TGF-β Signaling Regulates SLC8A3 Expression and Prevents Oxidative Stress in Developing Midbrain Dopaminergic and Dorsal Raphe Serotonergic Neurons
Abstract
:1. Introduction
2. Results
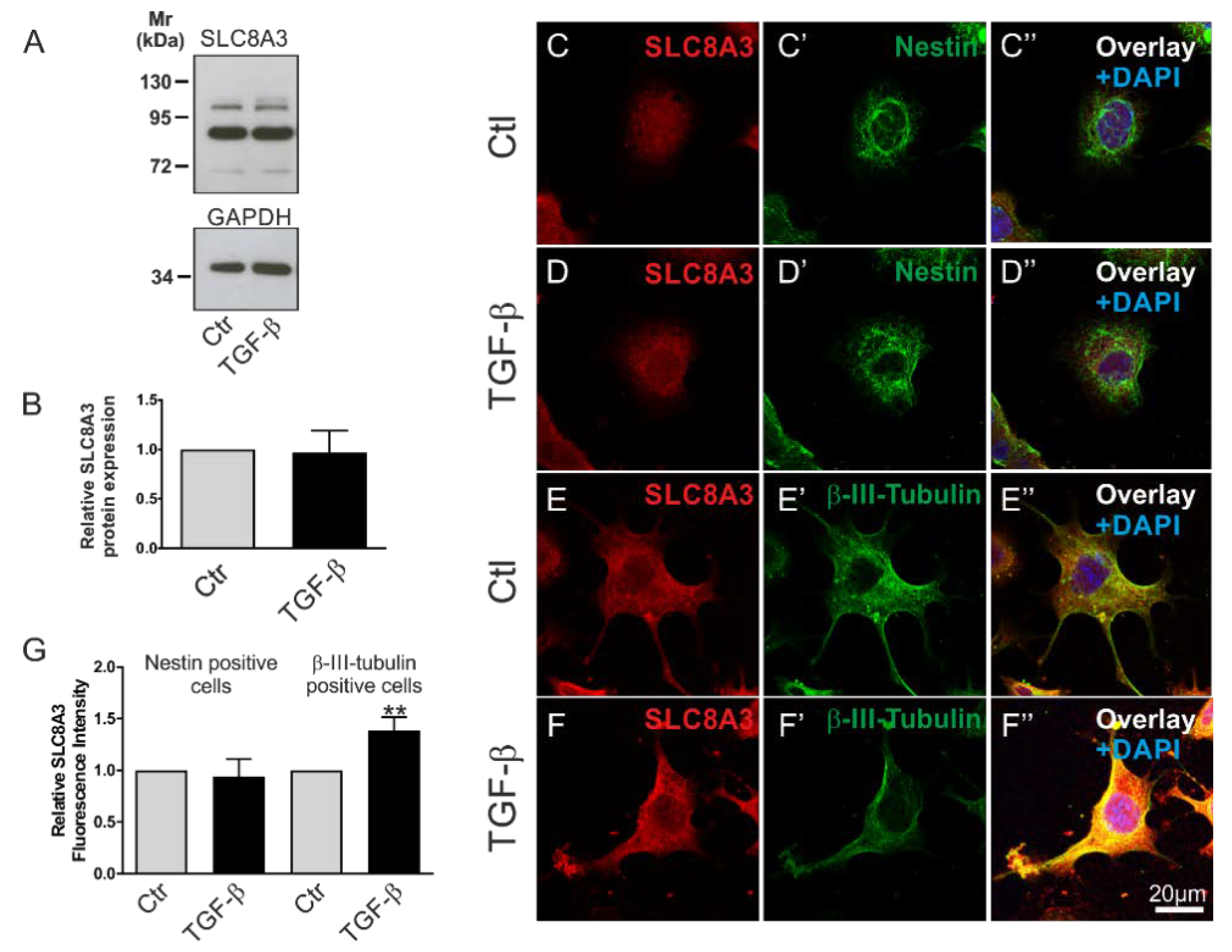
2.1. SLC8A3 Expression is Regulated by TGF-β Signaling
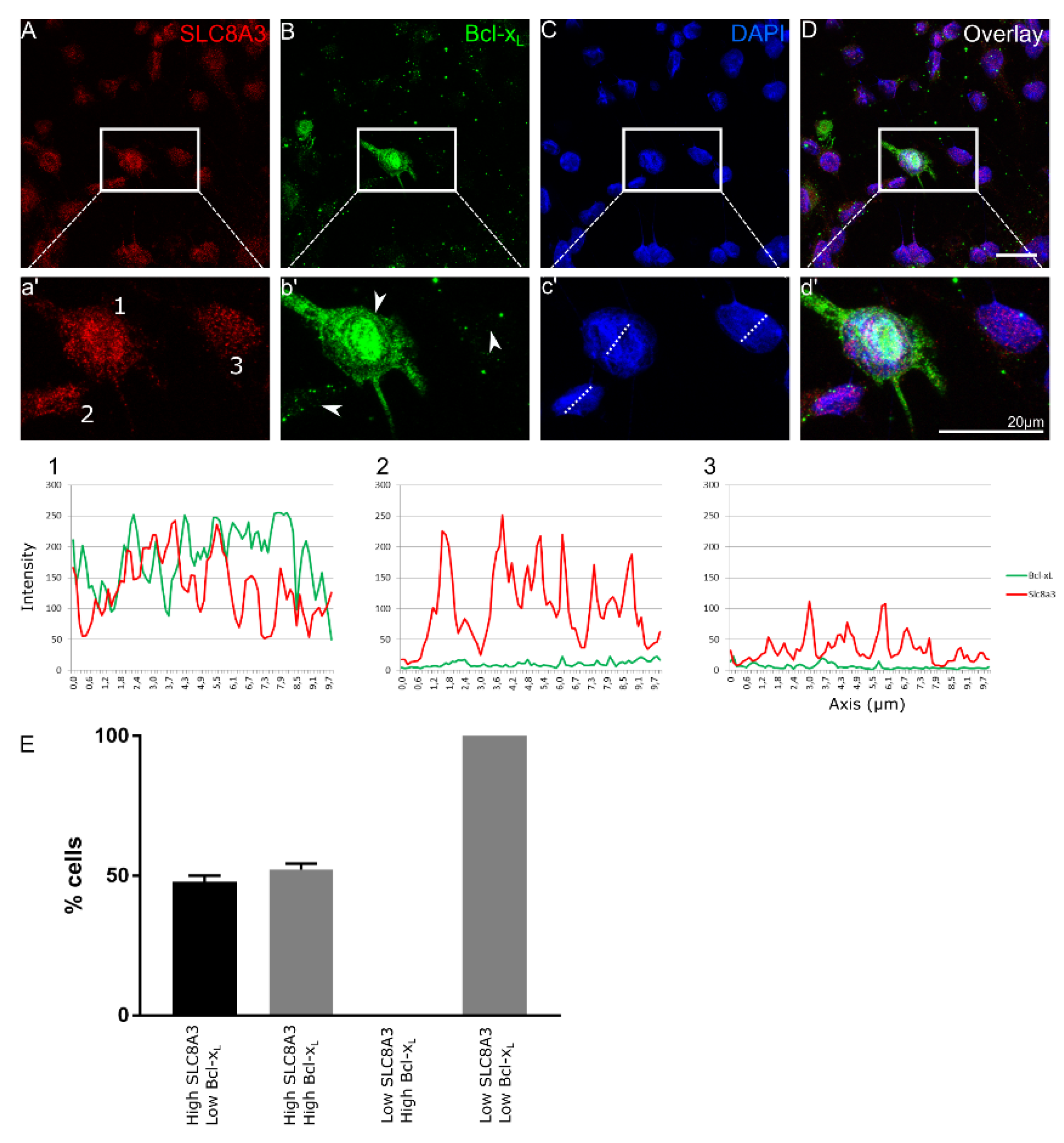
2.2. Anti-Apoptotic Action of SLC8A3
2.3. Cell-Type-Dependent Regulation of SLC8A3 by TGF-β
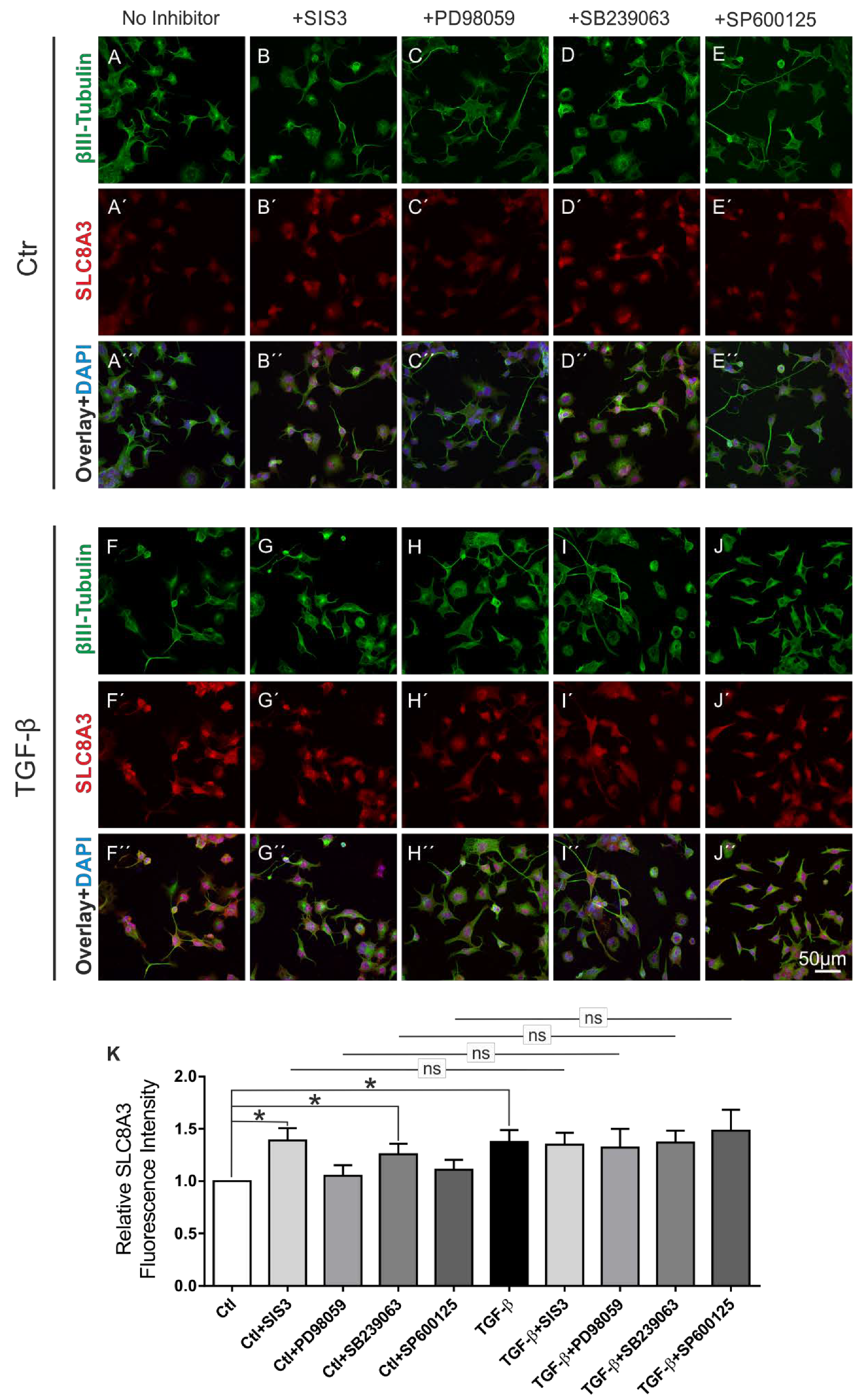
2.4. TGF-β Canonical Pathway and p38 Signaling Pathway Regulate Basal SLC8A3 Protein Expression
2.5. Smad4 Binds to Slc8a3 Promoter
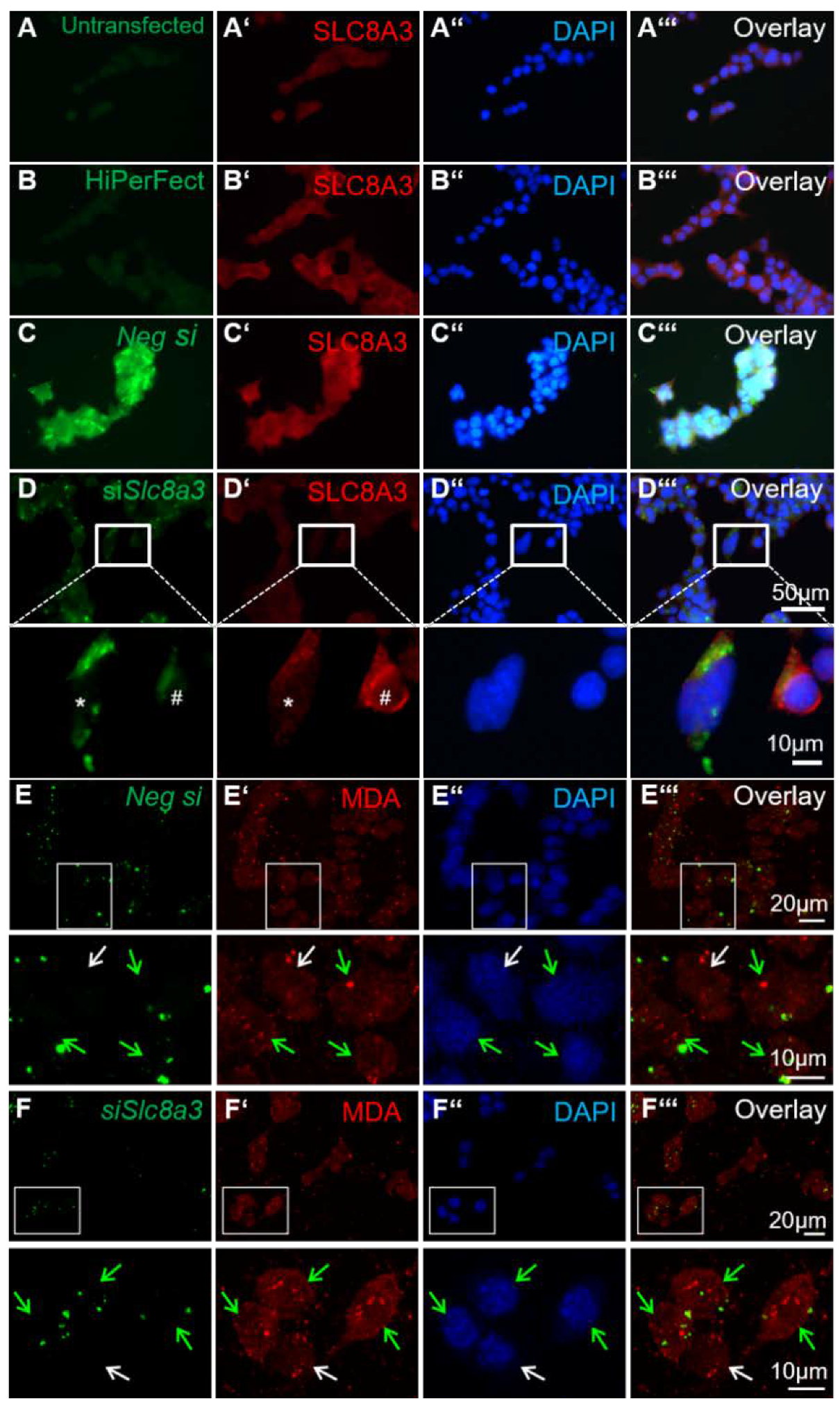
2.6. Knockdown of Slc8a3 is Associated with Oxidative Stress
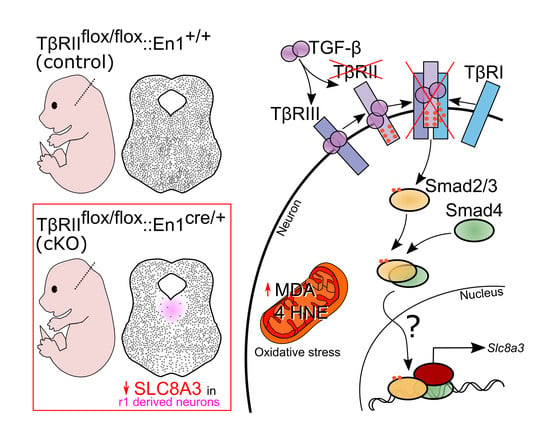
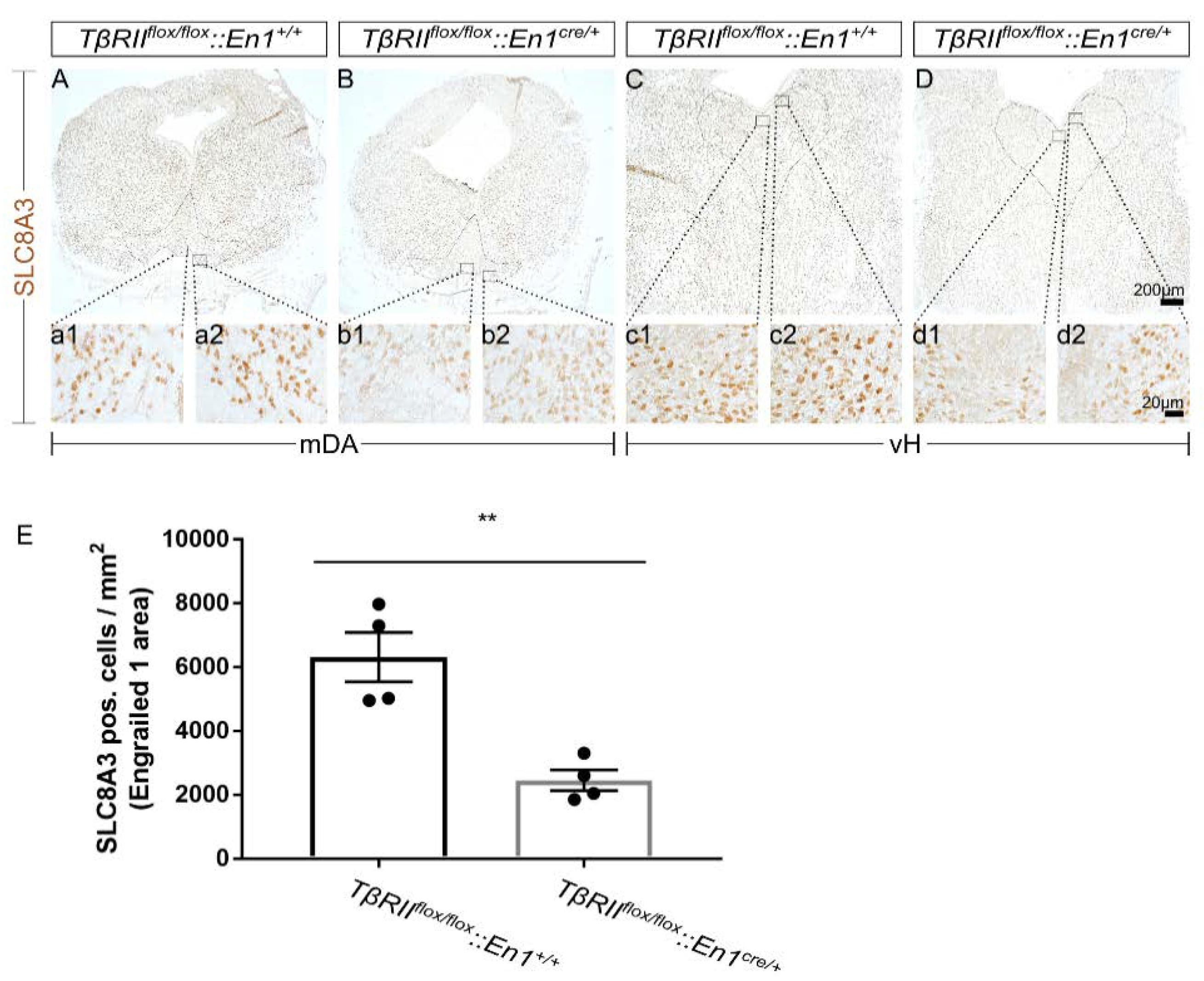
2.7. Cell-Type-Specific Deletion of TGF-β Signaling is Associated with Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Genotyping
4.3. Immunohistochemistry
4.4. Cell Culture of E14 Ventral Mesencephalon and Ventral Hindbrain
4.5. Cell Culture of MN9D and LUHMES Cells
4.6. Immunocytochemistry
4.7. Image Acquisition and Analysis
4.8. Immunoblotting
4.9. Chromatin Immunoprecipitation Assay
4.10. Transient Transfection of MN9D Cells
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 5-HT | 5-hydroxytryptamine |
| Bcl-xL | B-cell lymphoma-extra large |
| BDNF | Brain derived neurotrophic factor |
| cKO | Conditional knockout |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DR | Dorsal raphe |
| E# | Embryonic day |
| En1 | Engrailed 1 |
| ERK1/2 | Extracellular signal–regulated kinases-1/2 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| H3 | Histone 3 |
| JNK1/2 | c-Jun N-terminal kinases |
| L-type Ca2+ | Long-lasting calcium channel |
| LUHMES | Lund Human Mesencephalic |
