Sucrose Phosphorylase and Related Enzymes in Glycoside Hydrolase Family 13: Discovery, Application and Engineering
Abstract
:1. Introduction
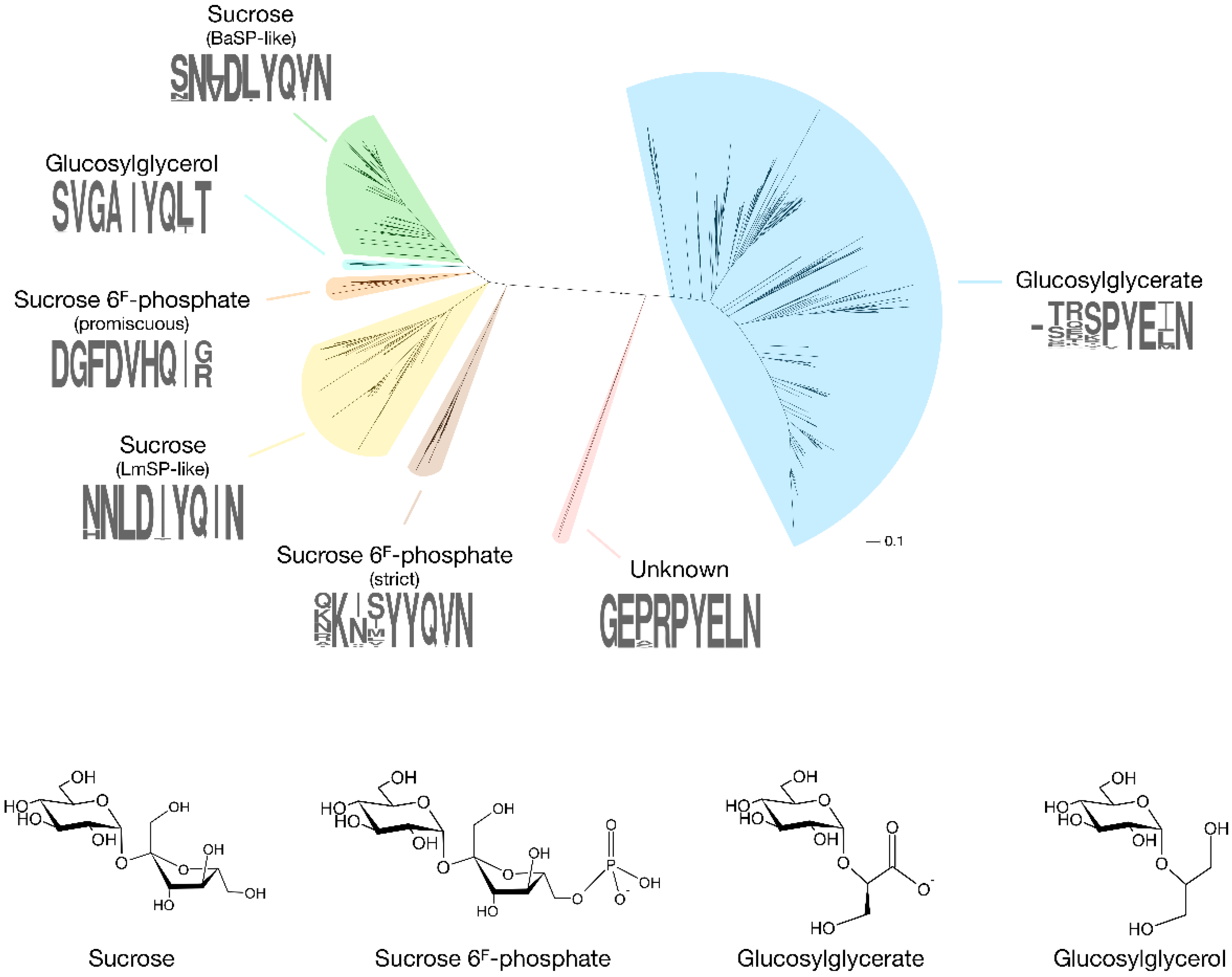
2. Discovery of Novel Glycoside Phosphorylases in GH13_18
2.1. Sucrose 6F-Phosphate Phosphorylase
2.2. 2-O-Glucosylglycerate Phosphorylase
2.3. 2-O-Glucosylglycerol Phosphorylase
2.4. Mysterious Myxobacterial Phosphorylases
3. Application of Sucrose Phosphorylase and Related Enzymes
3.1. Synthesis of Phosphorylated Sugars
3.2. Transglycosylation for the Synthesis of Glycosides and Rare Sugars
4. Engineering of Sucrose Phosphorylase and Related Enzymes
