Srpk3 Decrease Associated with Alpha-Synuclein Increase in Muscles of MPTP-Induced Parkinson’s Disease Mice
Abstract
:1. Introduction
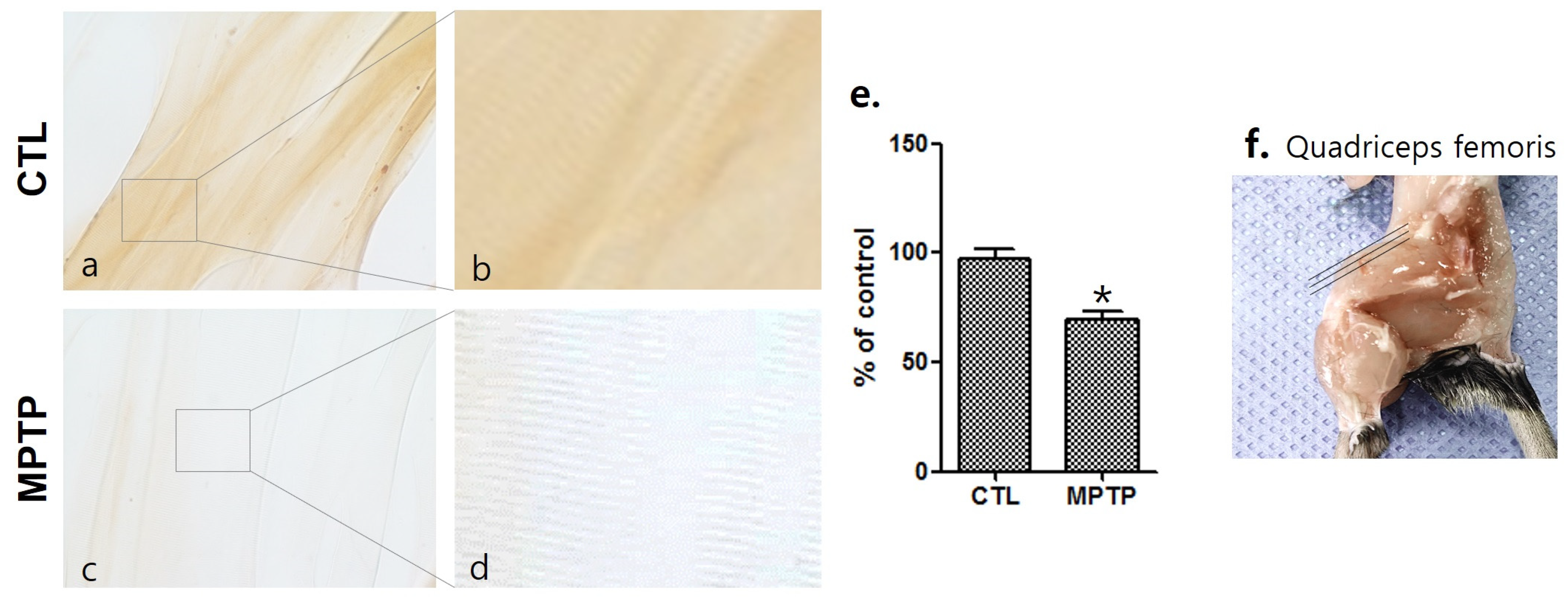
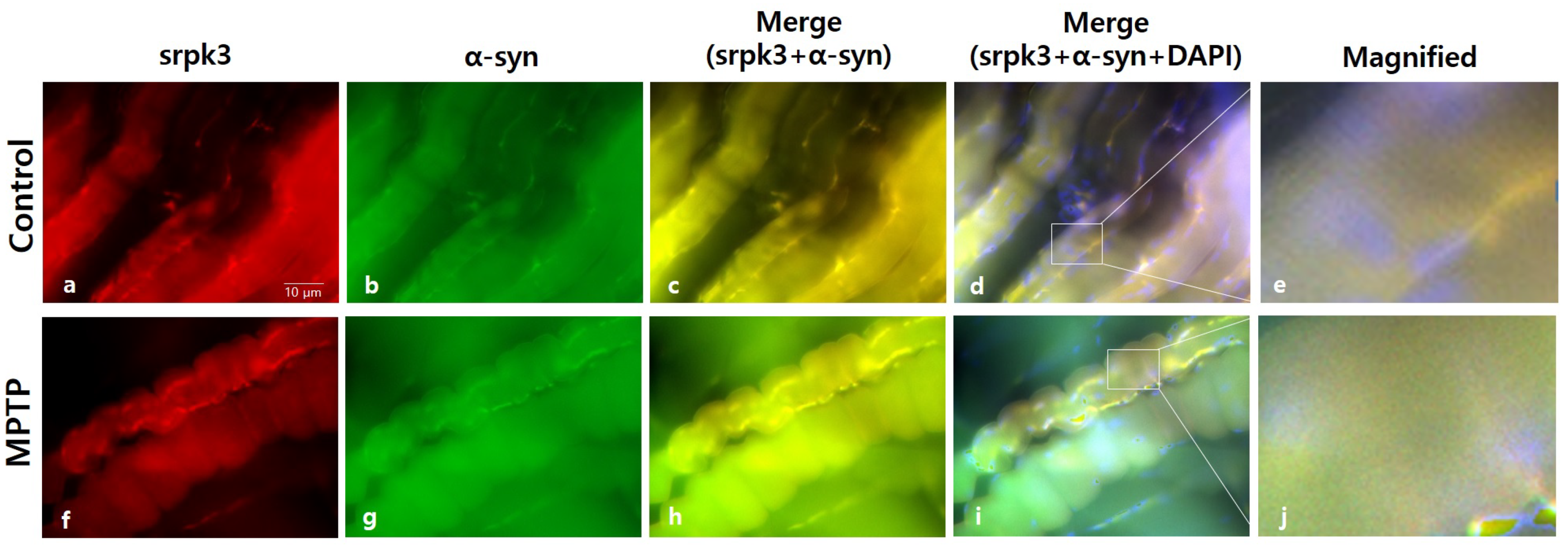
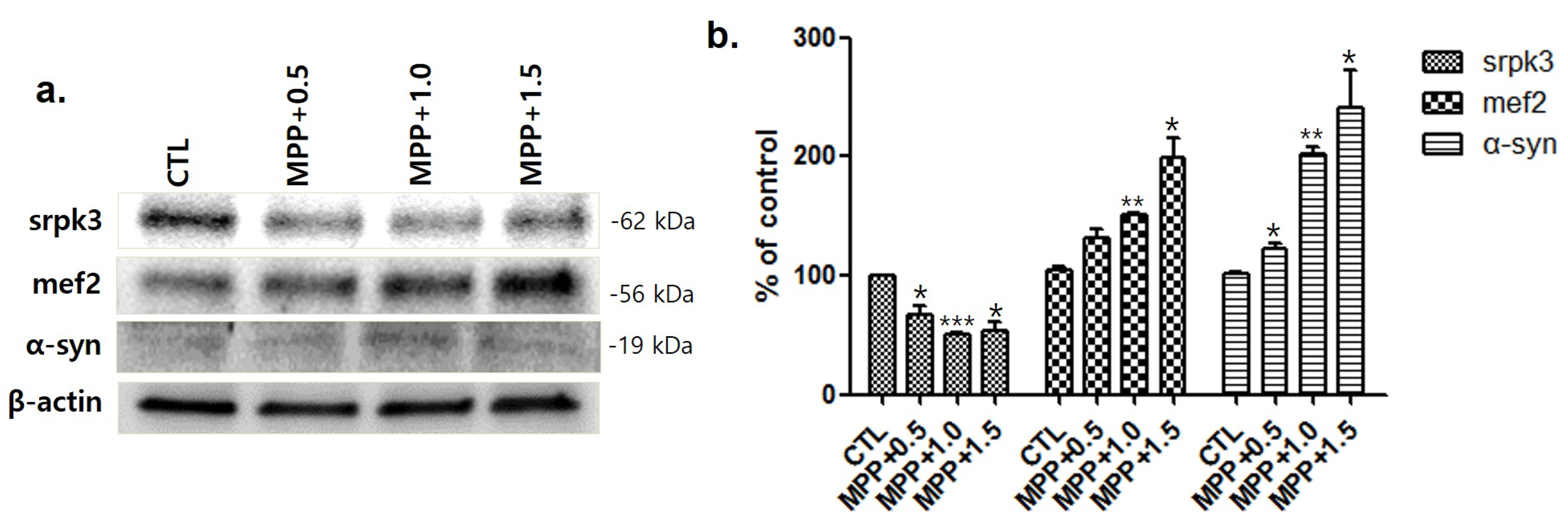
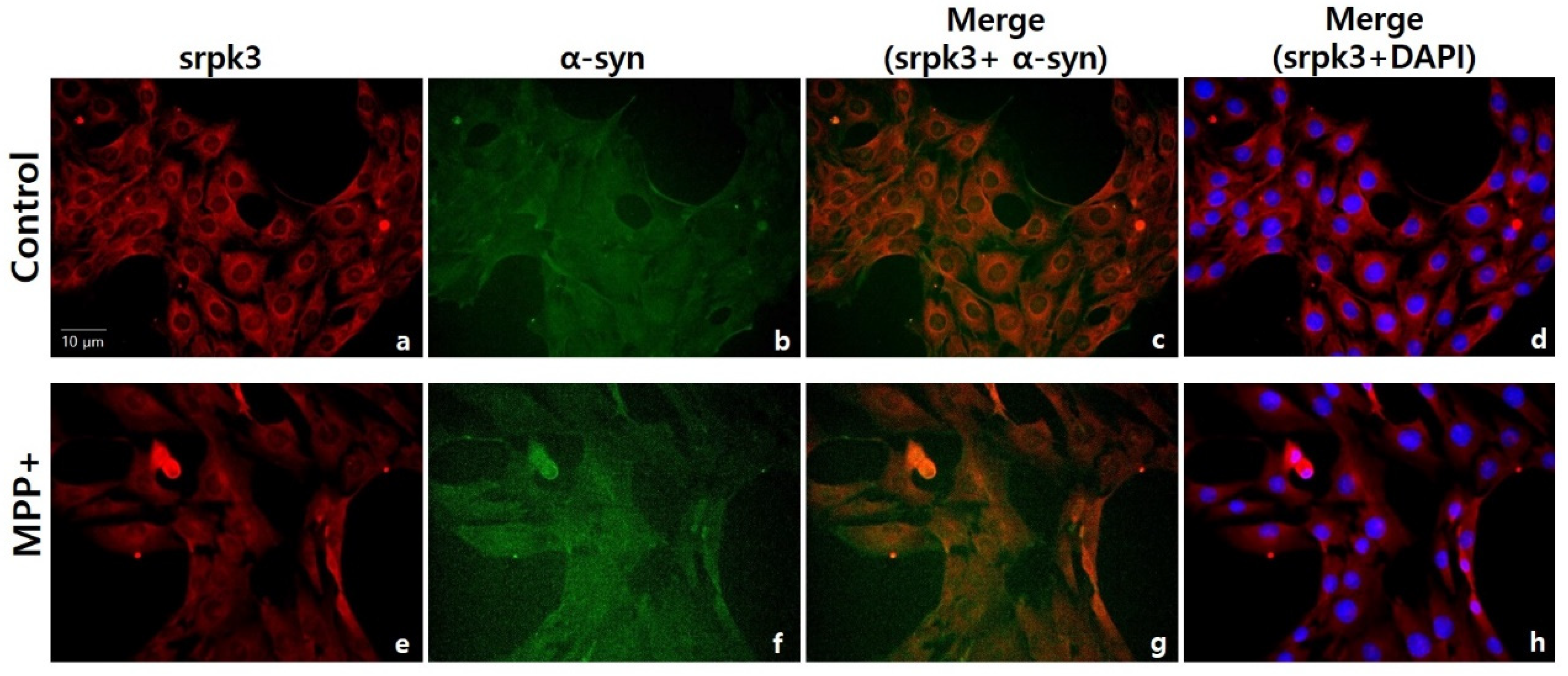
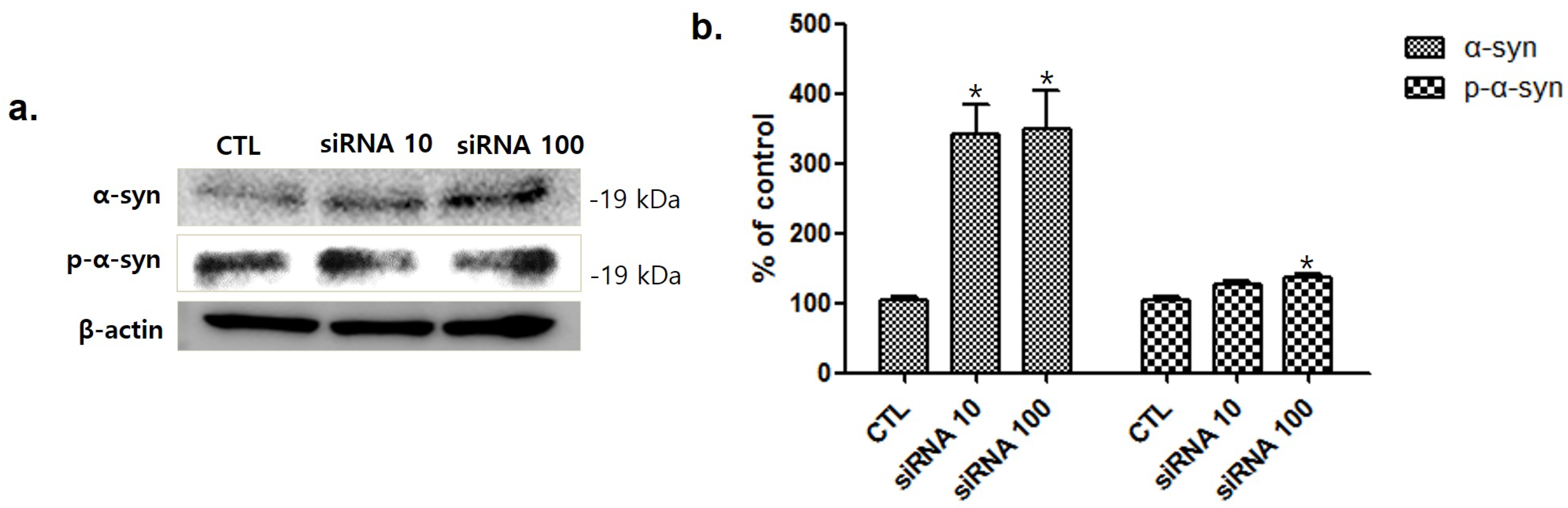
2. Results
3. Discussion
4. Materials and Methods
4.1. MPTP-Induced PD Mouse Model
4.2. Rotarod Test
4.3. Immunohistochemistry
4.4. Cell Lines and Cultures
4.5. MPP+ Treatment
4.6. Short Interfering RNA Knockdown
4.7. Western Blotting
4.8. Immunofluorescence
4.9. Imaging Software
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berardelli, A.; Rothwell, J.C.; Thompson, P.D.; Hallett, M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 2001, 124, 2131–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, K.; Takahashi, H.; Ohama, E.; Ikuta, F. Parkinson’s disease: An immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990, 79, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef]
- Bloch, A.M.; Probst, A.; Bissig, H.; Adams, H.; Tolnay, M. α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol. Appl. Neurobiol. 2006, 32, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Amino, T.; Orimo, S.; Itoh, Y.; Takahashi, A.; Uchihara, T.; Mizusawa, H. Profound Cardiac Sympathetic Denervation Occurs in Parkinson Disease. Brain Pathol. 2006, 15, 29–34. [Google Scholar] [CrossRef]
