Functional Analysis of Steroidogenic Factor 1 (sf-1) and 17α-Hydroxylase/Lyase (cyp17α) Promoters in Yellow Catfish Pelteobagrus fulvidraco
Abstract
:1. Introduction
2. Results
2.1. Cloning and Sequence Analysis of sf-1 and cyp17α Promoters
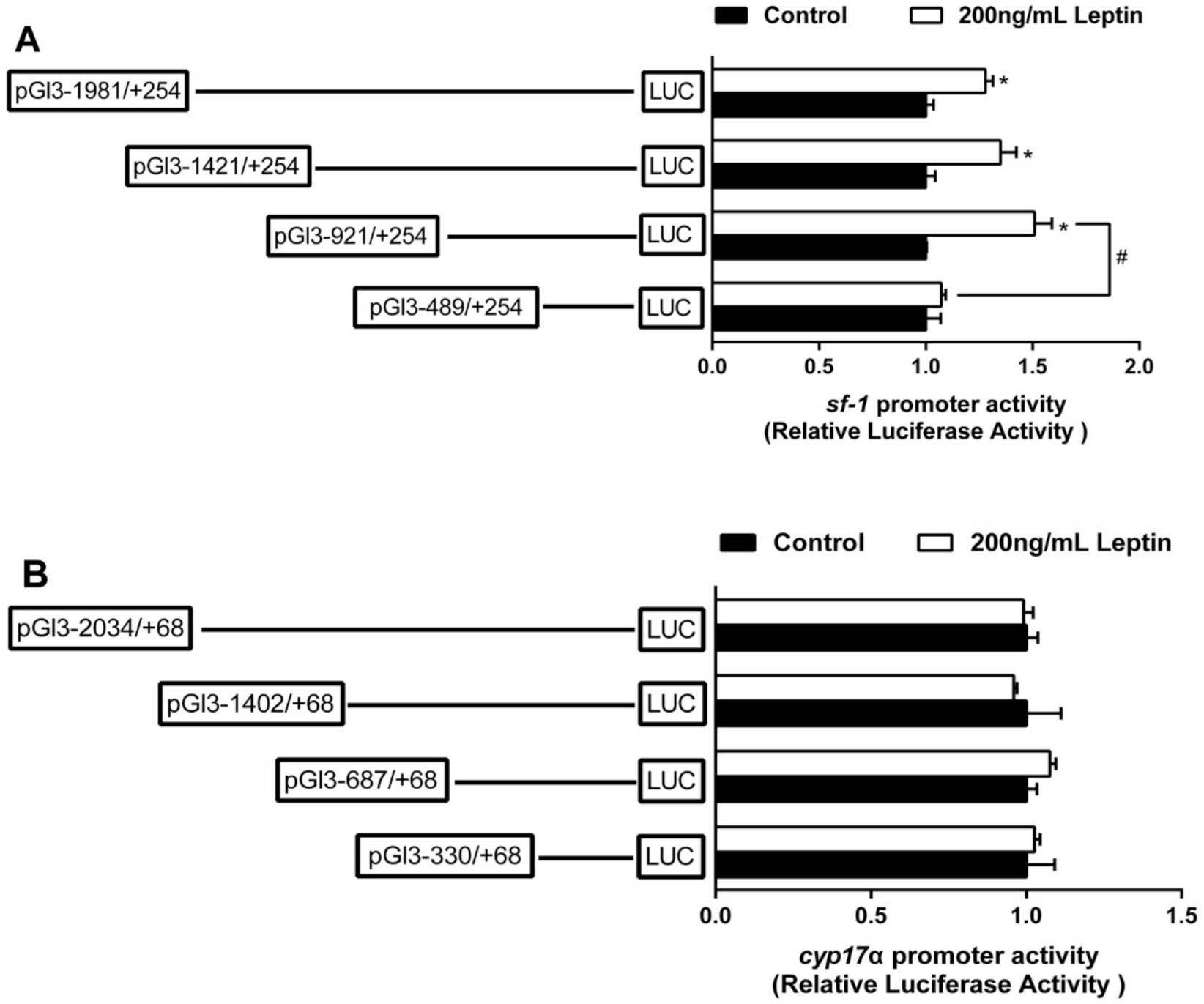
2.2. The Relative Luciferase Activity of 5’-Deletion Assay of the Regions of sf-1 and cyp17α Promoters
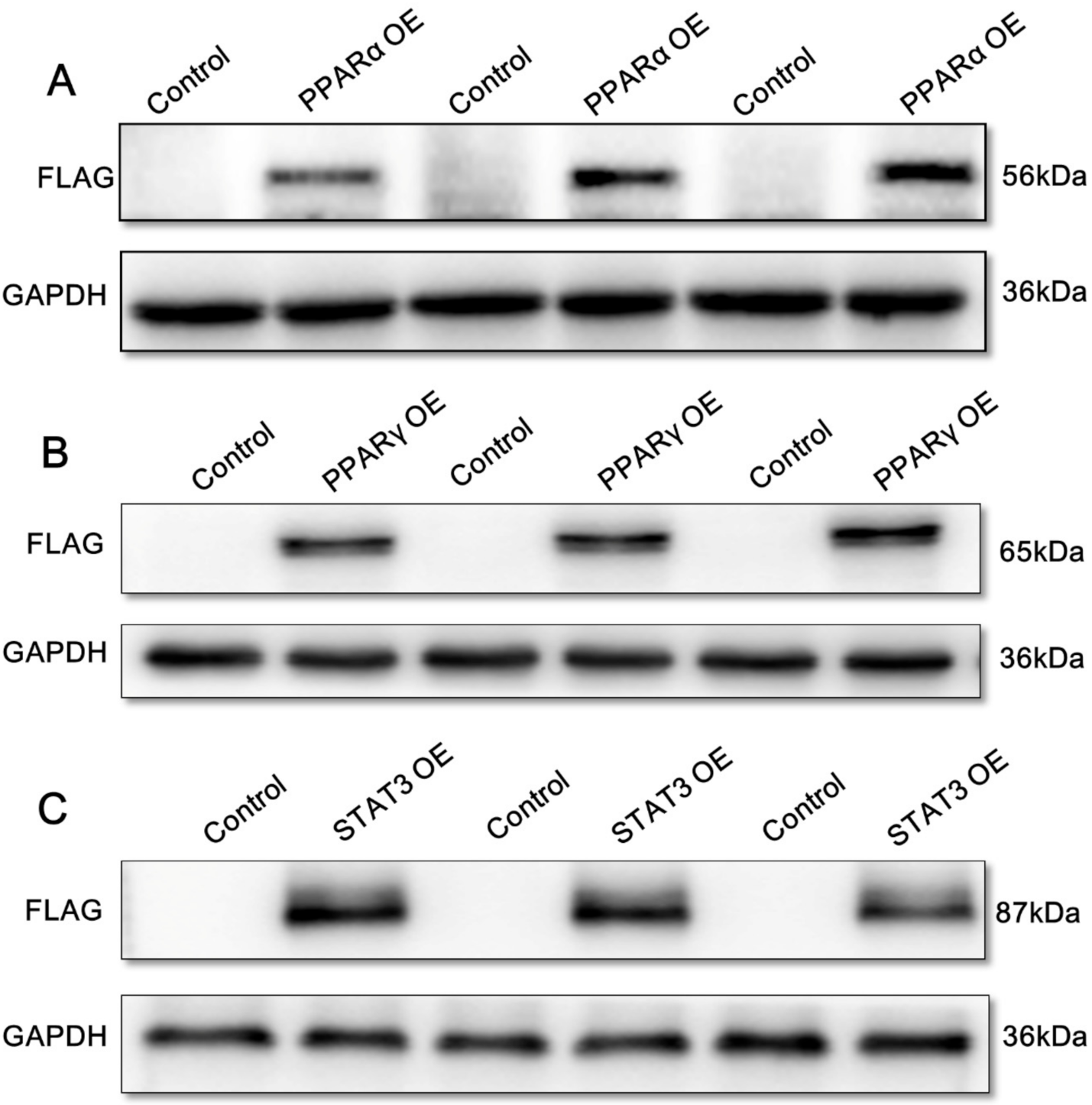
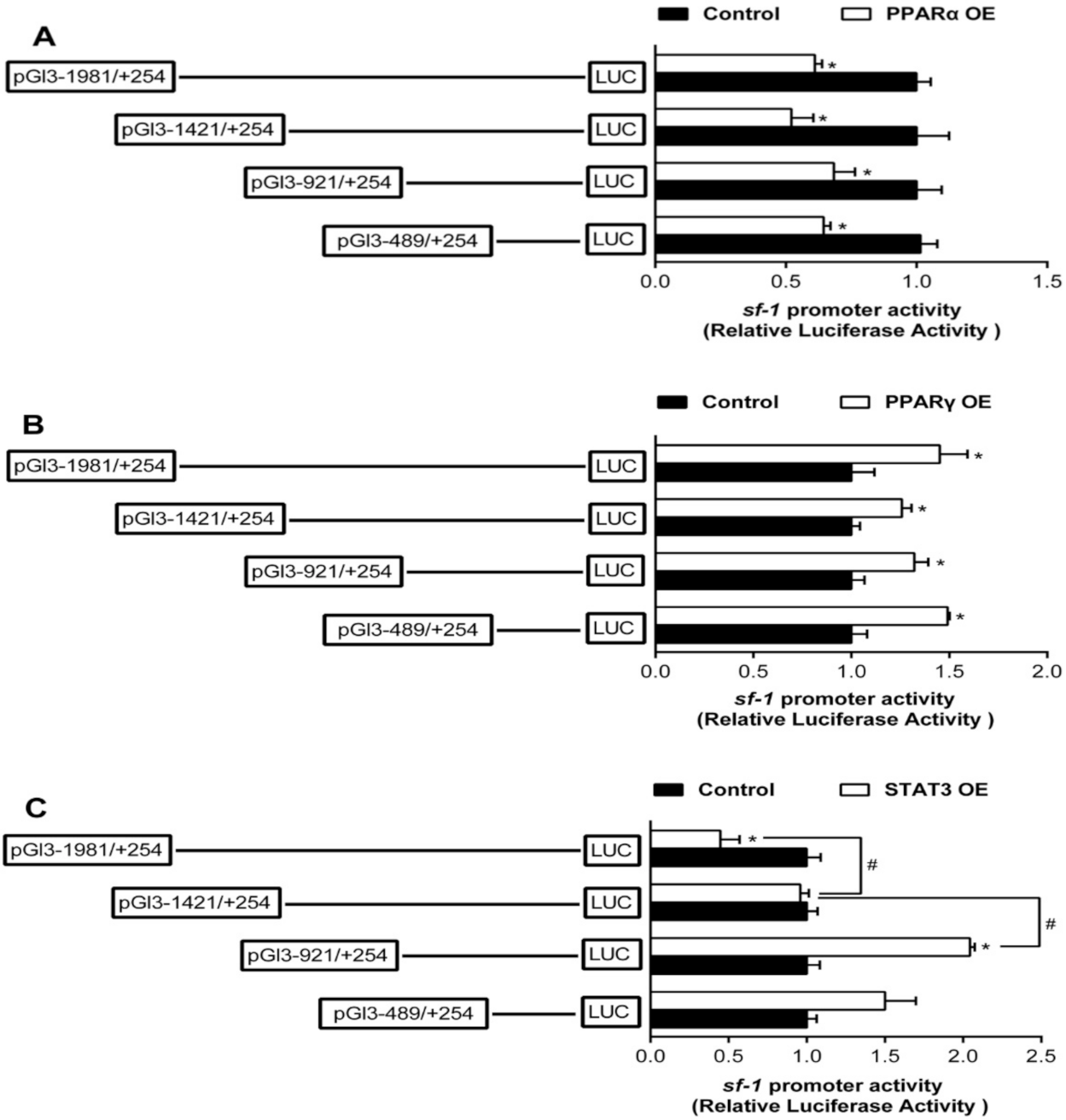
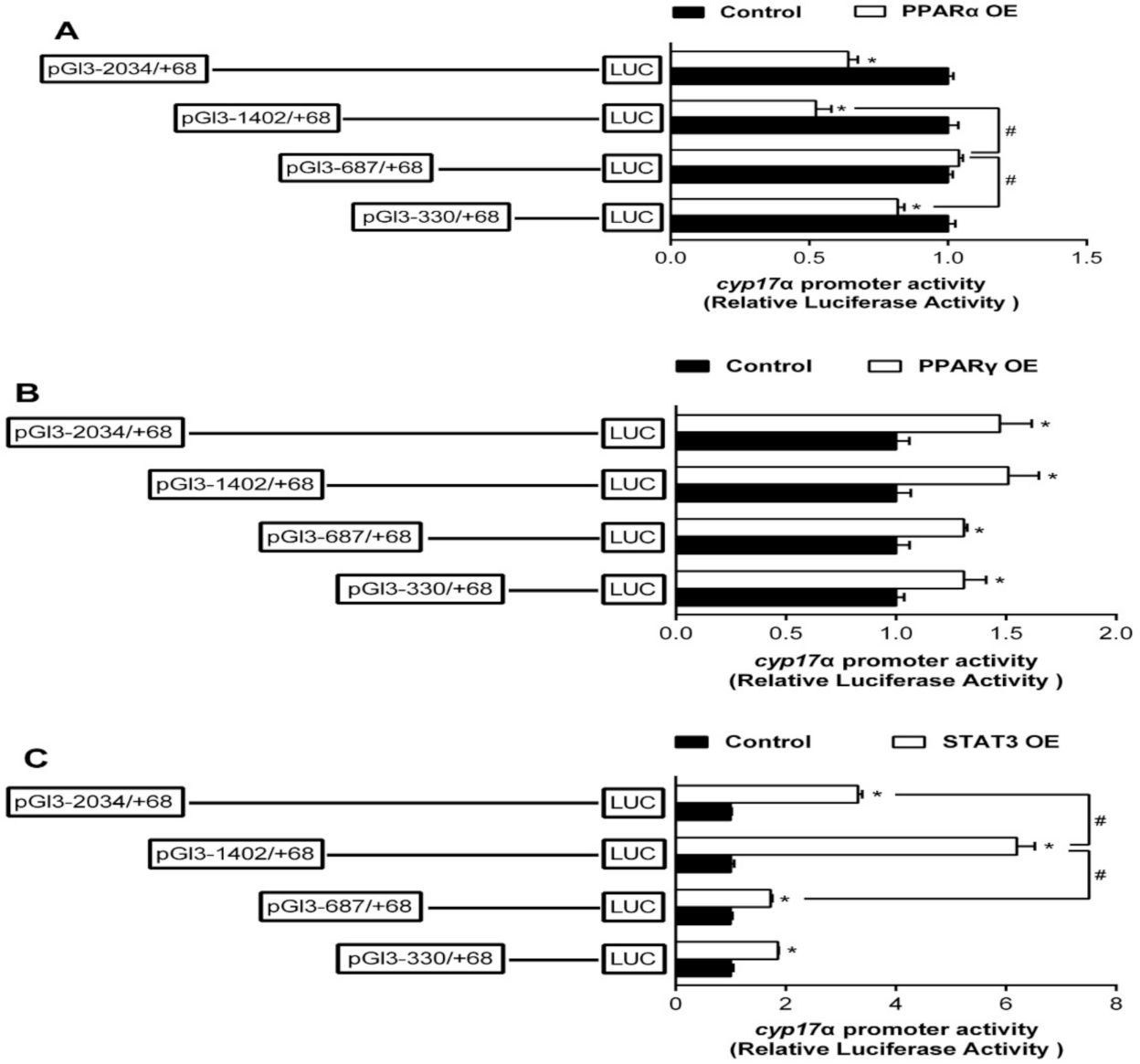
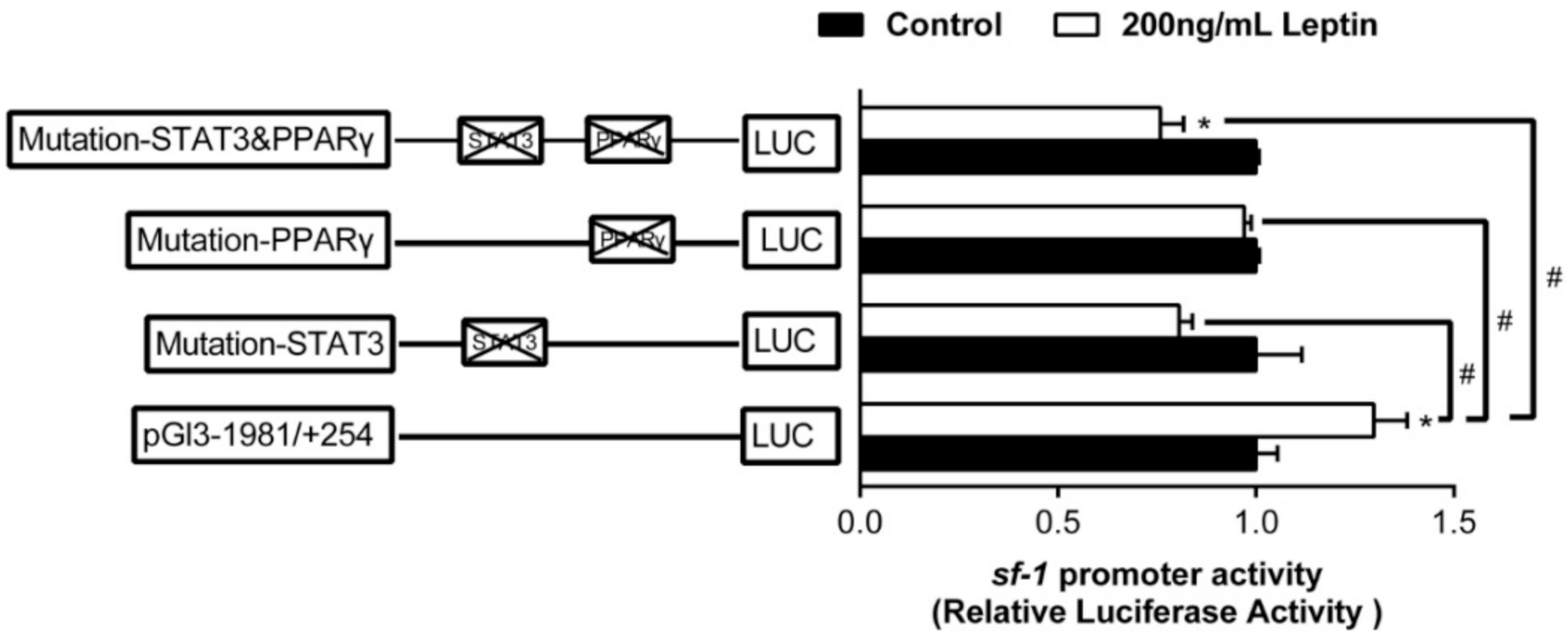
2.3. Overexpression Analysis of Yellow Catfish PPARα, PPARγ, and STAT3 of the Regions of sf-1 and cyp17α Promoters in HEK293T Cells
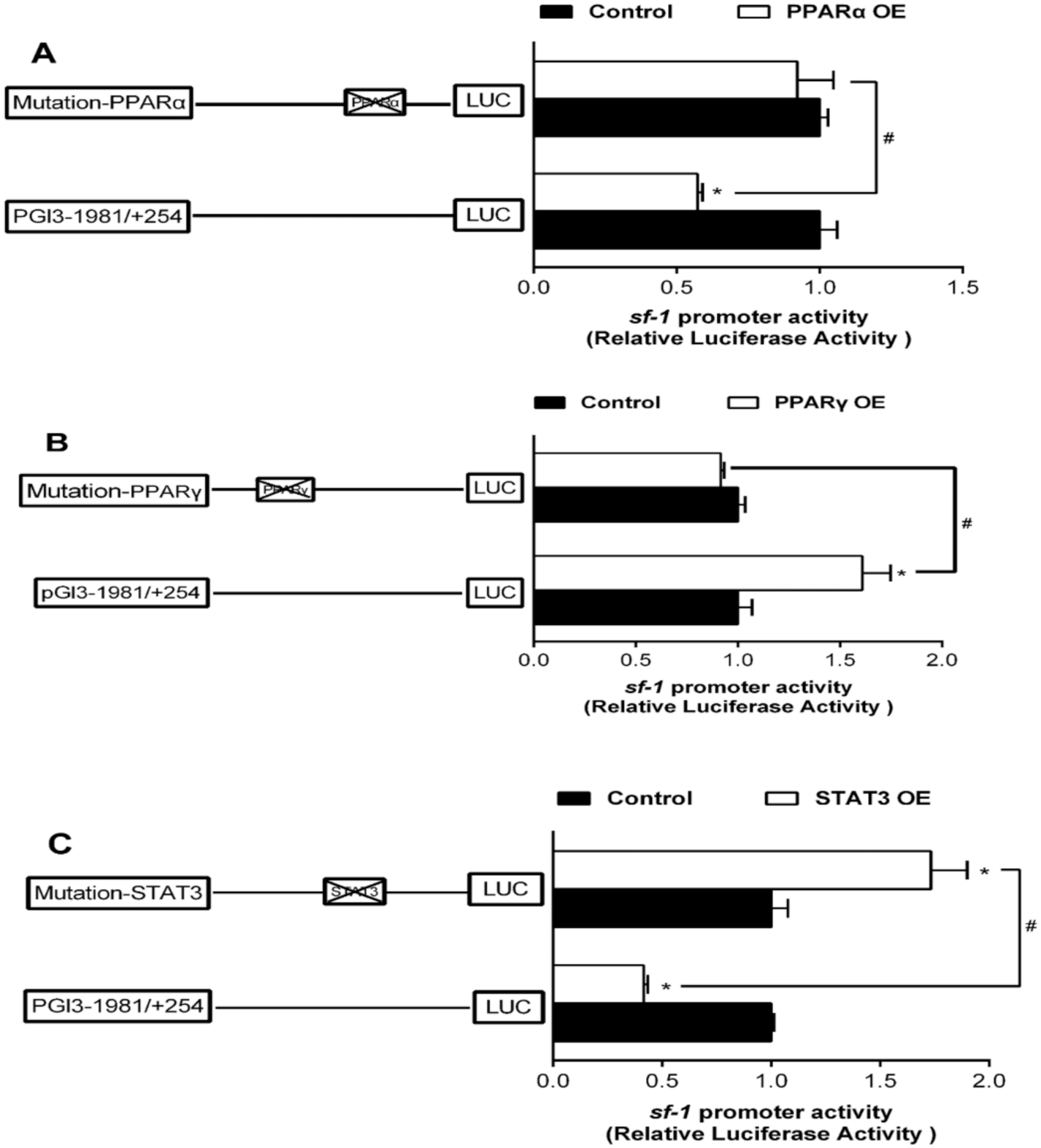
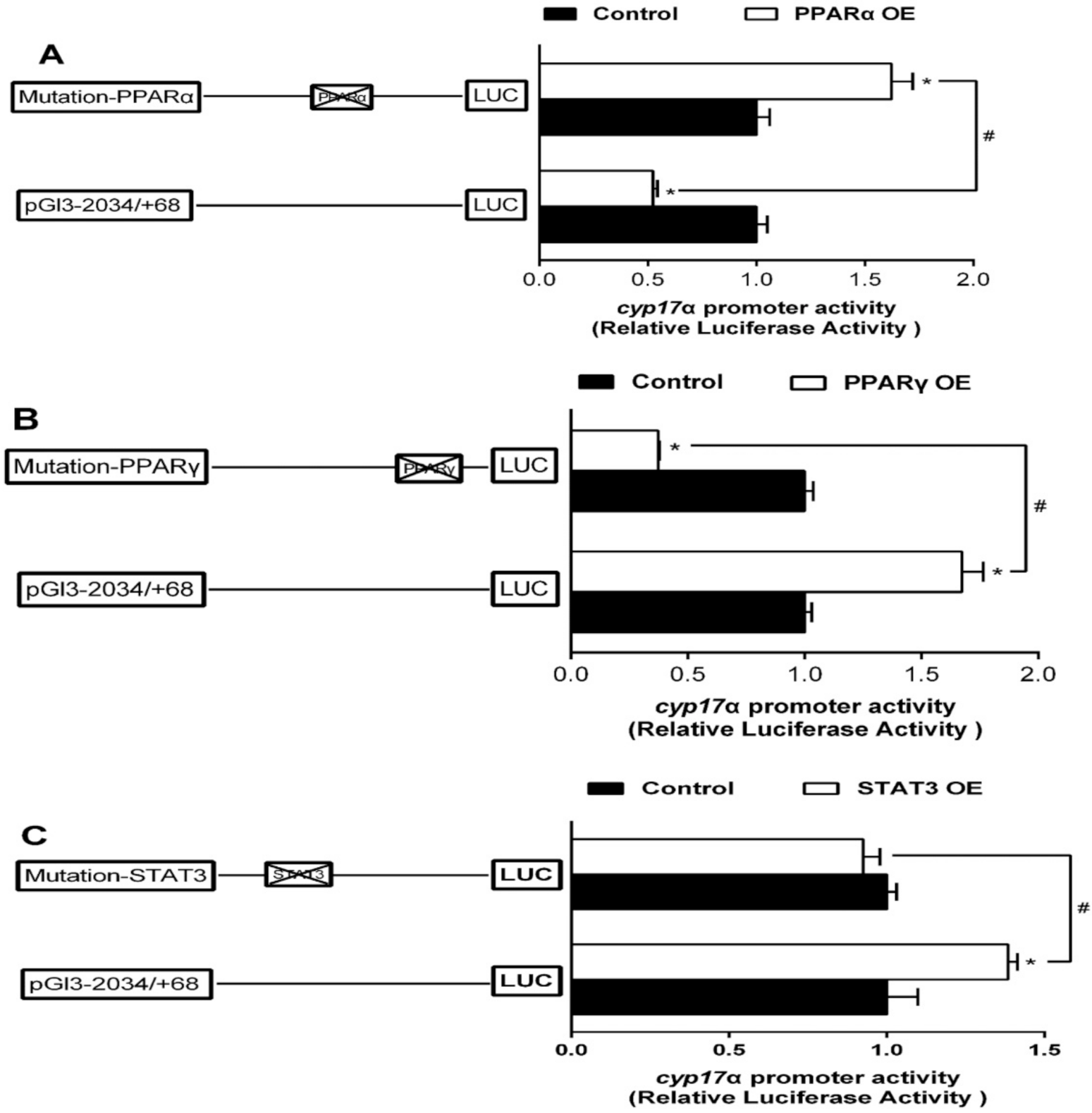
2.4. Site-Mutation Analysis of PPARα, PPARγ, and STAT3 on the Promoters of sf-1 and cyp17α
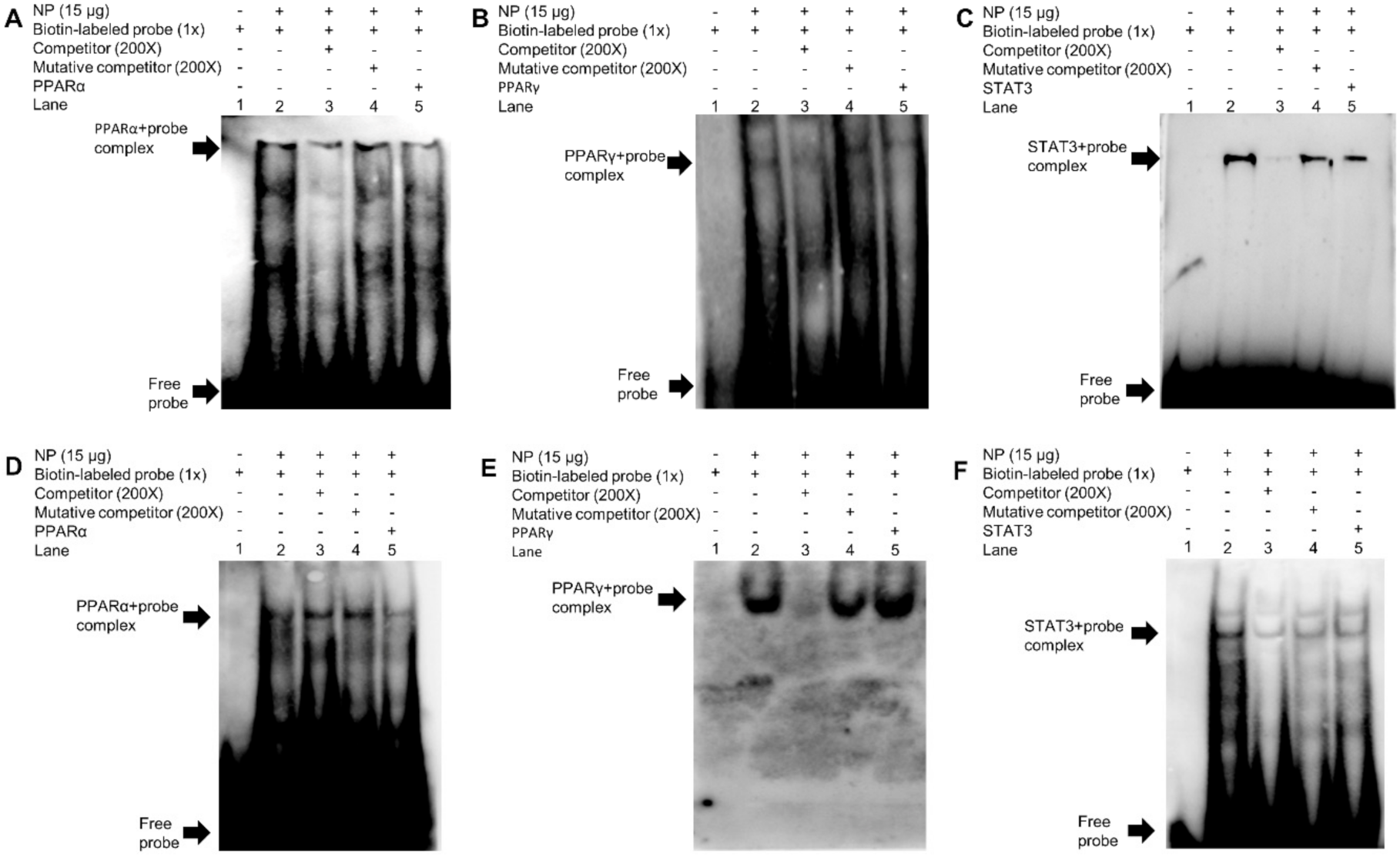
2.5. EMSA Analysis of Binding Sequence of Transcription Factors
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Reagents
4.2. Promoter Cloning and Plasmids Construction
4.3. Sequence Analysis
4.4. Plasmid Transfections and Assays of Luciferase Activities
4.5. Protein Expression of Yellow Catfish PPARα, PPARγ, and STAT3 in HEK293T Cells
4.6. Site-Mutation Analysis of Binding Sites on the sf-1 and cyp17α Promoters
4.7. Electrophoretic Mobility-Shift Assay (EMSA)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tokarz, J.; Möller, G.; de Angelis, M.H.