Optimal Brassinosteroid Levels Are Required for Soybean Growth and Mineral Nutrient Homeostasis
Abstract
:1. Introduction
2. Results
2.1. Optimal BR Levels Are Required for Soybean Growth
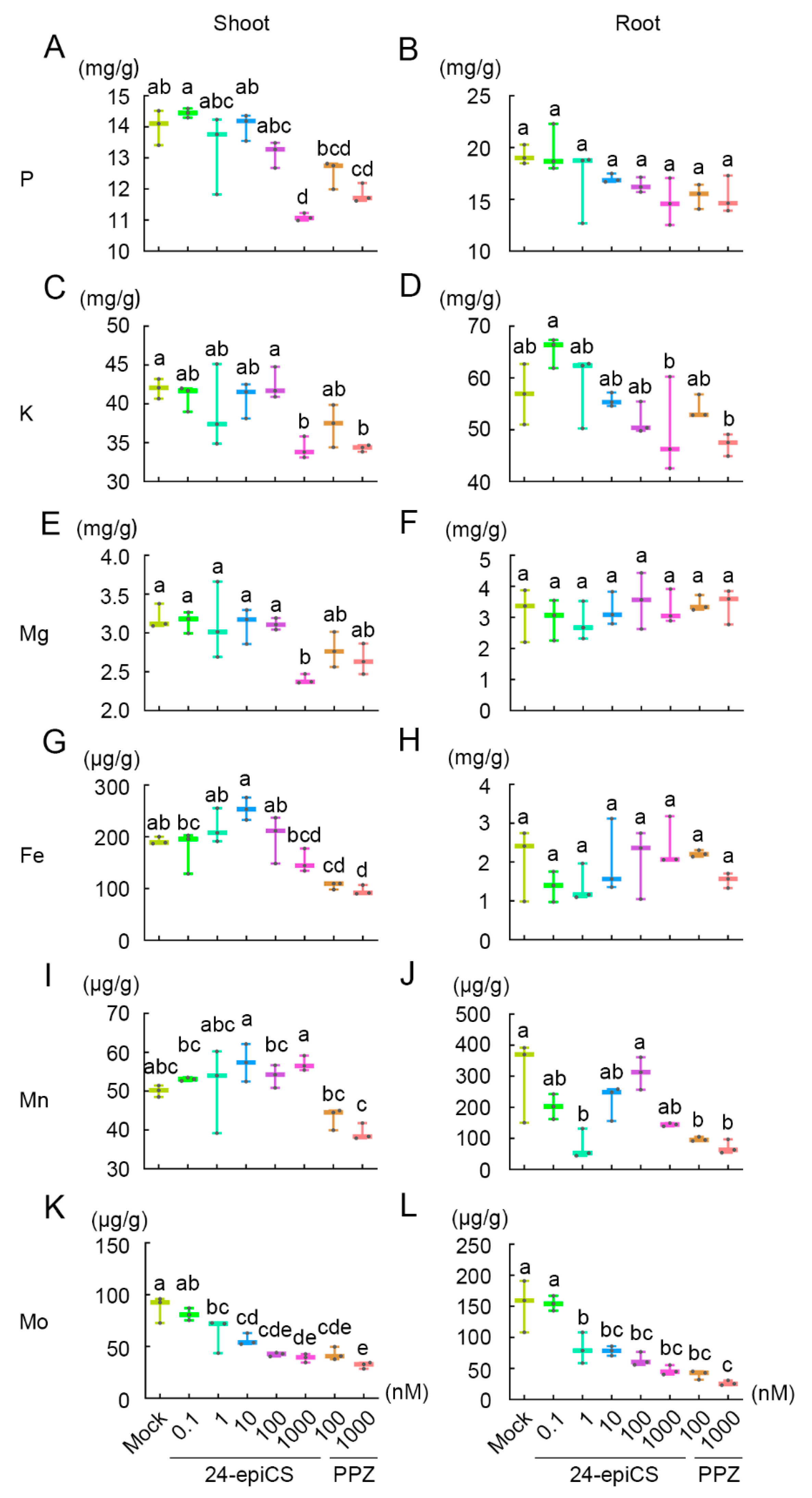
2.2. BR Regulates the Homeostasis of Nutrient Elements
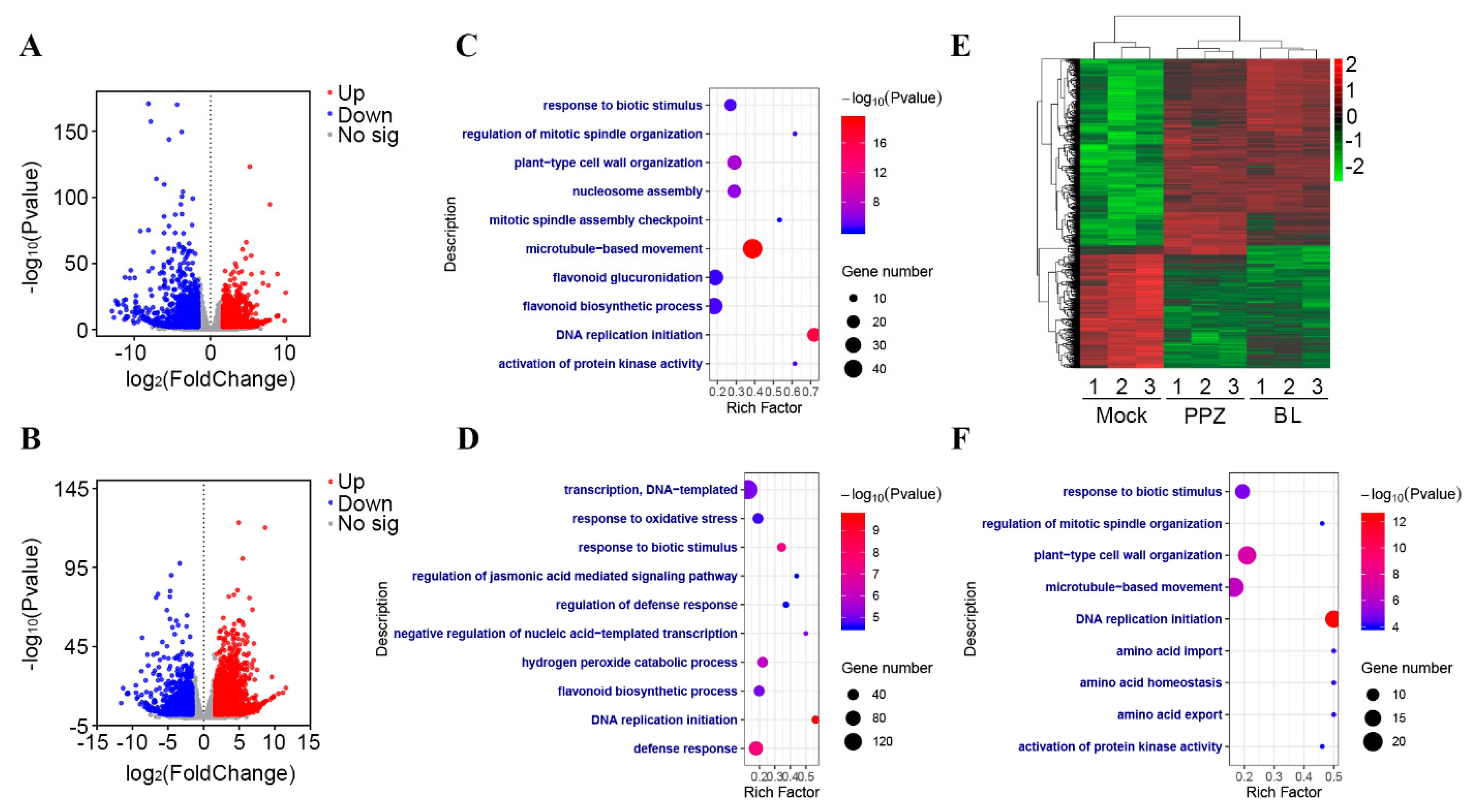
2.3. Transcriptomic Changes in the Soybean Root Tip by BR
2.4. Quantitative RT-PCR Analysis of Mineral Transporter Genes
3. Discussion
3.1. BR May Regulate Nutrient Concentration through the Transcriptional Regulation of Mineral Transporter Genes
3.2. BR Regulates the Nutrient Distribution between the Shoot and Root
4. Conclusions
5. Materials and Methods
5.1. Soybean Growth and Phenotype Analysis
5.2. Element Concentration Measurement
5.3. RNA Isolation, Library Construction, and RNA Sequencing
5.4. RNA-Seq Reads’ Mapping and Differential Counting
5.5. Functional Annotation and Gene Ontology (GO) Enrichment
5.6. Primer Design and Validation of the RNA-Seq Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 24-epiCS | 24-epicastasterone |
| BL | Brassinolide |
| BR | Brassinosteroid |
| BRs | Brassinosteroids |
| DEGs | Differentially expressed genes |
| GEO | Gene Expression Omnibus |
| GO | Gene ontology |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| KT | POTASSIUM (K) TRANSPORTER |
| MOT1 | MOLYBDATE TRANSPORTER 1 |
| PHT1 | PHOSPHATE TRANSPORTER 1 |
| PPZ | Propiconazole |
| VIT2 | VACUOLAR IRON TRANSPORTER 2 |
| ZIP | ZINC REGULATED TRANSPORTER/IRON-REGULATED TRANSPORTER-RELATED PROTEIN |
References
- Clouse, S.D.; Sasse, J.M. BRASSINOSTEROIDS: Essential regulators of plant growth and development. Annu Rev. Plant. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Fan, X.Y.; Cao, D.M.; Tang, W.; He, K.; Zhu, J.Y.; He, J.X.; Bai, M.Y.; Zhu, S.; Oh, E.; et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 2010, 19, 765–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, W.J. Influence of root-applied epibrassinolide and carbenoxolone on the nodulation and growth of soybean (Glycine max L.) seedlings. J. Agron. Crop. Sci. 2001, 186, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Li, G.; Zhang, W.H. Brassinosteroids are involved in Fe homeostasis in rice (Oryza sativa L.). J. Exp. Bot. 2015, 66, 2749–2761. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Wang, L.; Li, X. GmBEHL1, a BES1/BZR1 family protein, negatively regulates soybean nodulation. Sci. Rep. 2018, 8, 7614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhai, Z.; Tian, X.; Duan, L.; Li, Z. Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant. Growth Regul. 2008, 56, 257–264. [Google Scholar] [CrossRef]