| MAPK | Mitogen-activated protein kinase |
| MEK | Mitogen-activated protein kinase kinase |
| mDA | Midbrain dopaminergic |
| MDA | Malondialdehyde |
| NCX3 | Na+/Ca2+ exchanger 3 |
| NGF | Nerve growth factor |
| PC12 | Pheochromocytoma of the rat adrenal medulla |
| PD | Parkinson’s disease |
| PINK1 | PTEN-induced kinase 1 or PTEN-induced putative kinase 1 |
| ROS | Reactive oxygen species |
| siRNA | Silencing RNA |
| SLC8A3 | Solute carrier Family 8 member A3 |
| Smad | Small mother against decapentaplegic |
| SNc | Substantia nigra pars compacta |
| TGF-β | Transforming growth factor beta |
| TRPC | Transient receptor potential canonical channels |
| TβRII | Type II TGF-β receptor |
| vH | Ventral hindbrain |
| VTA | Ventral tegmental area |
| Wt | Wildtype |
References
- Derynck, R.; Miyazono, K. TGF-β and The TGF-β Family. In The TGF-β Family; Derynck, R., Miyazono, K., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2008; pp. 29–43. [Google Scholar] [CrossRef]
- Krieglstein, K.; Zheng, F.; Unsicker, K.; Alzheimer, C. More than being protective: Functional roles of TGF-β/activin signaling pathways at central synapses. Trends Neurosci. 2011, 34, 421–429. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGF-β signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef] [Green Version]
- Chleilat, E.; Skatulla, L.; Rahhal, B.; Hussein, M.T.; Feuerstein, M.; Krieglstein, K.; Roussa, E. TGF-β signaling regulates development of midbrain dopaminergic and hindbrain serotonergic neuron subgroups. Neuroscience 2018, 381, 124–137. [Google Scholar] [CrossRef]
- Chleilat, E.; Mallmann, R.; Spanagel, R.; Klugbauer, N.; Krieglstein, K.; Roussa, E. Spatiotemporal role of transforming growth factor beta 2 in developing and mature mouse hindbrain serotonergic neurons. Front. Cell. Neurosci. 2018, 13, 427. [Google Scholar] [CrossRef]