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Plummer, S.; Sharpe, R.M.; Hallmark, N.; Mahood, I.K.; Elcombe, C. Time-dependent and compartment-specific effects of in utero exposure to di (n-butyl) phthalate on gene/protein expression in the fetal rat testis as revealed by transcription profiling and laser capture microdissection. Toxicol. Sci. 2007, 97, 520–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.P. Steroidogenic Factor 1 (SF-1) Is Essential for Steroidogenesis, Gonadal Development and Sex Differentiation in Nile Tilapia. Ph.D. Thesis, Southwest University, Chongqing, China, 2016. [Google Scholar]
- Qin, F.; Shen, T.; Li, J.; Qian, J.; Zhang, J.; Zhou, G.; Tong, J. SF-1 mediates reproductive toxicity induced by Cerium oxide nanoparticles in male mice. J. Nanobiotechnol. 2019, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Thitiphuree, T.; Nagasawa, K.; Osada, M. Molecular identification of steroidogenesis-related genes in scallops and their potential roles in gametogenesis. J. Steroid. Biochem. Mol. Biol. 2019, 186, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Woodson, K.G.; Crawford, P.A.; Sadovsky, Y.; Milbrandt, J. Characterization of the promoter of SF-1, an orphan nuclear receptor required for adrenal and gonadal development. Mol. Endocrinol. 1997, 11, 117–126. [Google Scholar] [CrossRef]
- Scherrer, S.P.; Rice, D.A.; Heckert, L.L. Expression of steroidogenic factor 1 in the testis requires an interactive array of elements within its proximal promoter. Biol. Reprod. 2002, 67, 1509–1521. [Google Scholar] [CrossRef] [Green Version]