- Orimo, S.; Amino, T.; Itoh, Y.; Takahashi, A.; Kojo, T.; Uchihara, T.; Tsuchiya, K.; Mori, F.; Wakabayashi, K.; Takahashi, H. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 2005, 109, 583–588. [Google Scholar] [CrossRef]
- Orimo, S.; Takahashi, A.; Uchihara, T.; Mori, F.; Kakita, A.; Wakabayashi, K.; Takahashi, H. Degeneration of Cardiac Sympathetic Nerve Begins in the Early Disease Process of Parkinson’s Disease. Brain Pathol. 2007, 17, 24–30. [Google Scholar] [CrossRef]
- Dabby, R.; Djaldetti, R.; Shahmurov, M.; Treves, T.A.; Gabai, B.; Melamed, E.; Sadeh, M.; Avinoach, I. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J. Neural Transm. 2006, 113, 1169–1176. [Google Scholar] [CrossRef]
- Ikemura, M.; Saito, Y.; Sengoku, R.; Sakiyama, Y.; Hatsuta, H.; Kanemaru, K.; Sawabe, M.; Arai, T.; Ito, G.; Iwatsubo, T.; et al. Lewy Body Pathology Involves Cutaneous Nerves. J. Neuropathol. Exp. Neurol. 2008, 67, 945–953. [Google Scholar] [CrossRef]
- Mu, L.; Sobotka, S.; Chen, J.; Su, H.; Sanders, I.; Adler, C.H.; Shill, H.A.; Caviness, J.N.; Samanta, J.E.; Beach, T.G.; et al. Alpha-Synuclein Pathology and Axonal Degeneration of the Peripheral Motor Nerves Innervating Pharyngeal Muscles in Parkinson Disease. J. Neuropathol. Exp. Neurol. 2013, 72, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Burré, J.; Sharma, M.; Südhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2017, 8, a024091. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, L.S.; Dorsey, E.R. The Benefits of Exercise in Parkinson Disease. JAMA Neurol. 2013, 70, 156–157. [Google Scholar] [CrossRef] [Green Version]
