Androgen Receptor Signaling Induces Cisplatin Resistance via Down-Regulating GULP1 Expression in Bladder Cancer
Abstract
:1. Introduction
2. Results
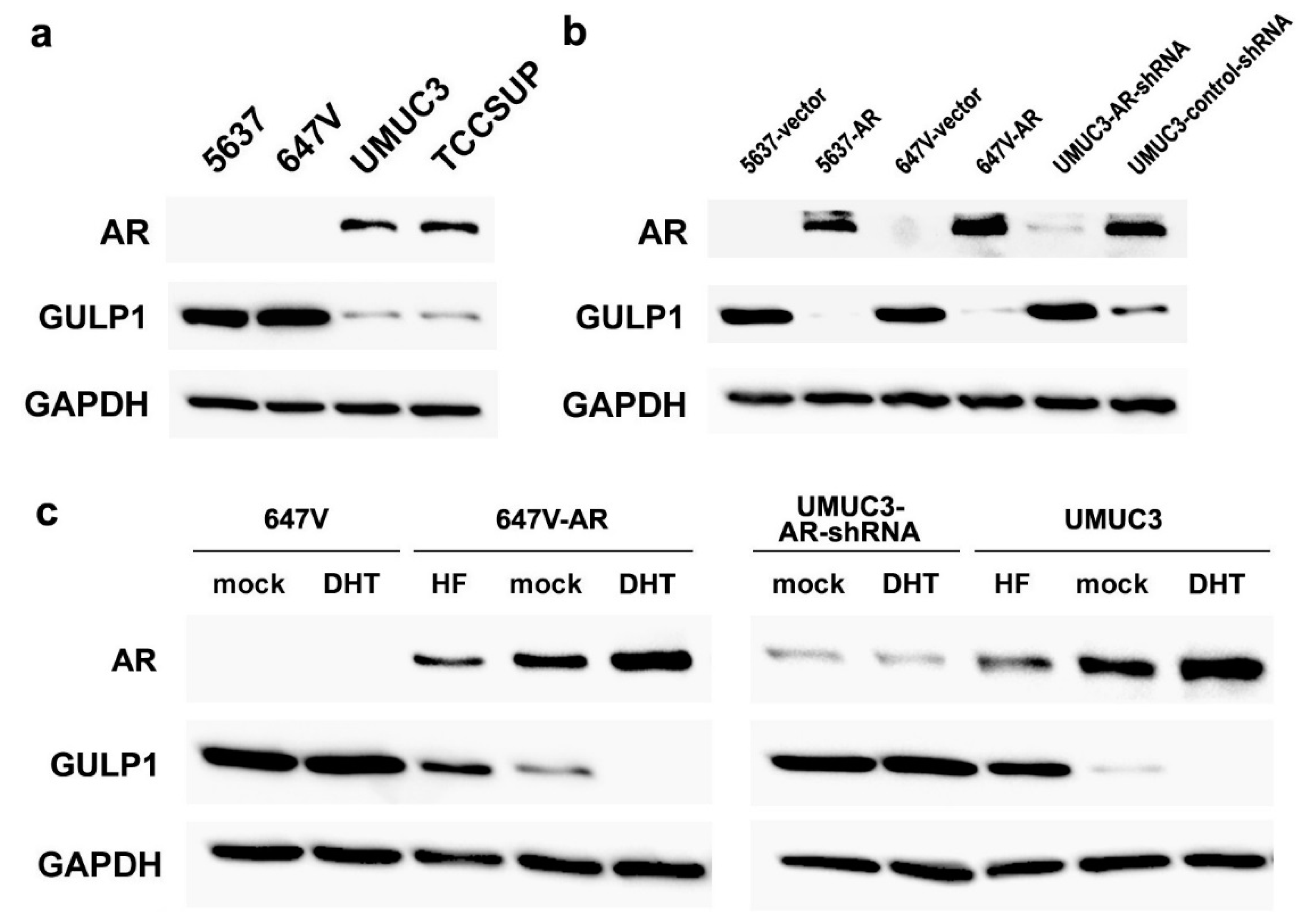
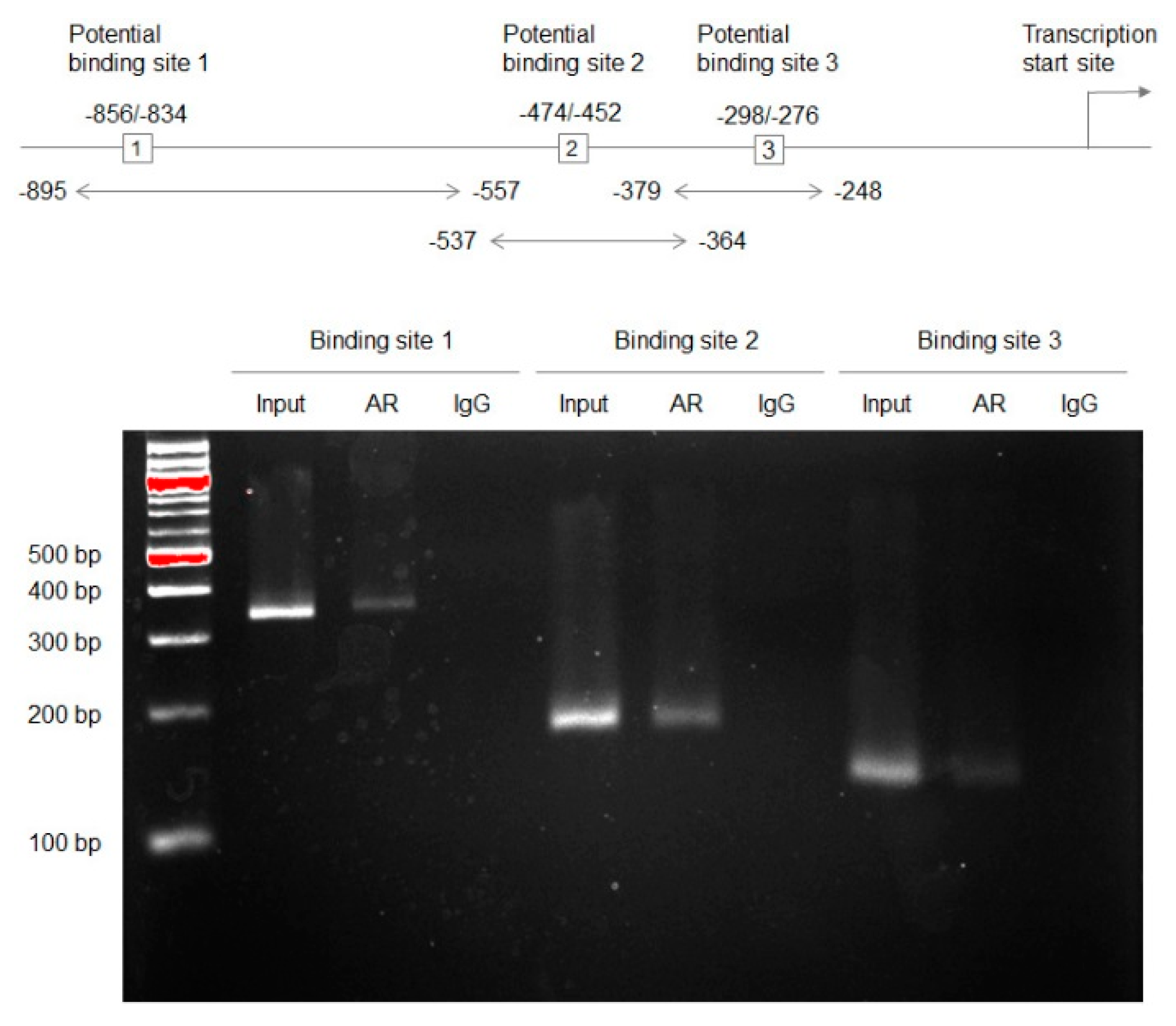