4.1. Thermostability
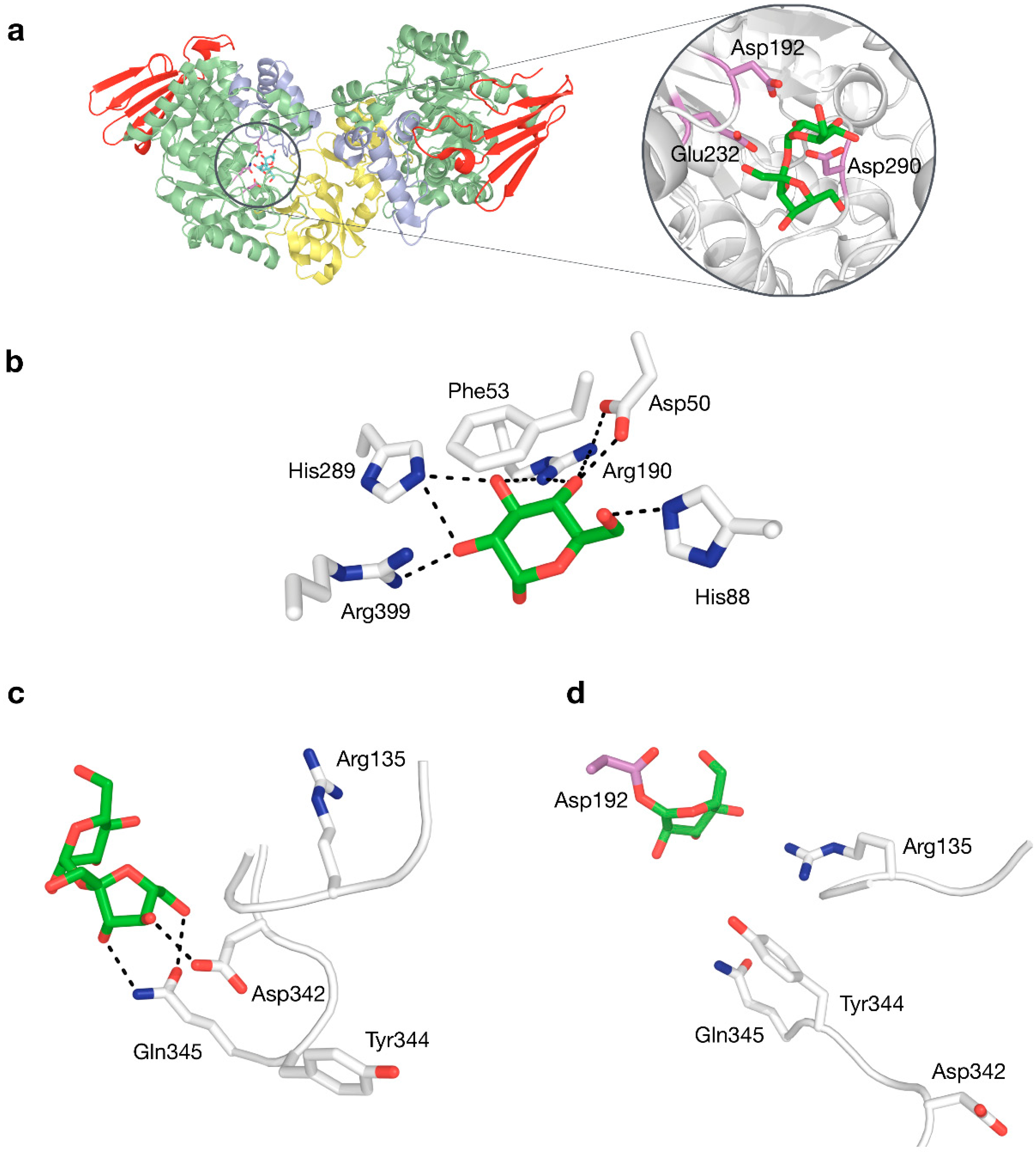
4.2. Structure–Function Relationships in the Active Site
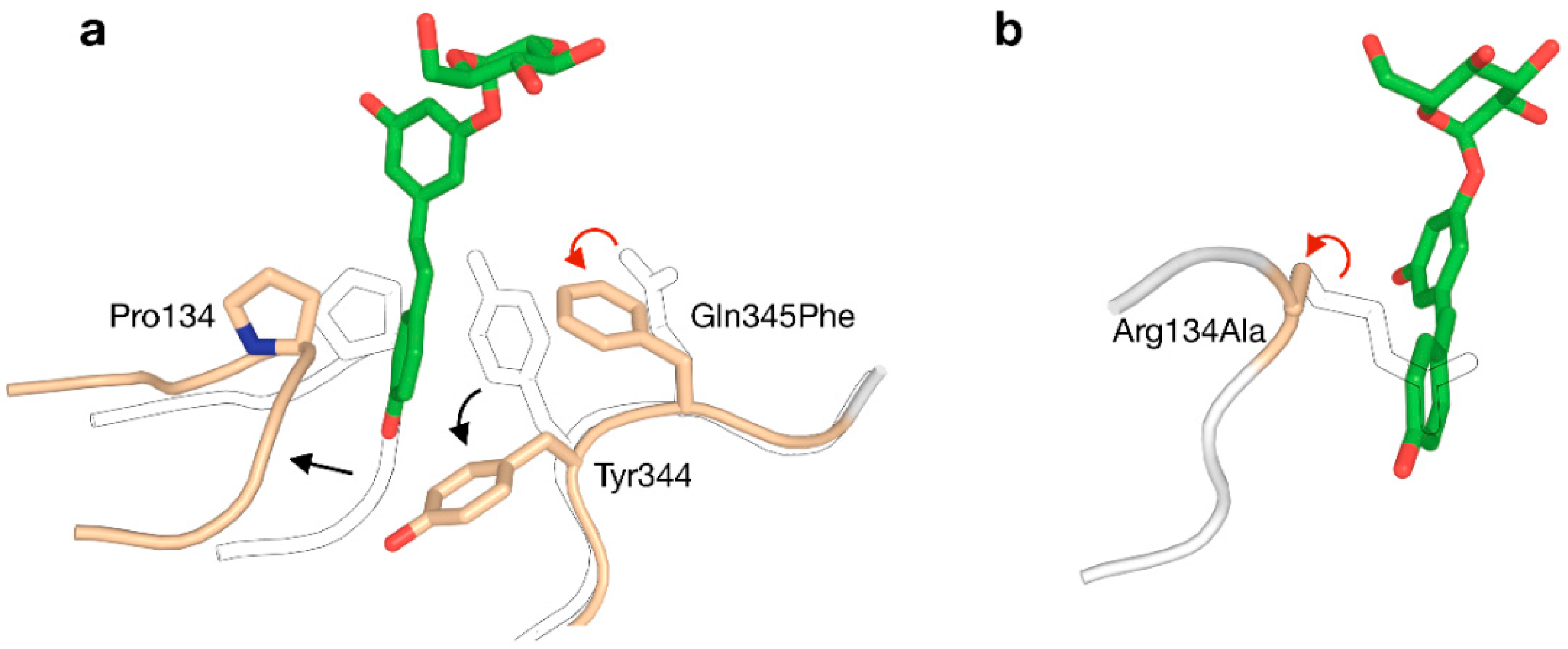
4.3. Engineering of Specificity or Selectivity
5. Concluding Remarks
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| BaSP | B. adolescentis SP |
| CAZy | Carbohydrate-active enzyme database |
| GGaP | 2-O-Glucosylglycerate phosphorylase |
| GGoP | 2-O-Glucosylglycerol phosphorylase |
| GH13_18 | Glycoside hydrolase family 13, subfamily 18 |
| Glc1P | α-d-Glucose 1-phosphate |
| iCLEA | Imprinted cross-linked enzyme aggregate |
| IcSPP | I. coccineus SPP |
| LmSP | L. mesenteroides SP |
| SP | Sucrose phosphorylase |
| SPP | Sucrose 6F-phosphate phosphorylase |
| TtSPP | T. thermosaccharolyticum SPP |
References
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Sheiham, A.; Liu, P.; Demarco, F.F.; Silva, A.E.R.; Assunção, M.C.; Menezes, A.M.; Barros, F.C.; Peres, K.G. Sugar consumption and changes in dental caries from childhood to adolescence. J. Dent. Res. 2016, 95, 388–394. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (W.H.O.). Guideline: Sugars Intake for Adults and Children. Available online: http://www.who.int/nutrition/publications/guidelines (accessed on 17 February 2020).