- David, F.J.; Robichaud, J.A.; Leurgans, S.E.; Poon, C.; Kohrt, W.M.; Goldman, J.G.; Comella, C.L.; Vaillancourt, D.E.; Corcos, D.M. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov. Disord. 2015, 30, 1657–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafferty, M.R.; Schmidt, P.N.; Luo, S.T.; Li, K.; Marras, C.; Davis, T.L.; Guttman, M.; Cubillos, F.; Simuni, T.; on behalf of All NPF-QII Investigators. Regular Exercise, Quality of Life, and Mobility in Parkinson’s Disease: A Longitudinal Analysis of National Parkinson Foundation Quality Improvement Initiative Data. J. Parkinsons Dis. 2017, 7, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients with De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Schootemeijer, S.; van der Kolk, N.M.; Bloem, B.R.; de Vries, N.M. Current Perspectives on Aerobic Exercise in People with Parkinson’s Disease. Neurotherapeutics 2020, 17, 1418–1433. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Xiong, Y.; Xie, H.; Ren, Z.; Xu, D.; Lei, M.; Zuo, B.; Feng, X. Molecular characterization and expression patterns of serine/arginine-rich specific kinase 3 (SPRK3) in porcine skeletal muscle. Mol. Biol. Rep. 2010, 38, 2903–2909. [Google Scholar] [CrossRef]