- Meinsohn, M.C.; Smith, O.E.; Bertolin, K.; Murphy, B.D. The orphan nuclear receptors steroidogenic factor-1 and liver receptor homolog-1: Structure, regulation, and essential roles in mammalian reproduction. Physiol. Rev. 2019, 99, 1249–1279. [Google Scholar] [CrossRef]
- Puttabyatappa, M.; Vande Voort, C.A.; Chaffin, C.L. hCG-induced down-regulation of PPARγ and liver X receptors promotes periovulatory progesterone synthesis by macaque granulosa cells. Endocrinology 2010, 151, 5865–5872. [Google Scholar] [CrossRef] [Green Version]
- Gatta, V.; Tatone, C.; Ciriminna, R.; Vento, M.; Franchi, S.; d’Aurora, M.; Stuppia, L. Gene expression profiles of cumulus cells obtained from women treated with recombinant human luteinizing hormone+ recombinant human follicle-stimulating hormone or highly purified human menopausal gonadotropin versus recombinant human follicle-stimulating hormone alone. Fertil. Steril. 2013, 99, 2000–2008. [Google Scholar]
- Tatone, C.; Benedetti, E.; Vitti, M.; Di Emidio, G.; Ciriminna, R.; Vento, M.E.; Cela, V.; Borzi, P.; Certa, G.; Lispi, M.; et al. Modulating intrafollicular hormonal milieu in controlled ovarian stimulation: Insights from PPAR expression in human granulosa cells. J. Cell. Physiol. 2016, 231, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Landry, D.A.; Sormany, F.; Haché, J.; Roumaud, P.; Martin, L.J. Steroidogenic genes expressions are repressed by high levels of leptin and the JAK/STAT signaling pathway in MA-10 Leydig cells. Mol. Cell. Biochem. 2017, 433, 79–95. [Google Scholar] [CrossRef]