- Ariyoshi, Y.; Itoyama, H.; Nakagawa, A.C.S.; Ario, N.; Kondo, Y.; Tomita, Y.; Tanaka, S.; Nakashima, M.; Tomioka, K.; Iwaya-Inoue, M.; et al. Regulation of brassinosteroid on pod growth through cell hypertrophy in soybean (Glycine max (L.) Merr.). Plant. Growth Regul. 2016, 80, 391–395. [Google Scholar] [CrossRef]
- Terakado, J.; Fujihara, S.; Goto, S.; Kuratani, R.; Suzuki, Y.; Yoshida, S.; Yoneyama, T. Systemic effect of a brassinosteroid on root nodule formation in soybean as revealed by the application of brassinolide and brassinazole. Soil Sci. Plant. Nutr. 2005, 51, 389–395. [Google Scholar] [CrossRef]
- Cheng, X.; Gou, X.; Yin, H.; Mysore, K.S.; Li, J.; Wen, J. Functional characterisation of brassinosteroid receptor MtBRI1 in Medicago truncatula. Sci. Rep. 2017, 7, 9327. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.J.; Ross, J.J.; Reid, J.B. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant. Physiol. 2005, 138, 2396–2405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yang, X.; Cheng, L.; Guo, Z.; Wang, H.; Wu, W.; Shin, K.; Zhu, J.; Zheng, X.; Bian, J.; et al. Physiological and transcriptomic analyses of brassinosteroid function in moso bamboo (Phyllostachys edulis) seedlings. Planta 2020, 252, 27. [Google Scholar] [CrossRef]
- Song, L.; Chen, W.; Yao, Q.; Guo, B.; Valliyodan, B.; Wang, Z.; Nguyen, H.T. Genome-wide transcriptional profiling for elucidating the effects of brassinosteroids on Glycine max during early vegetative development. Sci. Rep. 2019, 9, 16085. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Zhang, X.; Sun, S.; Wu, C.; Hou, W.; Wang, Q.; Han, T. Functional analysis of GmCPDs and investigation of their roles in flowering. PLoS ONE 2015, 10, e0118476. [Google Scholar] [CrossRef]
- Peng, S.; Tao, P.; Xu, F.; Wu, A.; Huo, W.; Wang, J. Functional Characterization of Soybean Glyma04g39610 as a Brassinosteroid Receptor Gene and Evolutionary Analysis of Soybean Brassinosteroid Receptors. Int. J. Mol. Sci. 2016, 17, 897. [Google Scholar] [CrossRef]
