Biomaterials in Neurodegenerative Disorders: A Promising Therapeutic Approach
Abstract
1. Introduction
1.1. Neurodegenerative Disorders
1.1.1. Alzheimer’s Disease
1.1.2. Parkinson’s Disease
1.1.3. Amyotrophic Lateral Sclerosis
1.2. Spinal Cord Injury
1.3. Current Treatments
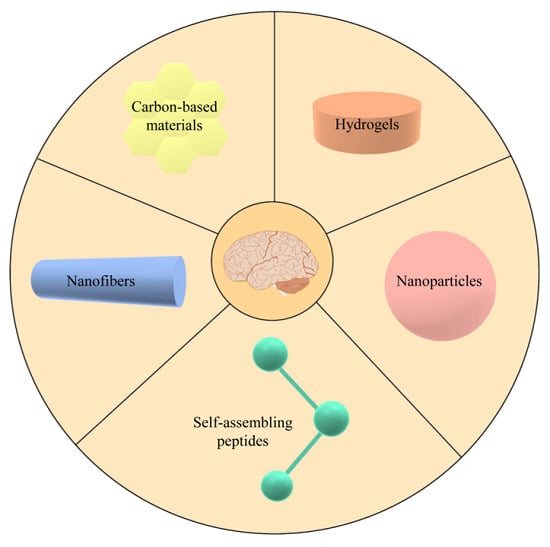
2. Biomaterials
2.1. Characteristics of Biomaterials
2.2. Hydrogels
2.3. Nanoparticles
2.4. Self-Assembling Peptides
2.5. Nanofibers
2.6. Carbon-Based Nanomaterials
3. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ND | Neurodegenerative Disease |
| AD | Alzheimer’s Disease |
| PD | Parkinson’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| SCI | Spinal Cord Injury |
| TDP-43 | TAR DNA-binding protein 43 |
| BBB | Blood–Brain Barrier |
| APP | Amyloid Precursor Protein |
| Aβ | Amyloid Beta |
| NMDA | N-methyl-d-aspartate |
| SOD1 | Superoxide Dismutase 1 |
| FUS | Fused in sarcoma |
| AAV | Adeno-Associated Viruses |
| CED | Convection-Enhanced Delivery |
| MSC | Mesenchymal Stem Cell |
| UC-MSC | Umbilical Cord Mesenchymal Stem Cell |
| BMSC | Bone Marrow Stem Cell |
| ASC | Adipose Stem Cell |
| ECM | Extracellular Matrix |
| HA | Hyaluronic Acid |
| VEGF | Vascular Endothelial Growth Factor |
| BDNF | Brain-Derived Neurotrophic Factor |
| GDNF | Glial Cell-Derived Neurotrophic Factor |
| iPSC | induced Pluripotent Stem Cell |
| ENSPC | Embryonic Neural Stem/Progenitor Cell |
| NP | Nanoparticle |
| PLGA | Poly-(Lactic-co-Glycolic Acid) |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| SAP | Self-Assembling Peptide |
| CNT | Carbon Nanotube |
References
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Molecular pathological classification of neurodegenerative diseases: Turning towards precision medicine. Int. J. Mol. Sci. 2016, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Focus on neurodegenerative disease. Nat. Neurosci. 2018, 21, 1293. [CrossRef] [PubMed]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Soto, C.; Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci 2018, 21, 1300–1309. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Nichols, E.; Szoeke, C.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Alzheimer’s Disease and Related Dementias: Experience and Caregiving, Epidemiology, and Models of Care: Proceedings of a Workshop–in Brief; The National Academies Press: Washington, DC, USA, 2020; p. 12. [Google Scholar]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Demen. (N. Y.) 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Grodzicki, W.; Dziendzikowska, K. The Role of Selected Bioactive Compounds in the Prevention of Alzheimer’s Disease. Antioxidants (Basel) 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef]
- Draoui, A.; El Hiba, O.; Aimrane, A.; El Khiat, A.; Gamrani, H. Parkinson’s disease: From bench to bedside. Rev. Neurol. (Paris) 2020. [Google Scholar] [CrossRef]
- Redenšek, S.; Trošt, M.; Dolžan, V. Genetic Determinants of Parkinson’s Disease: Can They Help to Stratify the Patients Based on the Underlying Molecular Defect? Front. Aging Neurosci. 2017, 9, 20. [Google Scholar] [CrossRef]
- Deng, H.; Wang, P.; Jankovic, J. The genetics of Parkinson disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. North Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Alam, Q.; Alam, M.Z.; Mushtaq, G.; Damanhouri, G.A.; Rasool, M.; Kamal, M.A.; Haque, A. Inflammatory Process in Alzheimer’s and Parkinson’s Diseases: Central Role of Cytokines. Curr. Pharm. Des. 2016, 22, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Mathis, S.; Goizet, C.; Soulages, A.; Vallat, J.M.; Masson, G.L. Genetics of amyotrophic lateral sclerosis: A review. J. Neurol. Sci. 2019, 399, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, E.; Ticozzi, N.; Mandrioli, J. Psychiatric Symptoms in Amyotrophic Lateral Sclerosis: Beyond a Motor Neuron Disorder. Front. Neurosci. 