- Seroussi, E.; Knytl, M.; Pitel, F.; Elleder, D.; Krylov, V.; Leroux, S.; Morisson, M.; Yosefi, S.; Miyara, S.; Ganesan, S.; et al. Avian Expression Patterns and Genomic Mapping Implicate Leptin in Digestion and TNF in Immunity, Suggesting That Their Interacting Adipokine Role Has Been Acquired Only in Mammals. Int. J. Mol. Sci. 2019, 20, 4489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, J.E.; Dunn, R.L.; Daniel, M.C.; Janis, G.M.; Smith, G.D. Direct effects of leptin on mouse reproductive function: Regulation of follicular, oocyte, and embryo development. Biol. Reprod. 2004, 71, 1446–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.A.; Sivakumar, A.V.N.; Pathipati, D.; Chakravarthi, V.P.; Brahmaiah, K.V.; Rao, V.H. Leptin induced in vitro development of ovarian follicles in sheep is related to the expression of P450 aromatase and steroidogenesis. Theriogenology 2019, 136, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J.; Francisco, C.C. The adipose obese gene product, leptin: Evidence of a direct inhibitory role in ovarian function. Endocrinology 1997, 138, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Vogel, K.; Weitsman, S.R.; Magoffin, D.A. Leptin antagonizes the insulin-like growth factor-i augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J. Clin. Endocr. Metab. 1999, 84, 1072–1076. [Google Scholar] [CrossRef]
- Ruiz-Cortes, Z.T.; Martel-Kennes, Y.; Nicolas, Y.G.; Downey, B.R.; Murphy, B.D. Biphasic effects of leptin in porcine granulosa cells. Biol. Reprod. 2003, 68, 789–796. [Google Scholar] [CrossRef]