- Song, L.; Chen, W.; Wang, B.; Yao, Q.M.; Valliyodan, B.; Bai, M.Y.; Zhao, M.Z.; Ye, H.; Wang, Z.Y.; Nguyen, H.T. GmBZL3 acts as a major BR signaling regulator through crosstalk with multiple pathways in Glycine max. BMC Plant. Biol. 2019, 19, 86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Y.J.; Yang, B.J.; Yu, X.X.; Wang, D.; Zu, S.H.; Xue, H.W.; Lin, W.H. Functional characterization of GmBZL2 (AtBZR1 like gene) reveals the conserved BR signaling regulation in Glycine max. Sci. Rep. 2016, 6, 31134. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Fridman, Y.; Friedlander-Shani, L.; Tarkowska, D.; Strnad, M.; Savaldi-Goldstein, S. Activity of the brassinosteroid transcription factors BRASSINAZOLE RESISTANT1 and BRASSINOSTEROID INSENSITIVE1-ETHYL METHANESULFONATE-SUPPRESSOR1/BRASSINAZOLE RESISTANT2 blocks developmental reprogramming in response to low phosphate availability. Plant. Physiol. 2014, 166, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Athar, H.; Ashraf, M. Influence of exogenously applied brassinosteroids on the mineral nutrient status of two wheat cultivars grown under saline conditions. Pak. J. Bot. 2006, 38, 1621–1632. [Google Scholar]
- Fariduddin, Q.; Ahmed, M.; Mir, B.A.; Yusuf, M.; Khan, T.A. 24-Epibrassinolide mitigates the adverse effects of manganese induced toxicity through improved antioxidant system and photosynthetic attributes in Brassica juncea. Environ. Sci. Pollut. Res. Int 2015, 22, 11349–11359. [Google Scholar] [CrossRef]
- Liu, J.; Gao, H.; Wang, X.; Zheng, Q.; Wang, C.; Wang, X.; Wang, Q. Effects of 24-epibrassinolide on plant growth, osmotic regulation and ion homeostasis of salt-stressed canola. Plant. Biol. 2014, 16, 440–450. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, S.; Shu, S.; Sun, J.; Guo, S. Regulation of 2,4-epibrassinolide on mineral nutrient uptake and ion distribution in Ca(NO3)2 stressed cucumber plants. J. Plant. Physiol. 2015, 188, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, T.; Corvalan, C.; Best, N.B.; Budka, J.S.; Zhu, J.Y.; Choe, S.; Schulz, B. Propiconazole is a specific and accessible brassinosteroid (BR) biosynthesis inhibitor for Arabidopsis and maize. PLoS ONE 2012, 7, e36625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roch, G.V.; Maharajan, T.; Ceasar, S.A.; Ignacimuthu, S. The Role of PHT1 family transporters in the acquisition and redistribution of phosphorus in plants. Crit. Rev. Plant. Sci. 2019, 38, 171–198. [Google Scholar] [CrossRef]