2.1. Associations between AR and GULP1 Expression
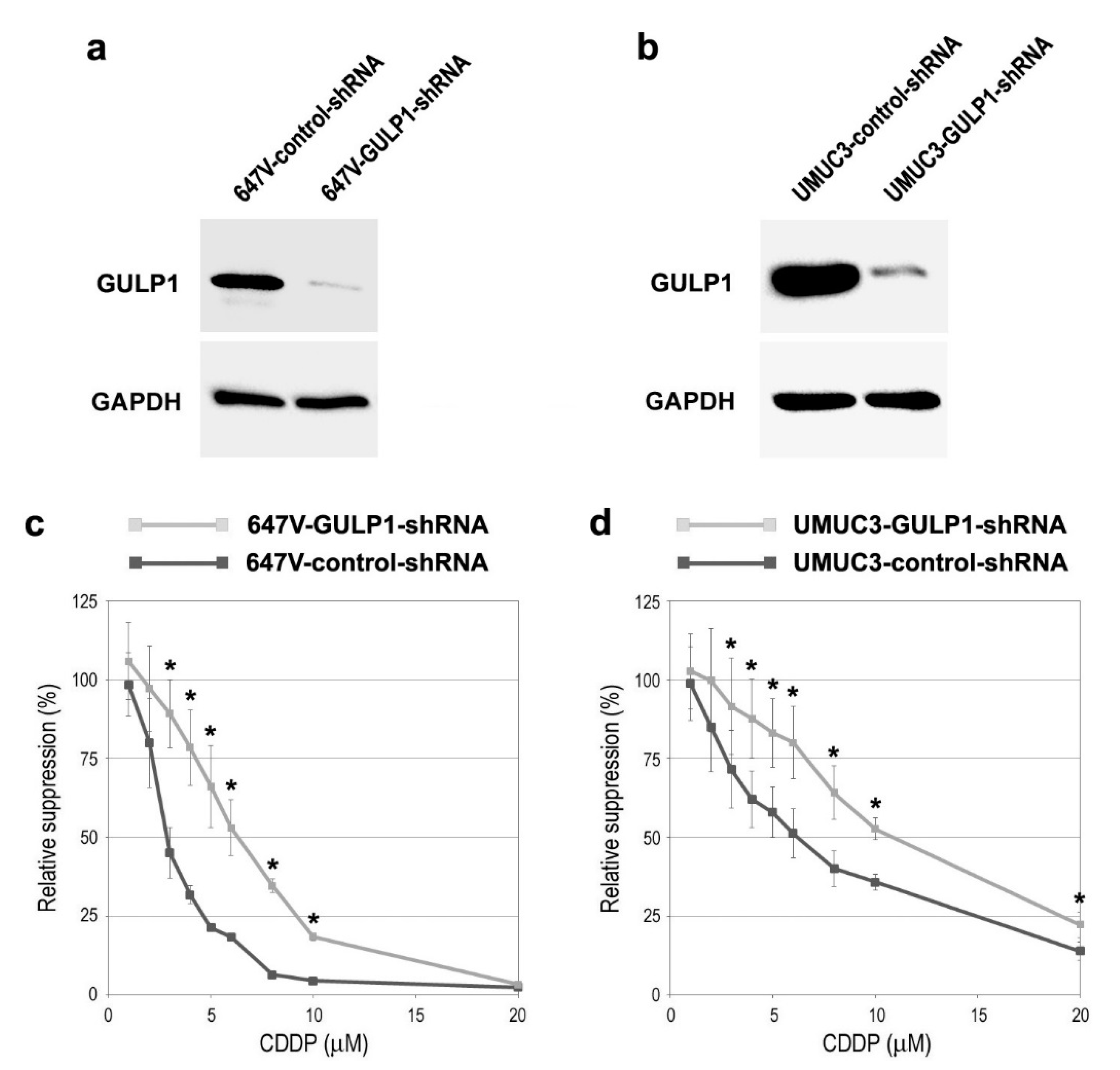
2.2. Associations between GULP1 Expression and CDDP Sensitivity
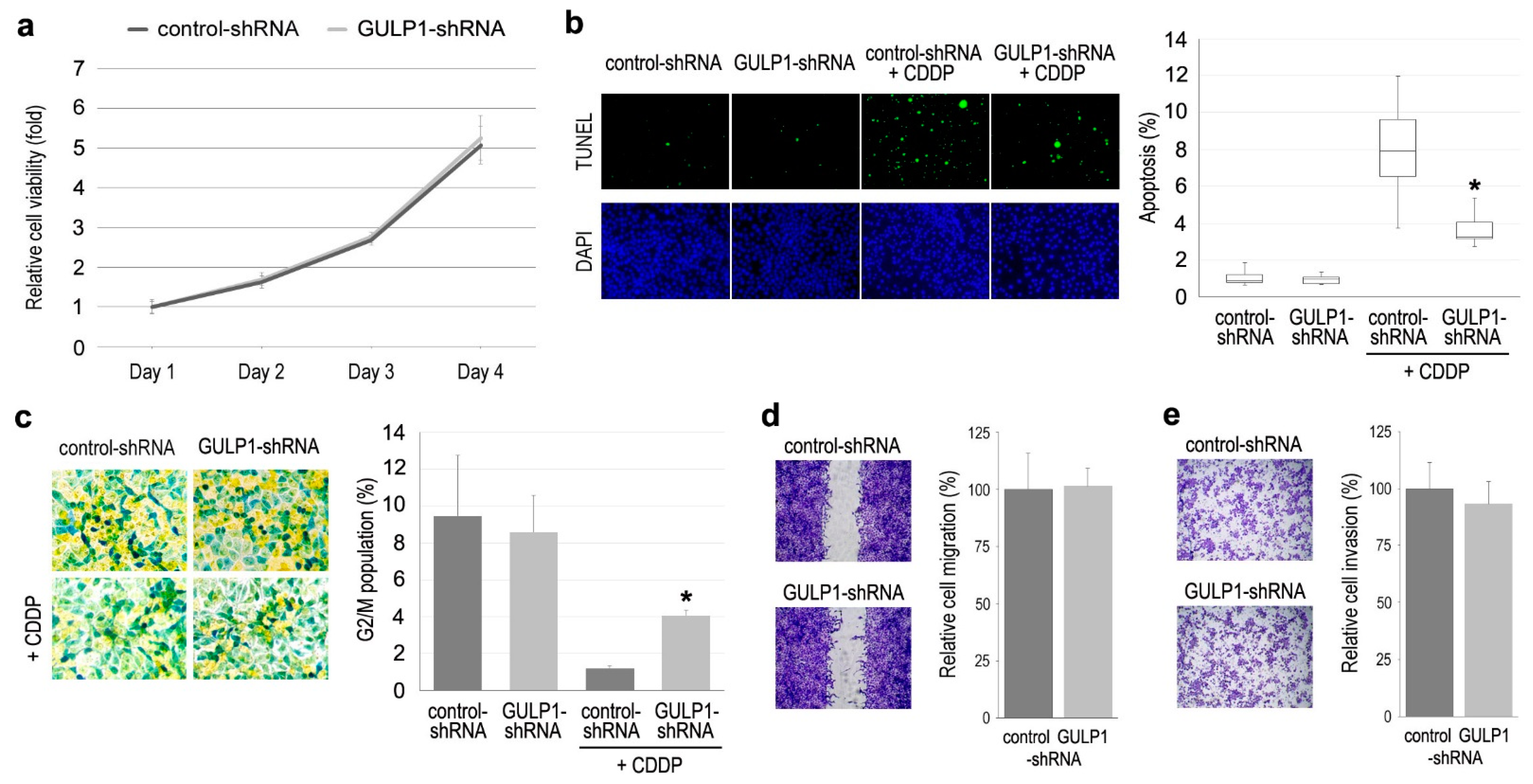