- Gregoraszczuk, E.L.; Rak, A. Superactive human leptin antagonist reverses leptin-induced excessive progesterone and testosterone secretion in porcine ovarian follicles by blocking leptin receptors. J. Physiol. Pharmacol. 2015, 66, 39–46. [Google Scholar]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.; Wang, Y.; Neel, B.G. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef]
- Wu, K.; Tan, X.Y.; Xu, Y.H.; Chen, Q.L.; Pan, Y.X. JAK and STAT members of yellow catfish Pelteobagrus fulvidraco and their roles in leptin affecting lipid metabolism. Gen. Comp. Endocrinol. 2016, 226, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Tan, X.Y.; Xu, Y.H.; Chen, G.H.; Zhuo, M.Q. Functional Analysis of Promoters of Genes in Lipid Metabolism and Their Transcriptional Response to STAT3 under Leptin Signals. Genes 2018, 9, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solberg, N.; Krauss, S. Luciferase assay to study the activity of a cloned promoter DNA fragment. Methods Mol. Biol. 2013, 977, 65–78. [Google Scholar] [PubMed]
- Delhove, J.M.K.M.; Karda, R.; Hawkins, K.E.; FitzPatrick, L.M.; Waddington, S.N.; McKay, T.R. Bioluminescence monitoring of promoter activity in vitro and in vivo. Methods Mol. Biol. 2017, 1651, 49–64. [Google Scholar] [PubMed]
- De Miguel, F.; Lee, S.O.; Onate, S.A.; Gao, A.C. Stat3 enhances transactivation of steroid hormone receptors. Nucl. Recept. 2003, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, J.A.; Tjian, R. 2010. Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat. Rev. Genet. 2010, 11, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Smale, S.T.; Kadonaga, J.T. The RNA polymerase II core promoter. Annu. Rev. Biochem. 2003, 72, 449–479. [Google Scholar] [CrossRef] [Green Version]
