholar] [CrossRef]
- Paclik, D.; Werner, L.; Guckelberger, O.; Wiedenmann, B.; Sturm, A. Galectins distinctively regulate central monocyte and macrophage function. Cell. Immunol. 2011, 271, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Svensson-Arvelund, J.; Ernerudh, J.; Buse, E.; Cline, J.M.; Haeger, J.-D.; Dixon, D.; Markert, U.R.; Pfarrer, C.; Vos, P.D.; Faas, M.M. The placenta in toxicology. Part II: Systemic and local immune adaptations in pregnancy. Toxicol. Pathol. 2013, 42, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ategbo, J.M.; Grissa, O.; Yessoufou, A.; Hichami, A.; Dramane, K.L.; Moutairou, K.; Miled, A.; Grissa, A.; Jerbi, M.; Tabka, Z.; et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J. Clin. Endocrinol. Metab. 2006, 91, 4137–4143. [Google Scholar] [CrossRef]
- Van der Laan, A.M.; Schirmer, S.H.; de Vries, M.R.; Koning, J.J.; Volger, O.L.; Fledderus, J.O.; Bastiaansen, A.J.; Hollander, M.R.; Baggen, J.M.; Koch, K.T.; et al. Galectin-2 expression is dependent on the rs7291467 polymorphism and acts as an inhibitor of arteriogenesis. Eur. Heart J. 2012, 33, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, M.R.; Jansen, M.F.; Hopman, L.; Dolk, E.; van de Ven, P.M.; Knaapen, P.; Horrevoets, A.J.; Lutgens, E.; van Royen, N. Stimulation of collateral vessel growth by inhibition of galectin 2 in mice using a single-domain llama-derived antibody. J. Am. Heart Assoc. 2019, 8, e012806. [Google Scholar] [CrossRef] [PubMed]
- Kleinwechter, H.; Schäfer-Graf, U.; Bührer, C.; Hoesli, I.; Kainer, F.; Kautzky-Willer, A.; Pawlowski, B.; Schunck, F.; Somville, T.; Sorger, M. Gestationsdiabetes mellitus (GDM) Evidenzbasierte Leitlinie zu Diagnostik, Therapie und Nachsorge; Deutschen Diabetes-Gesellschaft (DDG) & Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG): Berlin, Germany, 2011. [Google Scholar]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic. Report of A WHO Consultation; WHO: Geneva, Switzerland, 2000; ISBN 0512-3054. [Google Scholar]
- Hutter, S.; Knabl, J.; Andergassen, U.; Mayr, D.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Fetal gender specific expression of tandem-repeat galectins in placental tissue from normally progressed human pregnancies and intrauterine growth restriction (IUGR). Placenta 2015, 36, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]


| Number of Galectin-2 Positive Cells Evaluated | Galectin-2 and CK7 Double Positive Cells | Percentage of Galectin-2 and CK7 Positive Cells | |
|---|---|---|---|
| Control | 41 | 39 | 95,1 % |
| GDM | 79 | 77 | 97,5 % |
| GDM | Control | p-Value | |||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Birthweight (g) | 3662.1 ± 562 | 3635.9 ± 661 | 3339.8 ± 568 | 3294 ± 440 | p = 0.019 * |
| Duration of gestation at delivery (weeks) | 39.67 ± 1.30 | 39.83 ± 1.40 | 39.80 ± 1.54 | 39.75 ± 1.16 | p = 0.943 |
| Maternal BMI pre-pregnancy (kg/m2) | 29.38 ± 8.03 | 26.96 ± 4.73 | 21.92 ± 3.97 | 25.04 ± 7.90 | p < 0.001 * |
| pH in umbilical artery | 7.30 ± 0.07 | 7.30 ± 0.10 | 7.28 ± 0.10 | 7.30 ± 0.08 | p = 0.826 |
| Maternal Age (years) | 31.46 ± 4.12 | 33.21 ± 5.33 | 30.30 ± 6.11 | 32.00 ± 6.13 | p = 0.177 |
| Number of Patients in GDM Group | Number of Patients in Control Group | |
|---|---|---|
| Underweight (BMI < 18.5 kg/m2) | 0 | 4 |
| Normal BMI (18.5–24.9 kg/m2) | 16 | 25 |
| Overweight (25.0–29.9 kg/m2) | 10 | 3 |
| Obese (≥30.0 kg/m2) | 12 | 5 |
| Antibody | Dilution | Incubation | Manufacturer |
|---|---|---|---|
| Galectin-2—polyclonal Rabbit IgG | 1:200 | 16 h at 4 °C | Novus Biologicals—NBP1-89690 |
| CK7—Clone OVTL Mouse IgG | 1:30 | 16 h at 4 °C | Novocastra—NCL-L-CK7-OVTL |
| CD31—Clone JC/70A Mouse IgG | 1:50 | 16 h at 4 °C | Abcam—ab9498 |
| Cy-2-labelled goat-anti-rabbit | 1:100 | 30 min at RT | Dianova—115-226-062 |
| Cy-3-labelled goat-anti-mouse | 1:500 | 30 min at RT | Dianova—111-165-144 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hepp, P.; Unverdorben, L.; Hutter, S.; Kuhn, C.; Ditsch, N.; Groß, E.; Mahner, S.; Jeschke, U.; Knabl, J.; Heidegger, H.H. Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis. Int. J. Mol. Sci. 2020, 21, 2404. https://doi.org/10.3390/ijms21072404
Hepp P, Unverdorben L, Hutter S, Kuhn C, Ditsch N, Groß E, Mahner S, Jeschke U, Knabl J, Heidegger HH. Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis. International Journal of Molecular Sciences. 2020; 21(7):2404. https://doi.org/10.3390/ijms21072404
Chicago/Turabian StyleHepp, Paula, Laura Unverdorben, Stefan Hutter, Christina Kuhn, Nina Ditsch, Eva Groß, Sven Mahner, Udo Jeschke, Julia Knabl, and Helene H. Heidegger. 2020. "Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis" International Journal of Molecular Sciences 21, no. 7: 2404. https://doi.org/10.3390/ijms21072404