2.3. Role of GULP1 in Cell Growth
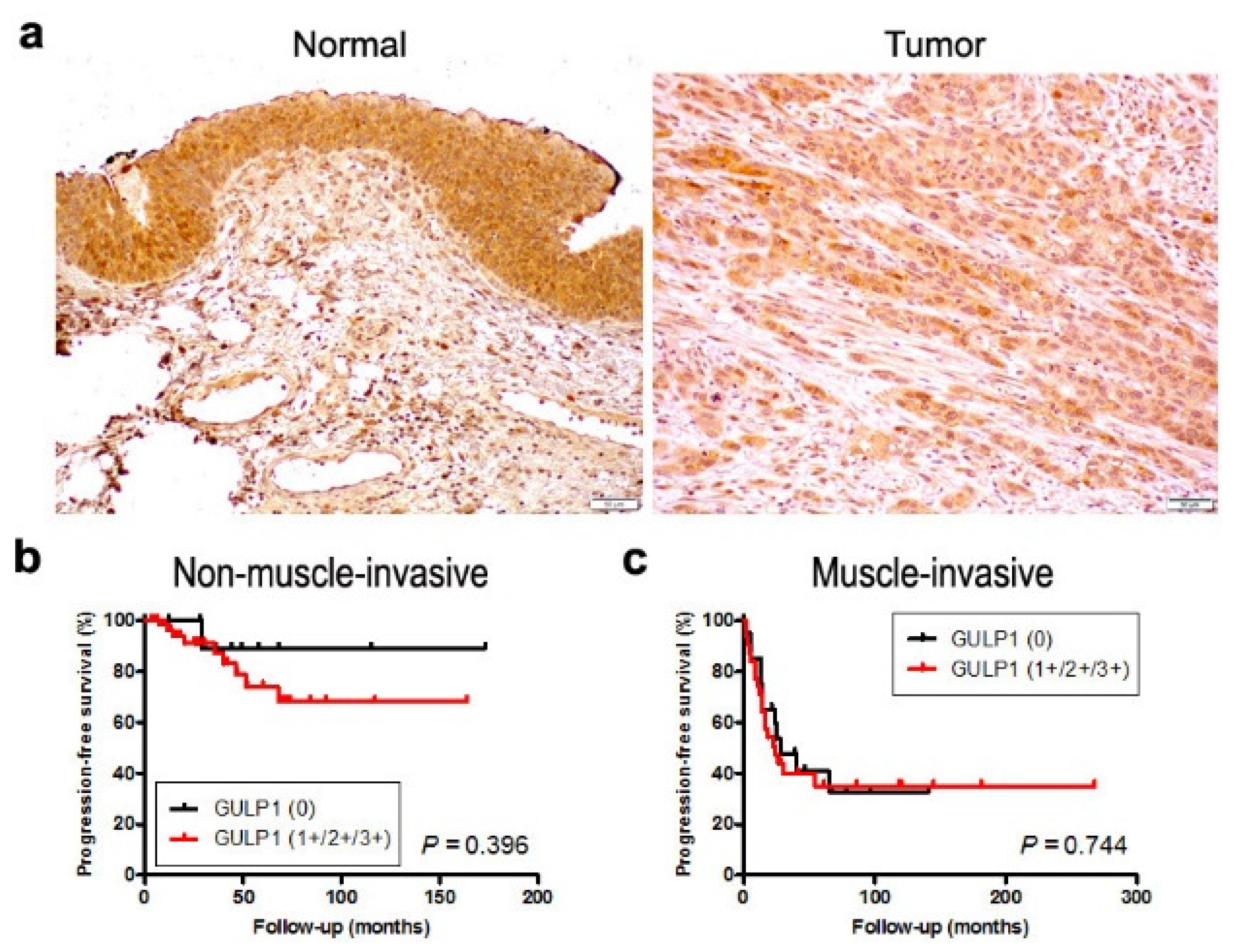
2.4. Expression of GULP1 in Bladder Cancer Specimens
3. Discussion
4. Materials and Methods
4.1. Antibodies and Chemicals
4.2. Cell Lines
4.3. Western Blot
4.4. ChIP
4.5. Cell Proliferation
4.6. Apoptosis and Cell Cycle Analysis
4.7. Cell Migration
4.8. Cell Invasion
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 397–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SEER Cancer Stat Facts: Bladder Cancer; National Cancer Institute: Bethesda, MD, USA. Available online: http://seer.cancer.gov/statfacts/html/urinb.html (accessed on 10 August 2021).