- Siegel, G.; Albers, R.W.; Brady, S.; Price, D. Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Elsevier: Amsterdam, The Netherland, 2005. [Google Scholar]
- Pon, J.R.; Marra, M.A. MEF2 transcription factors: Developmental regulators and emerging cancer genes. Oncotarget 2015, 7, 2297–2312. [Google Scholar] [CrossRef] [Green Version]
- Flavell, S.; Cowan, C.W.; Kim, T.-K.; Greer, P.L.; Lin, Y.; Paradis, S.; Griffith, E.; Hu, L.S.; Chen, C.; Greenberg, M.E. Activity-Dependent Regulation of MEF2 Transcription Factors Suppresses Excitatory Synapse Number. Science 2006, 311, 1008–1012. [Google Scholar] [CrossRef] [Green Version]
- Pulipparacharuvil, S.; Renthal, W.; Hale, C.F.; Taniguchi, M.; Xiao, G.; Kumar, A.; Russo, S.J.; Sikder, D.; Dewey, C.M.; Davis, M.M.; et al. Cocaine Regulates MEF2 to Control Synaptic and Behavioral Plasticity. Neuron 2008, 59, 621–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-K.; Chen, H.-L.; Lu, C.-H.; Chen, M.-H.; Chiang, P.-L.; Chen, Y.-S.; Lin, W.-C. Altered Body Composition of Psoas and Thigh Muscles in Relation to Frailty and Severity of Parkinson’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 3667. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, N.; Gram, H.; Sorrentino, Z.A.; Gregersen, E.; Schmidt, S.I.; Reimer, L.; Betzer, C.; Perez-Gozalbo, C.; Beltoja, M.; Nagaraj, M.; et al. Multiple system atrophy-associated oligodendroglial protein p25α stimulates formation of novel α-synuclein strain with enhanced neurodegenerative potential. Acta Neuropathol. 2021, 142, 87–115. [Google Scholar] [CrossRef] [PubMed]