2019, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Van Broeckhoven, C.; van der Zee, J. ALS Genes in the Genomic Era and their Implications for FTD. Trends Genet. 2018, 34, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Miki, Y.; Kon, T.; Tanji, K.; Wakabayashi, K. Autophagy Is a Common Degradation Pathway for Bunina Bodies and TDP-43 Inclusions in Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2019, 78, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Antonelli, A.C.; Afridi, A.; Vatsia, S.; Joshi, G.; Romanov, V.; Murray, I.V.J.; Khan, S.A. Protein misfolding and aggregation in neurodegenerative diseases: A review of pathogeneses, novel detection strategies, and potential therapeutics. Rev. Neurosci. 2019, 30, 339–358. [Google Scholar] [CrossRef]
- Lilley, E.; Andrews, M.R.; Bradbury, E.J.; Elliott, H.; Hawkins, P.; Ichiyama, R.M.; Keeley, J.; Michael-Titus, A.T.; Moon, L.D.; Pluchino, S. Refining rodent models of spinal cord injury. Exp. Neurol. 2020, 113273. [Google Scholar] [CrossRef]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Liverman, C.T.; Altevogt, B. Spinal Cord Injury: Progress, Promises and Priorities; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Rogers, W.K.; Todd, M. Acute spinal cord injury. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, V.; Richardson, R.M. Gene therapy for neurodegenerative diseases. Neurotherapeutics 2019, 16, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Budd Haeberlein, S.L.; Harris, T.J. Promising Targets for the Treatment of Neurodegenerative Diseases. Clin. Pharmacol. Ther. 2015, 98, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Poovaiah, N.; Davoudi, Z.; Peng, H.; Schlichtmann, B.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Treatment of neurodegenerative disorders through the blood-brain barrier using nanocarriers. Nanoscale 2018, 10, 16962–16983. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Pansarasa, O.; Dell’Orco, M.; Crippa, V.; Gagliardi, S.; Sproviero, D.; Bernuzzi, S.; Diamanti, L.; Ceroni, M.; Tedeschi, G.; et al. Nuclear Phospho-SOD1 Protects DNA from Oxidative Stress Damage in Amyotrophic Lateral Sclerosis. J. Clin. Med. 2019, 8, 729. [Google Scholar] [CrossRef]
- Bravo-Hernandez, M.; Tadokoro, T.; Navarro, M.R.; Platoshyn, O.; Kobayashi, Y.; Marsala, S.; Miyanohara, A.; Juhas, S.; Juhasova, J.; Skalnikova, H.; et al. Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nat. Med. 2020, 26, 118–130. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef]
- Mittermeyer, G.; Christine, C.W.; Rosenbluth, K.H.; Baker, S.L.; Starr, P.; Larson, P.; Kaplan, P.L.; Forsayeth, J.; Aminoff, M.J.; Bankiewicz, K.S. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum. Gene. Ther. 2012, 23, 377–381. [Google Scholar] [CrossRef]
- Warren Olanow, C.; Bartus, R.T.; Baumann, T.L.; Factor, S.; Boulis, N.; Stacy, M.; Turner, D.A.; Marks, W.; Larson, P.; Starr, P.A.; et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann. Neurol. 2015, 78, 248–257. [Google Scholar] [CrossRef]
- Rafii, M.S.; Tuszynski, M.H.; Thomas, R.G.; Barba, D.; Brewer, J.B.; Rissman, R.A.; Siffert, J.; Aisen, P.S. Adeno-Associated Viral Vector (Serotype 2)-Nerve Growth Factor for Patients With Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 834–841. [Google Scholar] [CrossRef]
- Manoutcharian, K.; Perez-Garmendia, R.; Gevorkian, G. Recombinant Antibody Fragments for Neurodegenerative Diseases. Curr. Neuropharmacol. 2017, 15, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Blundell, R.; Shah, M. Neurodegenerative diseases and stem cell transplantation. J. Stem Cell Res. Ther. 2015, 5, 2. [Google Scholar] [CrossRef]