- Roux, J.; Carles, M.; Koh, H.; Goolaerts, A.; Ganter, M.T.; Chesebro, B.B.; Howard, M.; Houseman, B.T.; Finkbeiner, W.; Shokat, K.M.; et al. Transforming growth factor beta1 inhibits cystic fibrosis transmembrane conductance regulator-dependent cAMP-stimulated alveolar epithelial fluid transport via a phosphatidylinositol 3-kinase-dependent mechanism. J. Biol. Chem. 2010, 285, 4278–4290. [Google Scholar] [CrossRef] [Green Version]
- Rathore, K.I.; Redensek, A.; David, S. Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-α and TGF-β1. Glia 2012, 60, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Pierucci-Alves, F.; Schultz, B.D. Transforming growth factor-β1 impairs CFTR-mediated monolayer via the p38 MAPK pathway. Am. J. Physiol. Cell Physiol. 2013, 305, C867–C876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khakipoor, S.; Ophoven, C.; Schrödl-Häußel, M.; Feuerstein, M.; Heimrich, B.; Deitmer, J.W.; Roussa, E. TGF-β Signaling directly regulates transcription and functional expression of the electrogenic sodium bicarbonate cotransporter 1, NBCe1 (SLC4A4), via Smad4 in mouse astrocytes. Glia 2017, 65, 1361–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Sheng, J.; Peng, G.; Yang, J.; Chen, W.; Li, K. TGF-β1 regulation of p-JNK and L-type calcium channel Cav1.2 in cortical neurons. J. Mol. Neurosci. 2018, 64, 374–384. [Google Scholar] [CrossRef]
- Yan, J.; Schmid, E.; Almilaji, A.; Shumilina, E.; Borst, O.; Laufer, S.; Gawaz, M.; Lang, F. Effect of TGF on calcium signaling in megakaryotes. Biochem. Biophys. Res. Commun. 2015, 461, 8–13. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakajima, T.; Yamamoto, Y.; Takano, N.; Tanaka, T.; Kikuchi, H.; Oguri, G.; Morita, T.; Nakamura, F.; Komuro, I. Roles of transient receptor potential canonical (TRPC) channels and reverse-mode Na+/Ca2+ exchanger on cell proliferation in human cardiac fibroblasts: Effects of transforming growth factor 1. Cell Calcium 2013, 54, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.Y.; Verkaart, S.; Koopman, W.J.; Willems, P.H.; Hoenderop, J.G.; Bindels, R.J. Function and regulation of the Na+-Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle. J. Biol. Chem. 2014, 289, 11293–11303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, L.Y.; Hoenderop, J.G.; Bindels, R.J. Towards understanding the role of the Na+-Ca2+ exchanger isoform 3. Rev. Physiol. Biochem. Pharmacol. 2015, 168, 31–57. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; D’Avenzo, C.; Pannaccione, A.; Secondo, A.; Casamassa, A.; Formisano, L.; Guida, N.; Annunziato, L. Silencing or knocking out the Na+/Ca2+ exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012, 19, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Sokolow, S.; Manto, M.; Gailly, P.; Molgo, J.; Vanderbrouck, C.; Vanderwinden, J.-M.; Herchuelz, A.; Schurmans, S. Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. J. Clin. Invest. 2004, 113, 265–293. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, P.; Viggiano, D.; Nistico, R.; Sirabella, R.; Secondo, A.; Boscia, F.; Pannaccione, A.; Scorziello, A.; Mehdawy, B.; Sokolow, S.; et al. Na+-Ca2+ exchanger (NCX3) knock-out mice display an impairment in hippocampal long-term potentiation and spatial learning and memory. J. Neurosci. 2011, 31, 7312–7321. [Google Scholar] [CrossRef]