- Briggs, A. Sugar tax could sweeten a market failure. Nature 2016, 531, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmet, T.; Soetaert, W. Enzymatic glycosyl transfer: Mechanisms and applications. Biocatal. Biotransformation 2011, 29, 1–18. [Google Scholar] [CrossRef]
- Arnold, W.N. The selection of sucrose as the translocate of higher plants. J. Theor. Biol. 1968, 21, 13–20. [Google Scholar] [CrossRef]
- Goedl, C.; Sawangwan, T.; Wildberger, P.; Nidetzky, B. Sucrose phosphorylase: A powerful transglucosylation catalyst for synthesis of α-D-glucosides as industrial fine chemicals. Biocatal. Biotransformation 2010, 28, 10–21. [Google Scholar] [CrossRef]
- Verhaeghe, T.; Aerts, D.; Diricks, M.; Soetaert, W.; Desmet, T. The quest for a thermostable sucrose phosphorylase reveals sucrose 6’-phosphate phosphorylase as a novel specificity. Appl. Microbiol. Biotechnol. 2014, 98, 7027–7037. [Google Scholar] [CrossRef]
- Mirza, O.; Skov, L.K.; Sprogøe, D.; van den Broek, L.A.; Beldman, G.; Kastrup, J.S.; Gajhede, M. Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion. J. Biol. Chem. 2006, 281, 35576–35584. [Google Scholar] [CrossRef] [Green Version]
- Tauzin, A.S.; Bruel, L.; Laville, E.; Nicoletti, C.; Navarro, D.; Henrissat, B.; Perrier, J.; Potocki-Veronese, G.; Giardina, T.; Lafond, M. Sucrose 6F-phosphate phosphorylase: A novel insight in the human gut microbiome. Microb. Genomics 2019, 5, 1–14. [Google Scholar] [CrossRef]
- Franceus, J.; Capra, N.; Desmet, T.; Thunnissen, A.-M.W.H. Structural Comparison of a Promiscuous and a Highly Specific Sucrose 6F-Phosphate Phosphorylase. Int. J. Mol. Sci. 2019, 20, 3906. [Google Scholar] [CrossRef] [Green Version]
- Sprogoe, D.; van den Broek, L.A.M.; Mirza, O.; Kastrup, J.S.; Voragen, A.G.J.; Gajhede, M.; Skov, L.K. Crystal structure of sucrose phosphorylase from Bifidobacterium adolescentis. Biochemistry 2004, 43, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceus, J.; Pinel, D.; Desmet, T. Glucosylglycerate Phosphorylase, an Enzyme with Novel Specificity Involved in Compatible Solute Metabolism. Appl. Environ. Microbiol. 2017, 83, e01434-17. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Narindoshvili, T.; Raushel, F.M. Discovery of a Kojibiose Phosphorylase in Escherichia coli K-12. Biochemistry 2018, 57, 2857–2867. [Google Scholar] [CrossRef]
- Nunes-Costa, D.; Maranha, A.; Costa, M.; Alarico, S.; Empadinhas, N. Glucosylglycerate metabolism, bioversatility and mycobacterial survival. Glycobiology 2017, 27, 213–227. [Google Scholar] [CrossRef]
- Franceus, J.; Decuyper, L.; D’hooghe, M.; Desmet, T. Exploring the sequence diversity in glycoside hydrolase family 13_18 reveals a novel glucosylglycerol phosphorylase. Appl. Microbiol. Biotechnol. 2018, 102, 3183–3191. [Google Scholar] [CrossRef]
- Nihira, T.; Saito, Y.; Ohtsubo, K.; Nakai, H.; Kitaoka, M. 2-O-α-D-glucosylglycerol phosphorylase from Bacillus selenitireducens MLS10 possessing hydrolytic activity on β-D-glucose 1-phosphate. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Franceus, J.; Desmet, T. A GH13 glycoside phosphorylase with unknown substrate specificity from Corallococcus coralloides. Amylase 2019, 32–40. [Google Scholar] [CrossRef]
- Herrmann, J.; Fayad, A.A.; Müller, R. Natural products from myxobacteria: Novel metabolites and bioactivities. Nat. Prod. Rep. 2017, 34, 135–160. [Google Scholar] [CrossRef]
- Plante, O.J.; Andrade, R.B.; Seeberger, P.H. Synthesis and use of glycosyl phosphates as glycosyl donors. Org. Lett. 1999, 1, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M. Diversity of phosphorylases in glycoside hydrolase families. Appl. Microbiol. Biotechnol. 2015, 99, 8377–8390. [Google Scholar] [CrossRef] [PubMed]
- Puchart, V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015, 33, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Fujinaka, H.; Nakamura, J.; Kobayashi, H.; Takizawa, M.; Murase, D.; Tokimitsu, I.; Suda, T. Glucose 1-phosphate increases active transport of calcium in intestine. Arch. Biochem. Biophys. 2007, 460, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ronchera-Oms, C.L.; Jiménez, N.V.; Peidro, J. Stability of parenteral nutrition admixtures containing organic phosphates. Clin. Nutr. 1995, 14, 373–380. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W. H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- De Winter, K.; Cerdobbel, A.; Soetaert, W.; Desmet, T. Operational stability of immobilized sucrose phosphorylase: Continuous production of α-glucose-1-phosphate at elevated temperatures. Process Biochem. 2011, 46, 1074–1078. [Google Scholar] [CrossRef]