- Berge, N.V.D.; Ferreira, N.; Gram, H.; Mikkelsen, T.W.; Alstrup, A.K.O.; Casadei, N.; Tsung-Pin, P.; Riess, O.; Nyengaard, J.R.; Tamgüney, G.; et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 2019, 138, 535–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, N.; Gonçalves, N.P.; Jan, A.; Jensen, N.M.; van der Laan, A.; Mohseni, S.; Vægter, C.B.; Jensen, P.H. Trans-synaptic spreading of alpha-synuclein pathology through sensory afferents leads to sensory nerve degeneration and neuropathic pain. Acta Neuropathol. Commun. 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.H.; Yeo, S. Srpk3 Decrease Associated with Alpha-Synuclein Increase in Muscles of MPTP-Induced Parkinson’s Disease Mice. Int. J. Mol. Sci. 2021, 22, 9375. https://doi.org/10.3390/ijms22179375
Seo MH, Yeo S. Srpk3 Decrease Associated with Alpha-Synuclein Increase in Muscles of MPTP-Induced Parkinson’s Disease Mice. International Journal of Molecular Sciences. 2021; 22(17):9375. https://doi.org/10.3390/ijms22179375
Chicago/Turabian StyleSeo, Min Hyung, and Sujung Yeo. 2021. "Srpk3 Decrease Associated with Alpha-Synuclein Increase in Muscles of MPTP-Induced Parkinson’s Disease Mice" International Journal of Molecular Sciences 22, no. 17: 9375. https://doi.org/10.3390/ijms22179375