- Wierstra, I. Sp1: Emerging roles-beyond constitutive activation of TATA-less housekeeping genes. Biochem. Biophys. Res. Commun. 2008, 372, 1–13. [Google Scholar] [CrossRef]
- Chan, T.M.; Leung, K.S.; Lee, K.H. TFBS identification based on genetic algorithm with combined representations and adaptive post-processing. Bioinformatics 2007, 24, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Lovekamp-Swan, T.; Davis, B.J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003, 111, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Kurzynska, A.; Bogacki, M.; Chojnowska, K.; Bogacka, I. Peroxisome proliferator activated receptor ligands affect progesterone and 17β-estradiol secretion by porcine corpus luteum during early pregnancy. J. Physiol. Pharmacol. 2014, 65, 709–717. [Google Scholar] [PubMed]
- Gorissen, M.; de Vrieze, E.; Flik, G.; Huising, M.O. STAT genes display differential evolutionary rates that correlate with their roles in the endocrine and immune system. J. Endocrinol. 2011, 209, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Álvarez, M.; García-García, R.M.; Torres-Rovira, L.; Gonzalez-Bulnes, A.; Rebollar, P.G.; Lorenzo, P.L. Influence of leptin on in vitro maturation and steroidogenic secretion of cumulus-oocyte complexes through JAK2/STAT3 and MEK 1/2 pathways in the rabbit model. Reproduction 2010, 139, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, K.P.; Tanphaichitr, N. Mechanisms of obesity-induced male infertility. Expert. Rev. Endocrinol. Metab. 