- Valikhani, D.; Bolivar, J.M.; Pfeiffer, M.; Nidetzky, B. Multivalency Effects on the Immobilization of Sucrose Phosphorylase in Flow Microchannels and Their Use in the Development of a High-Performance Biocatalytic Microreactor. ChemCatChem 2017, 9, 161–166. [Google Scholar] [CrossRef]
- Wildberger, P.; Pfeiffer, M.; Brecker, L.; Nidetzky, B. Diastereoselective Synthesis of Glycosyl Phosphates by Using a Phosphorylase-Phosphatase Combination Catalyst. Angew. Chemie Int. Ed. 2015, 54, 15867–15871. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Nishimoto, M.; Kitaoka, M. Facile enzymatic synthesis of sugar 1-phosphates as substrates for phosphorylases using anomeric kinases. Carbohydr. Res. 2015, 401, 1–4. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, T.; Tian, C.; Zhu, Y.; Zeng, Y.; Men, Y.; Chen, P.; Sun, Y.; Ma, Y. Multi-enzyme systems and recombinant cells for synthesis of valuable saccharides: Advances and perspectives. Biotechnol. Adv. 2019, 37, 107406. [Google Scholar] [CrossRef]
- Qi, P.; You, C.; Zhang, Y.H.P. One-pot enzymatic conversion of sucrose to synthetic amylose by using enzyme cascades. ACS Catal. 2014, 4, 1311–1317. [Google Scholar] [CrossRef]
- Zhong, C.; Nidetzky, B. Three-Enzyme Phosphorylase Cascade for Integrated Production of Short-Chain Cellodextrins. Biotechnol. J. 2019, 1900349, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Nishimoto, M.; Kitaoka, M. Practical preparation of D-galactosyl-β1→4-L-rhamnose employing the combined action of Phosphorylases. Biosci. Biotechnol. Biochem. 2010, 74, 1652–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nihira, T.; Suzuki, E.; Kitaoka, M.; Nishimoto, M.; Ohtsubo, K.; Nakai, H. Discovery of β-1,4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase involved in the metabolism of N-glycans. J. Biol. Chem. 2013, 288, 27366–27374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nihira, T.; Miyajima, F.; Chiku, K.; Nishimoto, M.; Kitaoka, M.; Ohtsubo, K.; Nakai, H. One Pot Enzymatic Production of Nigerose from Common Sugar Resources Employing Nigerose Phosphorylase. J. Appl. Glycosci. 2014, 61, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Su, H.H.; Guo, Z.W.; Wu, X.L.; Xu, P.; Li, N.; Zong, M.H.; Lou, W.Y. Efficient Bioconversion of Sucrose to High-Value-Added Glucaric Acid by In Vitro Metabolic Engineering. ChemSusChem 2019, 12, 2278–2285. [Google Scholar] [CrossRef]
- Nishimoto, M.; Kitaoka, M. Practical preparation of lacto-N-biose I, a candidate for the bifidus factor in human milk. Biosci. Biotechnol. Biochem. 2007, 71, 2101–2104. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto, M. Large scale production of lacto-N-biose I, a building block of type I human milk oligosaccharides, using sugar phosphorylases. Biosci. Biotechnol. Biochem. 2020, 84, 17–24. [Google Scholar] [CrossRef]
- Weyler, C.; Heinzle, E. Multistep Synthesis of UDP-Glucose Using Tailored, Permeabilized Cells of E. coli. Appl. Biochem. Biotechnol. 2015, 175, 3729–3736. [Google Scholar] [CrossRef]
- Desmet, T.; Soetaert, W.; Bojarová, P.; Křen, V.; Dijkhuizen, L.; Eastwick-Field, V.; Schiller, A. Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chem. - A Eur. J. 2012, 18, 10786–10801. [Google Scholar] [CrossRef]
- Goedl, C.; Sawangwan, T.; Mueller, M.; Schwarz, A.; Nidetzky, B. A high-yielding biocatalytic process for the production of 2-O-(α-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew. Chem. Int. Ed. Engl. 2008, 47, 10086–10089. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Luley-Goedl, C.; Leitner, E.; Sawangwan, T.; Nidetzky, B. Production of glucosyl glycerol by immobilized sucrose phosphorylase: Options for enzyme fixation on a solid support and application in microscale flow format. J. Biotechnol. 2017, 257, 131–138. [Google Scholar] [CrossRef] [PubMed]