- Lo Furno, D.; Pellitteri, R.; Graziano, A.C.; Giuffrida, R.; Vancheri, C.; Gili, E.; Cardile, V. Differentiation of human adipose stem cells into neural phenotype by neuroblastoma-or olfactory ensheathing cells-conditioned medium. J. Cell. Physiol. 2013, 228, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Lo Furno, D.; Mannino, G.; Giuffrida, R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J. Cell. Physiol. 2018, 233, 3982–3999. [Google Scholar] [CrossRef] [PubMed]
- Volkman, R.; Offen, D. Concise review: Mesenchymal stem cells in neurodegenerative diseases. Stem Cells 2017, 35, 1867–1880. [Google Scholar] [CrossRef]
- Carradori, D.; Eyer, J.; Saulnier, P.; Préat, V.; des Rieux, A. The therapeutic contribution of nanomedicine to treat neurodegenerative diseases via neural stem cell differentiation. Biomaterials 2017, 123, 77–91. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Maclean, F.L.; Rodriguez, A.L.; Parish, C.L.; Williams, R.J.; Nisbet, D.R. Integrating biomaterials and stem cells for neural regeneration. Stem Cells Dev. 2016, 25, 214–226. [Google Scholar] [CrossRef]
- Williams, D.F. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 1999. [Google Scholar]
- Masaeli, R.; Zandsalimi, K.; Tayebi, L. Biomaterials Evaluation: Conceptual Refinements and Practical Reforms. Ther. Innov. Regul. Sci. 2019, 53, 120–127. [Google Scholar] [CrossRef]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Rai, R.; Tallawi, M.; Roether, J.A.; Detsch, R.; Barbani, N.; Rosellini, E.; Kaschta, J.; Schubert, D.W.; Boccaccini, A.R. Sterilization effects on the physical properties and cytotoxicity of poly(glycerol sebacate). Mater. Lett. 2013, 105, 32–35. [Google Scholar] [CrossRef]
- Dos Santos, V.; Brandalise, R.N.; Savaris, M. Engineering of Biomaterials; Springer: Berlin, Germany, 2017. [Google Scholar]
- Wang, Y.X.; Robertson, J.L.; Spillman, W.B., Jr.; Claus, R.O. Effects of the chemical structure and the surface properties of polymeric biomaterials on their biocompatibility. Pharm. Res. 2004, 21, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Serra, R.; Gallego, R.; Lozano, P.; Gonzalez-Nieto, D. Hydrogels for neuroprotection and functional rewiring: A new era for brain engineering. Neural Regen. Res. 2020, 15, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Bariya, A.; Allam, A.; Othman, S.; Butani, S.B. In situ nanostructured hydrogel of resveratrol for brain targeting: In vitro-in vivo characterization. Drug Deliv. Transl. Res. 2018, 8, 1460–1470. [Google Scholar] [CrossRef]
- Spuch, C.; Antequera, D.; Portero, A.; Orive, G.; Hernández, R.M.; Molina, J.A.; Bermejo-Pareja, F.; Pedraz, J.L.; Carro, E. The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer’s disease. Biomaterials 2010, 31, 5608–5618. [Google Scholar] [CrossRef]
- Cui, G.H.; Shao, S.J.; Yang, J.J.; Liu, J.R.; Guo, H.D. Designer Self-Assemble Peptides Maximize the Therapeutic Benefits of Neural Stem Cell Transplantation for Alzheimer’s Disease via Enhancing Neuron Differentiation and Paracrine Action. Mol. Neurobiol. 2016, 53, 1108–1123. [Google Scholar] [CrossRef]
- Senthilkumar, K.S.; Saravanan, K.S.; Chandra, G.; Sindhu, K.M.; Jayakrishnan, A.; Mohanakumar, K.P. Unilateral implantation of dopamine-loaded biodegradable hydrogel in the striatum attenuates motor abnormalities in the 6-hydroxydopamine model of hemi-parkinsonism. Behav. Brain Res. 2007, 184, 11–18. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Humpel, C. Therapeutic efficacy of glial cell-derived neurotrophic factor loaded collagen scaffolds in ex vivo organotypic brain slice Parkinson’s disease models. Brain Res. Bull. 2019, 149, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Bruggeman, K.F.; Kauhausen, J.A.; Rodriguez, A.L.; Nisbet, D.R.; Parish, C.L. Functionalized composite scaffolds improve the engraftment of transplanted dopaminergic progenitors in a mouse model of Parkinson’s disease. Biomaterials 2016, 74, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Struzyna, L.A.; Browne, K.D.; Brodnik, Z.D.; Burrell, J.C.; Harris, J.P.; Chen, H.I.; Wolf, J.A.; Panzer, K.V.; Lim, J.; Duda, J.E.; et al. Tissue engineered nigrostriatal pathway for treatment of Parkinson’s disease. J. Tissue Eng. Regen. Med. 2018, 12, 1702–1716. [Google Scholar] [CrossRef]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef]