- Jeffs, G.J.; Meloni, B.P.; Sokolow, S.; Herchuelz, A.; Schurmans, S.; Knuckey, N.W. NCX3 knockout mice exhibit increased hippocampal CA1 and CA2 neuronal damage compared to wild-type mice following global cerebral ischemia. Exp. Neurol. 2008, 210, 268–273. [Google Scholar] [CrossRef]
- Molinaro, P.; Cuomo, O.; Pignataro, G.; Boscia, F.; Sirabella, R.; Pannaccione, A.; Secondo, A.; Scorziello, A.; Adornetto, A.; Gala, R.; et al. Targeted Disruption of Na+/Ca2+ exchanger (NCX3) gene leads to a worsening of ischemic brain damage. J. Neurosci. 2008, 28, 1179–1184. [Google Scholar] [CrossRef] [Green Version]
- Gabellini, N.; Bortoluzzi, S.; Danieli, G.A.; Carafioli, E. Control of the Na+/Ca2+ exchanger 3 promoter by cyclic adenosine monophosphate and Ca2+ in differentiating neurons. J. Neurochem. 2003, 84, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Formisano, L.; Saggese, M.; Secondo, A.; Sirabella, R.; Vito, P.; Valsecchi, V.; Molinaro, P.; Di Renzo, G.; Annunziato, L. The two isoforms of the Na+/Ca2+ exchanger, NCX1 and NCX3, constitute novel additional targets for the prosurvival action of Akt/protein kinase B pathway. Mol. Pharm. 2008, 73, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Sirabella, R.; Secondo, A.; Pannaccione, A.; Molinaro, P.; Formisano, L.; Guida, N.; Di Renzo, G.; Annunziato, L.; Cataldi, M. ERK1/2, p38, and JNK regulate the expression and the activity of the three isoforms of the Na+/Ca2+ exchanger, NCX1, NCX2, and NCX3, in neuronal PC12 cells. J. Neurochem. 2012, 122, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.A.; Porter, G.A.; Alavian, K.N. Bcl-xL in neuroprotection and plasticity. Front. Physiol. 2014, 5, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood-Kaczmar, A.; Deas, E.; Wood, N.W.; Abramov, A.Y. The role of mitochondrial NCX in the mechanism of neurodegeneration in Parkinson’s disease. Adv. Exp. Med. 2013, 961, 241–249. [Google Scholar] [CrossRef]