- De Winter, K.; Soetaert, W.; Desmet, T. An imprinted cross-linked enzyme aggregate (iCLEA) of sucrose phosphorylase: Combining improved stability with altered specificity. Int. J. Mol. Sci. 2012, 13, 11333–11342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruschitz, A.; Nidetzky, B. Removal of glycerol from enzymatically produced 2-α-D-glucosyl-glycerol by discontinuous diafiltration. Sep. Purif. Technol. 2020, 116749. [Google Scholar] [CrossRef]
- Luley-Goedl, C.; Sawangwan, T.; Brecker, L.; Wildberger, P.; Nidetzky, B. Regioselective O-glucosylation by sucrose phosphorylase: A promising route for functional diversification of a range of 1,2-propanediols. Carbohydr. Res. 2010, 345, 1736–1740. [Google Scholar] [CrossRef]
- Renirie, R.; Pukin, A.; Van Lagen, B.; Franssen, M.C.R. Regio- and stereoselective glucosylation of diols by sucrose phosphorylase using sucrose or glucose 1-phosphate as glucosyl donor. J. Mol. Catal. B Enzym. 2010, 67, 219–224. [Google Scholar] [CrossRef]
- Wildberger, P.; Brecker, L.; Nidetzky, B. Chiral resolution through stereoselective transglycosylation by sucrose phosphorylase: Application to the synthesis of a new biomimetic compatible solute, (R)-2-O-α-d-glucopyranosyl glyceric acid amide. Chem. Commun. 2014, 50, 436–438. [Google Scholar] [CrossRef] [Green Version]
- Cerdobbel, A.; De Winter, K.; Aerts, D.; Kuipers, R.; Joosten, H.J.; Soetaert, W.; Desmet, T. Increasing the thermostability of sucrose phosphorylase by a combination of sequence- and structure-based mutagenesis. Protein Eng. Des. Sel. 2011, 24, 829–834. [Google Scholar] [CrossRef] [Green Version]
- De Winter, K.; Verlinden, K.; Kren, V.; Weignerova, L.; Soetaert, W.; Desmet, T. Ionic liquids as cosolvents for glycosylation by sucrose phosphorylase: Balancing acceptor solubility and enzyme stability. Green Chem. 2013, 15, 1949–1955. [Google Scholar] [CrossRef]
- De Winter, K.; Desmet, T. Biphasic Catalysis with Disaccharide Phosphorylases: Chemoenzymatic Synthesis of α-D-Glucosides Using Sucrose Phosphorylase. Org. Process Res. Dev. 2014, 18, 781–787. [Google Scholar] [CrossRef]
- De Winter, K.; Dewitte, G.; Dirks-Hofmeister, M.E.; De Laet, S.; Pelantová, H.; Křen, V.; Desmet, T. Enzymatic Glycosylation of Phenolic Antioxidants: Phosphorylase-Mediated Synthesis and Characterization. J. Agric. Food Chem. 2015, 63, 10131–10139. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Muto, N.; Yamamoto, I. Characterization of Bacillus stearothermophilus cyclodextrin glucanotransferase in ascorbic acid 2-O-α-glucoside formation. Biochim. Biophys. Acta - Protein Struct. Mol. Enzymol. 1991, 1078, 127–132. [Google Scholar] [CrossRef]
- Gudiminchi, R.K.; Nidetzky, B. Walking a Fine Line with Sucrose Phosphorylase: Efficient Single-Step Biocatalytic Production of l-Ascorbic Acid 2-Glucoside from Sucrose. ChemBioChem 2017, 18, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Kitao, S.; Yoshida, S.; Horiuchi, T.; Sekine, H.; Kusakabe, I. Formation of Kojibiose and Nigerose by Sucrose Phosphorylase. Biosci. Biotechnol. Biochem. 1994, 58, 790–791. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, K.; Yoshihara, A.; Furumoto, T.; Takata, G. Production and application of a rare disaccharide using sucrose phosphorylase from Leuconostoc mesenteroides. J. Biosci. Bioeng. 2015, 119, 652–656. [Google Scholar] [CrossRef]
- Aerts, D.; Verhaeghe, T.F.; Roman, B.I.; Stevens, C.V.; Desmet, T.; Soetaert, W. Transglucosylation potential of six sucrose phosphorylases toward different classes of acceptors. Carbohydr. Res. 2011, 346, 1860–1867. [Google Scholar] [CrossRef]
- Cerdobbel, A.; Desmet, T.; De Winter, K.; Maertens, J.; Soetaert, W. Increasing the thermostability of sucrose phosphorylase by multipoint covalent immobilization. J. Biotechnol. 2010, 150, 125–130. [Google Scholar] [CrossRef]
- Lehmann, M.; Pasamontes, L.; Lassen, S.F.; Wyss, M. The consensus concept for thermostability engineering of proteins. Biochim. Bio 2000, 1543, 408–415. [Google Scholar] [CrossRef]
- Aerts, D.; Verhaeghe, T.; Joosten, H.-J.; Vriend, G.; Soetaert, W.; Desmet, T. Consensus engineering of sucrose phosphorylase: The outcome reflects the sequence input. Biotechnol. Bioeng. 2013, 110, 2563–2572. [Google Scholar] [CrossRef]
- Schwarz, A.; Brecker, L.; Nidetzky, B. Acid–base catalysis in Leuconostoc mesenteroides sucrose phosphorylase probed by site-directed mutagenesis and detailed kinetic comparison of wild-type and Glu237→Gln mutant enzymes. Biochem. J. 2007, 403, 441–449. [Google Scholar] [CrossRef]