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; Van Rhijn, B.W.G.; Mostafid, A.H.; et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 Update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef]
- Hanna, K.S. Updates and novel treatments in urothelial carcinoma. J. Oncol. Pharm. Pract. 2019, 25, 648–656. [Google Scholar] [CrossRef]
- Goto, T.; Miyamoto, H. Why has the prognosis for bladder cancer not significantly improved after decades of diagnostic and therapeutic advancements? Expert Rev. Anticancer Ther. 2020, 20, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargar, H.; Espiritu, P.N.; Fairey, A.S.; Mertens, L.S.; Dinney, C.P.; Mir, M.C.; Krabbe, L.M.; Cookson, M.S.; Jacobsen, N.E.; Gandhi, N.M.; et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur. Urol. 2015, 67, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voskuilen, C.S.; Oo, H.Z.; Genitsch, V.; Smit, L.A.; Vidal, A.; Meneses, M.; Necchi, A.; Colecchia, M.; Xylinas, E.; Fontugne, J.; et al. Multicenter validation of histopathologic tumor regression grade after neoadjuvant chemotherapy in muscle-invasive bladder carcinoma. Am. J. Surg. Pathol. 2019, 43, 1600–1610. [Google Scholar] [CrossRef]
- Kashiwagi, E.; Inoue, S.; Mizushima, T.; Chen, J.; Ide, H.; Kawahara, T.; Reis, L.O.; Baras, A.S.; Netto, G.J.; Miyamoto, H. Prostaglandin receptors induce urothelial tumourigenesis as well as bladder cancer progression and cisplatin resistance presumably via modulating PTEN expression. Br. J. Cancer 2018, 118, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Sarin, N.; Engel, F.; Rothweiler, F.; Cinatl, J.; Michaelis, M.; Frötschl, R.; Frötschl, H.; Kalayda, G.V. Key players of cisplatin resistance: Towards a systems pharmacology approach. Int. J. Mol. Sci. 2018, 19, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, H.; Miyamoto, H. The role of steroid hormone receptors in urothelial tumorigenesis. Cancers 2020, 12, 2155. [Google Scholar] [CrossRef]
- Ide, H.; Miyamoto, H. Sex hormone receptor signaling in bladder cancer: A potential target for enhancing the efficacy of conventional non-surgical therapy. Cells 2021, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, E.; Ide, H.; Inoue, S.; Kawahara, T.; Zheng, Y.; Reis, L.O.; Baras, A.S.; Miyamoto, H. Androgen receptor activity modulates responses to cisplatin treatment in bladder cancer. Oncotarget 2016, 7, 49169–49179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekino, Y.; Sakamoto, N.; Ishikawa, A.; Honma, R.; Shigematsu, Y.; Hayashi, T.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; et al. Transcribed ultraconserved region Uc.63+ promotes resistance to cisplatin through regulation of androgen receptor signaling in bladder cancer. Oncol. Rep. 2019, 41, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Chandrasekaran, B.; Kolluru, V.; Rai, S.; Jordan, A.C.; Houda, A.; Messer, J.; Ankem, M.; Damodaran, C.; Haddad, A. Combination of androgen receptor inhibitor and cisplatin, an effective treatment strategy for urothelial carcinoma of the bladder. Urol. Oncol. 2019, 37, 492–502. [Google Scholar] [CrossRef]
- Mizushima, T.; Jiang, G.; Kawahara, T.; Li, P.; Han, B.; Inoue, S.; Ide, H.; Kato, I.; Jalalizadeh, M.; Miyagi, E.; et al. Androgen receptor signaling reduces the efficacy of bacillus Calmette-Guѐrin therapy for bladder cancer via modulating Rab27b-induced exocytosis. Mol. Cancer Ther. 2020, 19, 1930–1942. [Google Scholar] [CrossRef]