- Krajewska, M.; Mai, J.K.; Zapata, J.M.; Ashwell, K.W.S.; Schendel, S.L.; Reed, J.C.; Krajewski, S. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax and Bak during development of murine nervous system. Cell Death Differ. 2002, 9, 145–157. [Google Scholar] [CrossRef]
- Lotharius, J.; Barg, S.; Wiekop, P.; Lundberg, C.; Raymon, H.K.; Brundin, P. Effect of mutant a-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J. Biol. Chem. 2002, 41, 38884–38894. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.K.; Won, L.A.; Kontur, P.J.; Hammond, D.N.; Fox, A.P.; Wainer, B.H.; Hoffmann, P.C.; Heller, A. Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res. 1991, 552, 67–76. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Detecting reactive oxygen species by immunohistochemistry. Methods Mol. Biol. 2015, 1292, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Munoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-noneal. Oxid. Med. Cell. Longev. 2014, 360438. [Google Scholar] [CrossRef]
- Secondo, A.; Staiano, R.I.; Scorziello, A.; Sirabella, R.; Boscia, F.; Adornetto, A.; Valsecchi, V.; Molinaro, P.; Canzoniero, L.M.; Di Renzo, G.; et al. BHK cells transfected with NCX3 are more resistant to hypoxia followed by reoxygenation than those transfected with NCX1 and NCX2: Possible relationship with mitochondrial membrane potential. Cell Calcium 2007, 42, 521–535. [Google Scholar] [CrossRef]
- Cai, Q.; Ma, T.; Tian, Y.; Li, C.; Li, H. Catalpol inhibits ischemia-induced premyelinating oligodendrocyte damage through regulation of intracellular calcium homoeostasis via Na+/Ca2+ exchanger 3. Int. J. Mol. Sci. 2018, 19, 1925. [Google Scholar] [CrossRef] [Green Version]