- Schwarz, A.; Nidetzky, B. Asp-196 - Ala mutant of Leuconostoc mesenteroides sucrose phosphorylase exhibits altered stereochemical course and kinetic mechanism of glucosyl transfer to and from phosphate. FEBS Lett. 2006, 580, 3905–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.; Nidetzky, B. The role of Asp-295 in the catalytic mechanism of Leuconostoc mesenteroides sucrose phosphorylase probed with site-directed mutagenesis. FEBS Lett. 2007, 581, 1403–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Broek, L.A.; van Boxtel, E.L.; Kievit, R.P.; Verhoef, R.; Beldman, G.; Voragen, A.G. Physico-chemical and transglucosylation properties of recombinant sucrose phosphorylase from Bifidobacterium adolescentis DSM20083. Appl. Microbiol. Biotechnol. 2004, 65, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Doudoroff, M.; Hassid, W.Z.; Barker, H.A. Studies with bacterial sucrose phosphorylase. II. Enzymatic synthesis of a new reducing and of a new non-reducing disaccharide. J. Biol. Chem. 1947, 168, 733–746. [Google Scholar]
- Weimberg, R.; Doudoroff, M. Studies with three bacterial sucrose phosphorylases. J. Bacteriol. 1954, 68, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Wildberger, P.; Luley-Goedl, C.; Nidetzky, B. Aromatic interactions at the catalytic subsite of sucrose phosphorylase: Their roles in enzymatic glucosyl transfer probed with Phe52→Ala and Phe52→Asn mutants. FEBS Lett. 2011, 585, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Wildberger, P.; Todea, A.; Nidetzky, B. Probing enzyme–substrate interactions at the catalytic subsite of Leuconostoc mesenteroides sucrose phosphorylase with site-directed mutagenesis: The roles of Asp49 and Arg395. Biocatal. Biotransformation 2012, 30, 326–337. [Google Scholar] [CrossRef]
- Verhaeghe, T.; Diricks, M.; Aerts, D.; Soetaert, W.; Desmet, T. Mapping the acceptor site of sucrose phosphorylase from Bifidobacterium adolescentis by alanine scanning. J. Mol. Catal. B Enzym. 2013, 96, 81–88. [Google Scholar] [CrossRef]
- Verhaeghe, T.; De Winter, K.; Berland, M.; De Vreese, R.; D’hooghe, M.; Offmann, B.; Desmet, T. Converting bulk sugars into prebiotics: Semi-rational design of a transglucosylase with controlled selectivity. Chem. Commun. 2016, 52, 3687–3689. [Google Scholar] [CrossRef]
- Beerens, K.; De Winter, K.; Van De Walle, D.; Grootaert, C.; Kamiloglu, S.; Miclotte, L.; Van De Wiele, T.; Van Camp, J.; Dewettinck, K.; Desmet, T. Biocatalytic Synthesis of the Rare Sugar Kojibiose: Process Scale-Up and Application Testing. J. Agric. Food Chem. 2017, 65, 6030–6041. [Google Scholar] [CrossRef]
- Kraus, M.; Grimm, C.; Seibel, J. Redesign of the Active Site of Sucrose Phosphorylase through a Clash-Induced Cascade of Loop Shifts. ChemBioChem 2016, 17, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Grimm, C.; Seibel, J. Switching enzyme specificity from phosphate to resveratrol glucosylation. Chem. Commun. 2017, 53, 12181–12184. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Grimm, C.; Seibel, J. Reversibility of a Point Mutation Induced Domain Shift: Expanding the Conformational Space of a Sucrose Phosphorylase. Sci. Rep. 2018, 8, 10490. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Gorl, J.; Timm, M.; Seibel, J. Synthesis of the rare disaccharide nigerose by structure-based design of a phosphorylase mutant with altered regioselectivity. Chem. Commun. 2016, 52, 4625–4627. [Google Scholar] [CrossRef] [PubMed]
- Franceus, J.; Dhaene, S.; Decadt, H.; Vandepitte, J.; Caroen, J.; Van der Eycken, J.; Beerens, K.; Desmet, T. Rational design of an improved transglucosylase for production of the rare sugar nigerose. Chem. Commun. 2019, 55, 4531–4533. [Google Scholar] [CrossRef]
- Dirks-Hofmeister, M.E.; Verhaeghe, T.; De Winter, K.; Desmet, T. Creating Space for Large Acceptors: Rational Biocatalyst Design for Resveratrol Glycosylation in an Aqueous System. Angew. Chemie 2015, 127, 9421–9424. [Google Scholar] [CrossRef]
- Decuyper, L.; Franceus, J.; Dhaene, S.; Debruyne, M.; Vandoorne, K.; Piens, N.; Dewitte, G.; Desmet, T.; D’hooghe, M. Chemoenzymatic Approach toward the Synthesis of 3-O-(α/β)-Glucosylated 3-Hydroxy-β-lactams. ACS Omega 2018, 3, 15235–15245. [Google Scholar] [CrossRef] [Green Version]