- Fantini, V.; Bordoni, M.; Scocozza, F.; Conti, M.; Scarian, E.; Carelli, S.; Di Giulio, A.M.; Marconi, S.; Pansarasa, O.; Auricchio, F.; et al. Bioink Composition and Printing Parameters for 3D Modeling Neural Tissue. Cells 2019, 8, 830. [Google Scholar] [CrossRef]
- Mothe, A.J.; Tam, R.Y.; Zahir, T.; Tator, C.H.; Shoichet, M.S. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 2013, 34, 3775–3783. [Google Scholar] [CrossRef]
- Johnson, J.O.; Mandrioli, J.; Benatar, M.; Abramzon, Y.; Van Deerlin, V.M.; Trojanowski, J.Q.; Gibbs, J.R.; Brunetti, M.; Gronka, S.; Wuu, J.; et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 2010, 68, 857–864. [Google Scholar] [CrossRef]
- Pertici, V.; Amendola, J.; Laurin, J.; Gigmes, D.; Madaschi, L.; Carelli, S.; Marqueste, T.; Gorio, A.; Decherchi, P. The use of poly(N-[2-hydroxypropyl]-methacrylamide) hydrogel to repair a T10 spinal cord hemisection in rat: A behavioural, electrophysiological and anatomical examination. ASN Neuro. 2013, 5, 149–166. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Silva Adaya, D.; Aguirre-Cruz, L.; Guevara, J.; Ortiz-Islas, E. Nanobiomaterials’ applications in neurodegenerative diseases. J. Biomater. Appl. 2017, 31, 953–984. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.A.; Chavhan, S.S.; Sawant, K.K. Rivastigmine-loaded PLGA and PBCA nanoparticles: Preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur. J. Pharm. Biopharm. 2010, 76, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.N.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef]
- Kulkarni, P.V.; Roney, C.A.; Antich, P.P.; Bonte, F.J.; Raghu, A.V.; Aminabhavi, T.M. Quinoline-n-butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer’s disease. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 35–47. [Google Scholar] [CrossRef]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef]
- Carrera, I.; Etcheverría, I.; Fernández-Novoa, L.; Lombardi, V.R.; Lakshmana, M.K.; Cacabelos, R.; Vigo, C. A comparative evaluation of a novel vaccine in APP/PS1 mouse models of Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 807146. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, R.; Cai, Y.; Liao, M.; Yuan, W.; Liu, Z. Controlled-release levodopa methyl ester/benserazide-loaded nanoparticles ameliorate levodopa-induced dyskinesia in rats. Int. J. Nanomedicine 2012, 7, 2077–2086. [Google Scholar] [CrossRef]
- Pehlivan, S.B. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm. Res. 2013, 30, 2499–2511. [Google Scholar] [CrossRef]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, H.E.; Anantharam, V.; Bronich, T.; Ghaisas, S.; Jin, H.; Kanthasamy, A.G.; Liu, X.; McMillan, J.; Mosley, R.L.; Narasimhan, B.; et al. Nanoneuromedicines for degenerative, inflammatory, and infectious nervous system diseases. Nanomedicine 2015, 11, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzo, S.; Isaia, D.; Bonanno, S.; Malacarne, C.; Cavalcante, P.; Zacheo, A.; Laquintana, V.; Denora, N.; Sanavio, B.; Salvati, E.; et al. FM19G11-Loaded Gold Nanoparticles Enhance the Proliferation and Self-Renewal of Ependymal Stem Progenitor Cells Derived from ALS Mice. Cells 2019, 8, 279. [Google Scholar] [CrossRef]