2010, 5, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Roumaud, P.; Martin, L.J. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm. Mol. Biol. Clin. Investig. 2015, 24, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.F.; Wu, K.; Tan, X.Y.; Zhang, L.H.; Zhuo, M.Q.; Pan, Y.X.; Chen, Q.L. Effects of recombinant human leptin administration on hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco: In vivo and in vitro studies. Gen. Comp. Endocrinol. 2015, 212, 92–99. [Google Scholar] [CrossRef]
- Song, Y.F.; Tan, X.Y.; Pan, Y.X.; Zhang, L.H.; Chen, Q.L. 2018. Fatty acid ß-oxidation is essential in leptin-mediated oocytes maturation of yellow catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2018, 19, 1457. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, H.; Zigman, J.M.; Ye, C.; Lee, C.E.; Lowell, B.B. Leptin directly activates sf1 neurons in the vmh, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006, 49, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Hausman, G.J.; Barb, C.R.; Lents, C.A. Leptin and reproductive function. Biochimie 2012, 94, 2075–2081. [Google Scholar] [CrossRef]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Reshma, R.; Mishra, S.R.; Thakur, N.; Parmar, M.S.; Somal, A.; Bharti, M.K.; Pandey, S.; Chouhan, V.S.; Verma, M.R.; Singh, G.; et al. Modulatory role of leptin on ovarian functions in water buffalo (bubalus bubalis). Theriogenology 2016, 86, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Luo, Z.; Wu, K.; Fan, Y.F.; You, W.J.; Zhang, L.H. Structure and functional analysis of promoters from two liver isoforms of CPT I in grass carp Ctenopharyngodon idella. Int. J. Mol. Sci. 2017, 18, 2405. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.C.; Luo, Z.; Hogstrand, C.; Xu, Y.H.; Wu, L.X.; Chen, G.H.; Song, Y.F. Zinc reduces hepatic lipid deposition and activates lipophagy via Zn2+/MTF-1/PPARα and Ca2+/CaMKKβ/AMPK pathways. FASEB J. 2018, 32, 6666–6680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wu, K.; Hogstrand, C.; Xu, Y.H.; Chen, G.H.; Wei, C.C.; Luo, Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell. Mol. Life Sci. 2020, 77, 1987–2003. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.-H.; Chen, G.-H.; Zhuo, M.-Q.; Xu, Y.-H.; Xu, Y.-C.; Tan, X.-Y. Functional Analysis of Steroidogenic Factor 1 (sf-1) and 17α-Hydroxylase/Lyase (cyp17α) Promoters in Yellow Catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2021, 22, 195. https://doi.org/10.3390/ijms22010195
Lv W-H, Chen G-H, Zhuo M-Q, Xu Y-H, Xu Y-C, Tan X-Y. Functional Analysis of Steroidogenic Factor 1 (sf-1) and 17α-Hydroxylase/Lyase (cyp17α) Promoters in Yellow Catfish Pelteobagrus fulvidraco. International Journal of Molecular Sciences. 2021; 22(1):195. https://doi.org/10.3390/ijms22010195
Chicago/Turabian StyleLv, Wu-Hong, Guang-Hui Chen, Mei-Qin Zhuo, Yi-Huan Xu, Yi-Chuang Xu, and Xiao-Ying Tan. 2021. "Functional Analysis of Steroidogenic Factor 1 (sf-1) and 17α-Hydroxylase/Lyase (cyp17α) Promoters in Yellow Catfish Pelteobagrus fulvidraco" International Journal of Molecular Sciences 22, no. 1: 195. https://doi.org/10.3390/ijms22010195