| Product | Enzymes | Substrates and Cofactors | Reaction Conditions | Yield | Ref. |
|---|---|---|---|---|---|
| β(1,4)-Galactosyl l-rhamnose | B. longum SP, UDP-glucose—hexose 1-phosphate uridylyltransferase, UDP-glucose 4-epimerase, Galactosyl l-rhamnose phosphorylase | 1.1 M sucrose, 1 M l-rhamnose, 30 mM phosphate, 1 mM UDP glucose, 10 mM MgCl2 | 30 °C, pH 7 | 65% | [35] |
| β(1,4)-Mannosyl N-acetylglucosamine | SP (unknown source), α-phosphoglucomutase, glucose 6-phosphate isomerase, mannose 6-phosphate isomerase, α-phosphomannomutase, β(1,4)-mannosyl N-acetylglucosamine phosphorylase | 250 mM sucrose, 250 mM N-acetylglucosamine, 25 mM phosphate, 60 µM glucose 1,6-bisphosphate | 30 °C, pH 7 | 23% | [36] |
| Nigerose | B. longum SP, nigerose phosphorylase, xylose isomerase, α-phosphoglucomutase, β-phosphoglucomutase | 500 mM sucrose, 25 mM phosphate, 10 mM MgCl2, 41 µM glucose 1,6-bisphosphate | 30 °C, pH 7 | 67% | [37] |
| Amylose | T. thermosaccharolyticum SPP, potato α-glucan phosphorylase | 100 mM sucrose, 50 mM phosphate, 200 µM maltodextrins | 37 °C, pH 7.4 | 0.22 g/g 1 | [33] |
| Short-chain cellodextrins | B. adolescentis SP, cellobiose phosphorylase, cellodextrin phosphorylase | 200 mM sucrose, 50 mM phosphate, 65–80 mM glucose | 45 °C, pH 7 | ~90% | [34] |
| Glucaric acid | L. mesenteroides SP, phosphoglucomutase, myo-inositol 1-phosphate synthase, myo-inositol monophosphatase, myo-inositol oxygenase, uronate dehydrogenase, NADH oxidase | 50 mM sucrose, 2 mM MgCl2, 2 mM Fe2+, 3 mM NAD+ | 30 °C, pH 7.5 | 75% 2 | [38] |
| Enzyme | Residue | (Potential) Function | Ref. |
|---|---|---|---|
| All | Asp192 | Catalytic nucleophile | [63] |
| Glu232 | Catalytic acid/base | [62] | |
| Asp290 | Transition state stabiliser | [64] | |
| Phe53 | Hydrophobic platform, transition state stabilisation, stacking interaction in subsite −1 | [68] | |
| SP | Asp50 | Binding of glucosyl moiety (H-bond with OH4) | [69] |
| His88 | Binding of glucosyl moiety (H-bond with OH6) | - | |
| Arg190 | Binding of glucosyl moiety (H-bond with OH2) | - | |
| His289 | Binding of glucosyl moiety (H-bond with OH2 and OH3) | - | |
| Arg399 | Binding of glucosyl moiety (H-bond with OH3 and OH4) | [69] | |
| Tyr132 | Indirectly involved in binding of fructosyl moiety (via Tyr196) | [70] | |
| Pro134 | Indirectly involved in binding of phosphate and fructosyl moiety | [70] | |
| Arg135 | Binding of phosphate | [70] | |
| Tyr196 | Binding of fructosyl moiety (hydrophobic interaction with C1) and indirectly influences phosphate binding (via Tyr344) | [70] | |
| His234 | Crucial for overall activity (reason unknown) | [70] | |
| Asp342 | Binding of fructosyl moiety (H-bond with OH4) | [70] | |
| Leu343 | Indirectly involved in binding of phosphate (via Tyr344) | [70] | |
| Tyr344 | Binding of phosphate | [70] | |
| Gln345 | Binding of fructosyl moiety (H-bond with OH3 and OH6) and indirectly influences phosphate binding (via Tyr344) | [70] | |
| SPP | Arg134 | Binding of the phosphate group of fructose 6-phosphate | [8] |
| (promiscuous) | His344 | Binding of the phosphate group of fructose 6-phosphate | [8] |
| SPP | Arg152 | Binding of fructose 6-phosphate | [11] |
| (strict) | Lys364 | Binding of fructose 6-phosphate | [11] |
| Lys434 | Binding of fructose 6-phosphate | [11] | |
| GGoP | Tyr194 | Binding of glycerol | [18] |
| Ala333 | Binding of glycerol | [18] | |
| Gln336 | Binding of glycerol | [18] | |
| GGaP | Asn275 | Binding of glycerate | [15] |
| Glu383 | Binding of glycerate | [15] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceus, J.; Desmet, T. Sucrose Phosphorylase and Related Enzymes in Glycoside Hydrolase Family 13: Discovery, Application and Engineering. Int. J. Mol. Sci. 2020, 21, 2526. https://doi.org/10.3390/ijms21072526
Franceus J, Desmet T. Sucrose Phosphorylase and Related Enzymes in Glycoside Hydrolase Family 13: Discovery, Application and Engineering. International Journal of Molecular Sciences. 2020; 21(7):2526. https://doi.org/10.3390/ijms21072526
Chicago/Turabian StyleFranceus, Jorick, and Tom Desmet. 2020. "Sucrose Phosphorylase and Related Enzymes in Glycoside Hydrolase Family 13: Discovery, Application and Engineering" International Journal of Molecular Sciences 21, no. 7: 2526. https://doi.org/10.3390/ijms21072526