- Nabi, B.; Rehman, S.; Fazil, M.; Khan, S.; Baboota, S.; Ali, J. Riluzole-loaded nanoparticles to alleviate the symptoms of neurological disorders by attenuating oxidative stress. Drug Dev. Ind. Pharm. 2020, 46, 471–483. [Google Scholar] [CrossRef]
- Naz, S.; Beach, J.; Heckert, B.; Tummala, T.; Pashchenko, O.; Banerjee, T.; Santra, S. Cerium oxide nanoparticles: A ‘radical’ approach to neurodegenerative disease treatment. Nanomedicine (Lond.) 2017, 12, 545–553. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian. J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef]
- DeCoteau, W.; Heckman, K.L.; Estevez, A.Y.; Reed, K.J.; Costanzo, W.; Sandford, D.; Studlack, P.; Clauss, J.; Nichols, E.; Lipps, J.; et al. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine 2016, 12, 2311–2320. [Google Scholar] [CrossRef]
- Papa, S.; Rossi, F.; Vismara, I.; Forloni, G.; Veglianese, P. Nanovector-Mediated Drug Delivery in Spinal Cord Injury: A Multitarget Approach. ACS Chem. Neurosci. 2019, 10, 1173–1182. [Google Scholar] [CrossRef]
- Kim, Y.T.; Caldwell, J.M.; Bellamkonda, R.V. Nanoparticle-mediated local delivery of Methylprednisolone after spinal cord injury. Biomaterials 2009, 30, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, S.R.; Oliveira, J.M.; Silva, N.A.; Leite-Almeida, H.; Ribeiro-Samy, S.; Almeida, A.; Mano, J.F.; Sousa, N.; Salgado, A.J.; Reis, R.L. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small 2013, 9, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Rossi, F.; Ferrari, R.; Mariani, A.; De Paola, M.; Caron, I.; Fiordaliso, F.; Bisighini, C.; Sammali, E.; Colombo, C.; et al. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano 2013, 7, 9881–9895. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhang, Y.; Saito, E.; Gurczynski, S.J.; Moore, B.B.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2019, 116, 14947–14954. [Google Scholar] [CrossRef] [PubMed]
- Tukmachev, D.; Lunov, O.; Zablotskii, V.; Dejneka, A.; Babic, M.; Syková, E.; Kubinová, Š. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015, 7, 3954–3958. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Barraud, P.; Chari, D.M. The transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticles. Biomaterials 2011, 32, 2274–2284. [Google Scholar] [CrossRef]
- Pal, A.; Kumar, S.; Jain, S.; Nag, T.C.; Mathur, R. Neuroregenerative Effects of Electromagnetic Field and Magnetic Nanoparticles on Spinal Cord Injury in Rats. J. Nanosci. Nanotechnol. 2018, 18, 6756–6764. [Google Scholar] [CrossRef]
- Xu, D.; Wu, D.; Qin, M.; Nih, L.R.; Liu, C.; Cao, Z.; Ren, J.; Chen, X.; He, Z.; Yu, W.; et al. Delivery of Nerve Growth Factors to the Central Nervous System for Neural Regeneration. Adv. Mater. 2019, 31, 1900727. [Google Scholar] [CrossRef]
- Xu, Z.X.; Zhang, L.Q.; Zhou, Y.N.; Chen, X.M.; Xu, W.H. Histological and functional outcomes in a rat model of hemisected spinal cord with sustained VEGF/NT-3 release from tissue-engineered grafts. Artif. Cells Nanomed. Biotechnol. 2020, 48, 362–376. [Google Scholar] [CrossRef]
- Azizi, M.; Farahmandghavi, F.; Joghataei, M.T.; Zandi, M.; Imani, M.; Bakhtiari, M.; Omidian, H. ChABC-loaded PLGA nanoparticles: A comprehensive study on biocompatibility, functional recovery, and axonal regeneration in animal model of spinal cord injury. Int. J. Pharm. 2020, 577, 119037. [Google Scholar] [CrossRef]
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.B.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, D.R.; Williams, R.J. Self-assembled peptides: Characterisation and in vivo response. Biointerphases 2012, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Design of self-assembling surfactant-like peptides and their applications. Curr. Opin. Colloid. Interface Sci. 2009, 14, 340–348. [Google Scholar] [CrossRef]