- Grabov, A. Plant KT/KUP/HAK potassium transporters: Single family—Multiple functions. Ann. Bot. 2007, 99, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Wang, X.; Hu, R.; Wang, Y.; Xiao, C.; Jiang, Y.; Zhang, X.; Zheng, C.; Fu, Y.F. The pattern of Phosphate transporter 1 genes evolutionary divergence in Glycine max L. BMC Plant. Biol. 2013, 13, 48. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Qin, L.; Zhou, L.; Li, X.; Chen, Z.; Sun, L.; Wang, W.; Lin, Z.; Zhao, J.; Yamaji, N.; et al. A nodule-localized phosphate transporter GmPT7 plays an important role in enhancing symbiotic N2 fixation and yield in soybean. New Phytol. 2019, 221, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Azhar, N.; Su, N.; Shabala, L.; Shabala, S. Exogenously applied 24-epibrassinolide (EBL) ameliorates detrimental effects of salinity by reducing K+ efflux via depolarization-activated K+ channels. Plant. Cell Physiol. 2017, 58, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Grund, B.; Narayanan, R. Photosynthesis and nitrogenase activity in soybean treated with sulphur dioxide and molybdenum. Plant. Sci. 1991, 79, 157–161. [Google Scholar] [CrossRef]
- Trevisan, S.; Forestan, C.; Brojanigo, S.; Quaggiotti, S.; Varotto, S. Brassinosteroid application affects the growth and gravitropic response of maize by regulating gene expression in the roots, shoots and leaves. Plant. Growth Regul. 2020, 92, 117–130. [Google Scholar] [CrossRef]
- Symons, G.M.; Reid, J.B. Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels. Plant. Physiol. 2004, 135, 2196–2206. [Google Scholar] [CrossRef] [Green Version]
- Symons, G.M.; Ross, J.J.; Jager, C.E.; Reid, J.B. Brassinosteroid transport. J. Exp. Bot. 2007, 59, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Li, M.; Min, W.; Wang, M.; Chen, R.; Wang, W. Optimal Brassinosteroid Levels Are Required for Soybean Growth and Mineral Nutrient Homeostasis. Int. J. Mol. Sci. 2021, 22, 8400. https://doi.org/10.3390/ijms22168400
Cheng L, Li M, Min W, Wang M, Chen R, Wang W. Optimal Brassinosteroid Levels Are Required for Soybean Growth and Mineral Nutrient Homeostasis. International Journal of Molecular Sciences. 2021; 22(16):8400. https://doi.org/10.3390/ijms22168400
Chicago/Turabian StyleCheng, Ling, Man Li, Wanling Min, Mengke Wang, Rongqing Chen, and Wenfei Wang. 2021. "Optimal Brassinosteroid Levels Are Required for Soybean Growth and Mineral Nutrient Homeostasis" International Journal of Molecular Sciences 22, no. 16: 8400. https://doi.org/10.3390/ijms22168400