- Dünker, N.; Krieglstein, K. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur. J. Biochem. 2000, 267, 6982–6988. [Google Scholar] [CrossRef] [PubMed]
- Parpura, V.; Sekler, I.; Fern, R. Plasmalemmal and mitochondrial Na+/Ca2+ exchange in neuroglia. Glia 2016, 64, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Wood-Kaczmar, A.; Yao, Z.; Plun-Favreau, H.; Deas, E.; Klupsch, K.; Downward, J.; Latchman, D.S.; Tabrizi, S.J.; Wood, N.W.; et al. Pink1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 2009, 33, 627–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.H.; Paxinos, G.; Watson, C.; Halliday, G.M. The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J. Chem. Neurol. 2016, 76, 98–107. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G.; Pizzimenti, S.; Ciamporcero, E.S.; Daga, M.; Ullio, C.; Arcaro, A.; Cetrangolo, G.P.; Ferretti, C.; Dianzani, C.; Lepore, A.; et al. Role of 4-Hydroxynonenal-Protein Adducts in Human Diseases. Antioxid. Redox Signal. 2014, 22, 1681–1702. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Zuo, M.-L.; Hu, G.-H.; Duan, X.-M.; Yang, Z.-B. Mir-193 targets ALDH2 and contributes to toxic aldehyde accumulation and tyrosine hydroxylase dysfunction in cerebral ischemia/reperfusion injury. Oncotarget 2017, 8, 99681–99692. [Google Scholar] [CrossRef] [Green Version]
- Yoritaka, A.; Hattori, N.; Uchida, K.; Tanaka, M.; Stadman, E.R.; Mizuno, Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2696–2701. [Google Scholar] [CrossRef] [Green Version]
- Chytil, A.; Magnuson, M.A.; Wright, C.V.; Moses, H.L. Conditional inactivation of the TGF-beta type II receptor using Cre: Lox. Genesis 2002, 32, 73–75. [Google Scholar] [CrossRef]
- Kimmel, R.; Turnbull, D.H.; Blanquet, V.; Wurst, W.; Loomis, C.A.; Joyner, A.L. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 2000, 14, 1377–1389. [Google Scholar]