- Wang, J.; Han, S.; Meng, G.; Xu, H.; Xia, D.; Zhao, X.; Schweins, R.; Lu, J.R. Dynamic self-assembly of surfactant-like peptides A6K and A9K. Soft Matter 2009, 5, 3870–3878. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, X.; Gong, S.; Feng, L.; Zhong, Y.; Pang, Z. The proton permeability of self-assembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood-brain barrier after modification with lactoferrin. Nanoscale 2014, 6, 3250–3258. [Google Scholar] [CrossRef]
- Al-Halifa, S.; Babych, M.; Zottig, X.; Archambault, D.; Bourgault, S. Amyloid self-assembling peptides: Potential applications in nanovaccine engineering and biosensing. Pept. Sci. 2019, 111, e24095. [Google Scholar] [CrossRef]
- Jacob, R.S.; Ghosh, D.; Singh, P.K.; Basu, S.K.; Jha, N.N.; Das, S.; Sukul, P.K.; Patil, S.; Sathaye, S.; Kumar, A.; et al. Self healing hydrogels composed of amyloid nano fibrils for cell culture and stem cell differentiation. Biomaterials 2015, 54, 97–105. [Google Scholar] [CrossRef]
- Collins, S.J.; Tumpach, C.; Li, Q.X.; Lewis, V.; Ryan, T.M.; Roberts, B.; Drew, S.C.; Lawson, V.A.; Haigh, C.L. The prion protein regulates beta-amyloid-mediated self-renewal of neural stem cells in vitro. Stem Cell Res. Ther. 2015, 6, 60. [Google Scholar] [CrossRef]
- Hellmund, K.S.; Koksch, B. Self-Assembling Peptides as Extracellular Matrix Mimics to Influence Stem Cell’s Fate. Front. Chem. 2019, 7, 172. [Google Scholar] [CrossRef]
- Mehrban, N.; Zhu, B.; Tamagnini, F.; Young, F.I.; Wasmuth, A.; Hudson, K.L.; Thomson, A.R.; Birchall, M.A.; Randall, A.D.; Song, B.; et al. Functionalized α-Helical Peptide Hydrogels for Neural Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 431–439. [Google Scholar] [CrossRef]
- Guo, J.; Leung, K.K.; Su, H.; Yuan, Q.; Wang, L.; Chu, T.H.; Zhang, W.; Pu, J.K.; Ng, G.K.; Wong, W.M.; et al. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine 2009, 5, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Chen, M.H.; Chang, W.H.; Huang, M.Y.; Wang, T.W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013, 34, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Villa, O.; Silva, D.; Gelain, F. 3D culture of adult mouse neural stem cells within functionalized self-assembling peptide scaffolds. Int. J. Nanomedicine 2011, 6, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Gelain, F.; Bottai, D.; Vescovi, A.; Zhang, S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE 2006, 1, e119. [Google Scholar] [CrossRef]
- Zhao, X.; Yao, G.S.; Liu, Y.; Wang, J.; Satkunendrarajah, K.; Fehlings, M. The role of neural precursor cells and self assembling peptides in nerve regeneration. J. Otolaryngol. Head Neck Surg. 2013, 42, 60. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.; Satkunendrarajah, K.; Yao, G.S.; Bayon, Y.; Fehlings, M.G. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013, 9, 8075–8088. [Google Scholar] [CrossRef]
- Zweckberger, K.; Ahuja, C.S.; Liu, Y.; Wang, J.; Fehlings, M.G. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater. 2016, 42, 77–89. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Zadegan, S.A.; Vaccaro, A.R.; Rahimi-Movaghar, V.; Sabzevari, O. Biofunctionalized peptide-based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury 2019, 50, 278–285. [Google Scholar] [CrossRef]
- Tran, K.A.; Partyka, P.P.; Jin, Y.; Bouyer, J.; Fischer, I.; Galie, P.A. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 2020, 104, 76–84. [Google Scholar] [CrossRef]
- Chen, N.; Tian, L.; He, L.; Ramakrishna, S. Nanobiomaterials for neural regeneration. Neural Regen. Res. 2016, 11, 1372–1374. [Google Scholar] [CrossRef]
- Jin, G.; He, R.; Sha, B.; Li, W.; Qing, H.; Teng, R.; Xu, F. Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polymer Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.-H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Mostafavi, H.; Barati, G.; Eskandari, M.; Nadri, S. Differentiation of mesenchymal stem cells -derived trabecular meshwork into dopaminergic neuron-like cells on nanofibrous scaffolds. Biologicals 2017, 50, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Terraf, P.; Babaloo, H.; Kouhsari, S.M. Directed Differentiation of Dopamine-Secreting Cells from Nurr1/GPX1 Expressing Murine Embryonic Stem Cells Cultured on Matrigel-Coated PCL Scaffolds. Mol. Neurobiol. 2017, 54, 1119–1128. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication, characterization and in vitro evaluation of aligned PLGA-PCL nanofibers for neural regeneration. Ann. Biomed. Eng. 2012, 40, 2098–2110. [Google Scholar] [CrossRef]
- Lin, C.; Liu, C.; Zhang, L.; Huang, Z.; Zhao, P.; Chen, R.; Pang, M.; Chen, Z.; He, L.; Luo, C.; et al. Interaction of iPSC-derived neural stem cells on poly(L-lactic acid) nanofibrous scaffolds for possible use in neural tissue engineering. Int. J. Mol. Med. 2018, 41, 697–708. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, P.; Han, Z.; Wei, G.; Zhou, C.; Wen, J.; Su, B.; Wang, X.; Wang, Y. Collagen nanofibers facilitated presynaptic maturation in differentiated neurons from spinal-cord-derived neural stem cells through MAPK/ERK1/2-Synapsin I signaling pathway. Biomacromolecules 2014, 15, 2449–2460. [Google Scholar] [CrossRef]
- Lau, Y.T.; Kwok, L.F.; Tam, K.W.; Chan, Y.S.; Shum, D.K.; Shea, G.K. Genipin-treated chitosan nanofibers as a novel scaffold for nerve guidance channel design. Colloids Surf. B Biointerfaces 2018, 162, 126–134. [Google Scholar] [CrossRef]
- Farzamfar, S.; Salehi, M.; Tavangar, S.M.; Verdi, J.; Mansouri, K.; Ai, A.; Malekshahi, Z.V.; Ai, J. A novel polycaprolactone/carbon nanofiber composite as a conductive neural guidance channel: An in vitro and in vivo study. Prog. Biomater. 2019, 8, 239–248. [Google Scholar] [CrossRef]
- Chang, W.; Shah, M.B.; Zhou, G.; Walsh, K.; Rudraiah, S.; Kumbar, S.G.; Yu, X. Polymeric nanofibrous nerve conduits coupled with laminin for peripheral nerve regeneration. Biomed. Mater. 2020, 15, 035003. [Google Scholar] [CrossRef] [PubMed]
- Satish, A.; Korrapati, P.S. Strategic design of peptide-decorated aligned nanofibers impregnated with triiodothyronine for neural regeneration. J. Tissue Eng. Regen Med. 2019, 13, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Haggerty, A.E.; Yan, J.; Lan, M.; Seu, M.; Yang, M.; Marlow, M.M.; Maldonado-Lasunción, I.; Cho, B.; et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials 2020, 245, 119978. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.B.; Chang, W.; Zhou, G.; Glavy, J.S.; Cattabiani, T.M.; Yu, X. Novel spiral structured nerve guidance conduits with multichannels and inner longitudinally aligned nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1410–1419. [Google Scholar] [CrossRef]
- Thompson, B.C.; Murray, E.; Wallace, G.G. Graphite oxide to graphene. Biomaterials to bionics. Adv. Mater. 2015, 27, 7563–7582. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef]
- Zhang, Z.; Klausen, L.H.; Chen, M.; Dong, M. Electroactive Scaffolds for Neurogenesis and Myogenesis: Graphene-Based Nanomaterials. Small 2018, 14, 1801983. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, Y.; Guo, W.; Liang, X.-J. Biomimetic carbon nanotubes for neurological disease therapeutics as inherent medication. Acta Pharm. Sin. B 2019. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 2011, 32, 9374–9382. [Google Scholar] [CrossRef]
- Solanki, A.; Shah, S.; Memoli, K.A.; Park, S.Y.; Hong, S.; Lee, K.B. Controlling differentiation of neural stem cells using extracellular matrix protein patterns. Small 2010, 6, 2509–2513. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.B.; Kim, Y.; Jeong, J.R.; Lee, M.H.; Kang, K. Neurite guidance on laser-scribed reduced graphene oxide. Nano Lett. 2018, 18, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef]