- Roussa, E.; Wiehle, M.; Dünker, N.; Becker-Catins, S.; Oehlke, O.; Krieglstein, K. Transforming growth factor beta is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: Ectopic induction in dorsal mesencephalon. Stem. Cells 2006, 24, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Heien, M.L.; Maxson, M.M.; Ewing, A.G. Amperometric measurements of catecholamine release from single vesicles in MN9D cells. J. Neurochem. 2008, 107, 1589–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oehlke, O.; Schlosshardt, C.; Feuerstein, M.; Roussa, E. Acidosis-induced V-ATPase trafficking in salivary ducts is initiated by cAMP/PKA/CREB pathway via regulation of Rab11b expression. Int. J. Biochem. Cell Biol. 2012, 44, 1254–1265. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chleilat, E.; Pethe, A.; Pfeifer, D.; Krieglstein, K.; Roussa, E. TGF-β Signaling Regulates SLC8A3 Expression and Prevents Oxidative Stress in Developing Midbrain Dopaminergic and Dorsal Raphe Serotonergic Neurons. Int. J. Mol. Sci. 2020, 21, 2735. https://doi.org/10.3390/ijms21082735
Chleilat E, Pethe A, Pfeifer D, Krieglstein K, Roussa E. TGF-β Signaling Regulates SLC8A3 Expression and Prevents Oxidative Stress in Developing Midbrain Dopaminergic and Dorsal Raphe Serotonergic Neurons. International Journal of Molecular Sciences. 2020; 21(8):2735. https://doi.org/10.3390/ijms21082735
Chicago/Turabian StyleChleilat, Enaam, Abhishek Pethe, Dietmar Pfeifer, Kerstin Krieglstein, and Eleni Roussa. 2020. "TGF-β Signaling Regulates SLC8A3 Expression and Prevents Oxidative Stress in Developing Midbrain Dopaminergic and Dorsal Raphe Serotonergic Neurons" International Journal of Molecular Sciences 21, no. 8: 2735. https://doi.org/10.3390/ijms21082735