- Song, Q.; Jiang, Z.; Li, N.; Liu, P.; Liu, L.; Tang, M.; Cheng, G. Anti-inflammatory effects of three-dimensional graphene foams cultured with microglial cells. Biomaterials 2014, 35, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yang, L.; Jiang, Z.; Song, Q.; Xiao, M.; Zhang, D.; Ma, X.; Wen, T.; Cheng, G. Three-dimensional stiff graphene scaffold on neural stem cells behavior. ACS App. Mater. Interfaces 2016, 8, 34227–34233. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S.; Cho, S.-P.; Hong, B.H.; Choung, P.-H.; Chung, T.D. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Song, J.; Zhao, X.; Chen, W.; Ouyang, Y.; Yuan, W.; Fan, C. 3D fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv. Sci. (Wienh) 2018, 5, 1700499. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef]
- Imaninezhad, M.; Pemberton, K.; Xu, F.; Kalinowski, K.; Bera, R.; Zustiak, S.P. Directed and enhanced neurite outgrowth following exogenous electrical stimulation on carbon nanotube-hydrogel composites. J. Neural. Eng. 2018, 15, 056034. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Wu, W.; Park, S.; Miller Ii, A.L.; Terzic, A.; Lu, L. Effective nerve cell modulation by electrical stimulation of carbon nanotube embedded conductive polymeric scaffolds. Biomater. Sci. 2018, 6, 2375–2385. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Lee, J.; Joong, O.K.; Kim, B.-S.; Park, C.H.; Kim, C.S. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater. Sci. Eng. C Mater. Bio. Appl. 2019, 102, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Karabulut, E.; Kuzmenko, V.; Fantini, V.; Pansarasa, O.; Cereda, C.; Gatenholm, P. 3D Printed Conductive Nanocellulose Scaffolds for the Differentiation of Human Neuroblastoma Cells. Cells 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Elnegiry, A.A.; Zaghloul, A.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Farag, A.; Lashen, S.; Rezk, S.; Shouman, Z. Nanotubes impregnated human olfactory bulb neural stem cells promote neuronal differentiation in Trimethyltin-induced neurodegeneration rat model. J. Cell. Physiol. 2017, 232, 3586–3597. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.-S.; Hwang, J.-Y.; Kim, M.S.; Lee, J.-Y.; Kim, J.-W.; Kim, H.-S.; Shin, U.S.; Knowles, J.C.; Kim, H.-W.; Hyun, J.K. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015, 13, 324–334. [Google Scholar] [CrossRef]
- Lee, S.-J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural. Eng. 2018, 15, 016018. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, M.; Scarian, E.; Rey, F.; Gagliardi, S.; Carelli, S.; Pansarasa, O.; Cereda, C. Biomaterials in Neurodegenerative Disorders: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2020, 21, 3243. https://doi.org/10.3390/ijms21093243
Bordoni M, Scarian E, Rey F, Gagliardi S, Carelli S, Pansarasa O, Cereda C. Biomaterials in Neurodegenerative Disorders: A Promising Therapeutic Approach. International Journal of Molecular Sciences. 2020; 21(9):3243. https://doi.org/10.3390/ijms21093243
Chicago/Turabian StyleBordoni, Matteo, Eveljn Scarian, Federica Rey, Stella Gagliardi, Stephana Carelli, Orietta Pansarasa, and Cristina Cereda. 2020. "Biomaterials in Neurodegenerative Disorders: A Promising Therapeutic Approach" International Journal of Molecular Sciences 21, no. 9: 3243. https://doi.org/10.3390/ijms21093243
APA StyleBordoni, M., Scarian, E., Rey, F., Gagliardi, S., Carelli, S., Pansarasa, O., & Cereda, C. (2020). Biomaterials in Neurodegenerative Disorders: A Promising Therapeutic Approach. International Journal of Molecular Sciences, 21(9), 3243. https://doi.org/10.3390/ijms21093243