- Ide, H.; Inoue, S.; Mizushima, T.; Jiang, G.; Chuang, K.H.; Oya, M.; Miyamoto, H. Androgen receptor signaling reduces radiosensitivity in bladder cancer. Mol. Cancer Ther. 2018, 17, 1566–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Kim, S.Y.; Kang, K.B.; Kim, I.S. Adaptor protein GULP in involved in stabilin-1-mediated phagocytosis. Biochem. Biophys. Res. Commun. 2010, 398, 467–472. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yang, Z.; Chen, Y.T.; Ishiguro, H.; Uemura, H.; Kubota, Y.; Nagashima, Y.; Chang, Y.J.; Hu, Y.C.; Tsai, M.Y.; et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl. Cancer Inst. 2007, 99, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Wang, X.; Wang, Y.; Ma, D. Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2010, 392, 373–379. [Google Scholar] [CrossRef]
- Shiota, M.; Takeuchi, A.; Yokomizo, A.; Kashiwagi, E.; Tatsugami, K.; Kuroiwa, K.; Naito, S. Androgen receptor signaling regulates cell growth and vulnerability to doxorubicin in bladder cancer cells. J. Urol. 2012, 188, 276–286. [Google Scholar] [CrossRef]
- Kameyama, K.; Horie, K.; Mizutani, K.; Kato, T.; Fujita, Y.; Kawakami, K.; Kojima, T.; Miyazaki, T.; Deguchi, T.; Ito, M. Enzalutamide inhibits proliferation of gemcitabine-resistant bladder cancer cells with increased androgen receptor expression. Int. J. Oncol. 2017, 50, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, H.; Goto, T.; Teramoto, Y.; Mizushima, T.; Jiang, G.; Nagata, Y.; Inoue, S.; Baras, A.S.; Kashiwagi, E.; Miyamoto, H. FOXO1 inactivation induces cisplatin resistance in bladder cancer. Cancer Sci. 2020, 111, 3397–3400. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Teramoto, Y.; Goto, T.; Mizushima, T.; Inoue, S.; Ide, H.; Nagata, Y.; Kashiwagi, E.; Baras, A.S.; Netto, G.J.; et al. Identification of BXDC2 as a key downstream effector of the androgen receptor in modulating cisplatin sensitivity in bladder cancer. Cancers 2021, 13, 975. [Google Scholar] [CrossRef]
- Su, H.; Nakada-Tsukui, K.; Tosello-Trampont, A.C.; Li, Y.; Bu, G.; Hensen, P.M.; Ravichandran, K.S. Interaction of CED-6/GULP, an adaptor protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP). J. Biol. Chem. 2002, 277, 11772–11779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osada, Y.; Sunatani, T.; Kim, I.S.; Nakanishi, Y.; Shiratsuchi, A. Signaling pathway involving GULP, MAPK and Rac1 for SR-BI-induced phagocytosis of apoptotic cells. J. Biochem. 2009, 145, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.I.J.; Martin, C.L.; Ma, Z.; Hafiane, A.; Dai, M.; Lebrun, J.J.; Kiss, R.S. Engulfment protein GULP is regulator of transforming growth factor-β response in ovarian cells. J. Biol. Chem. 2012, 287, 20636–20651. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, L.; Brait, M.; Izumchenko, E.; Begum, S.; Chatterjee, A.; Sen, T.; Loyo, M.; Barbosa, A.; Poeta, M.L.; Makarev, E.; et al. Integrated transcriptomic and epigenomic analysis of ovarian cancer reveals epigenetically silenced GULP1. Cancer Lett. 2018, 433, 242–251. [Google Scholar] [CrossRef]
- Hayashi, M.; Guida, E.; Inokawa, Y.; Goldberg, R.; Reis, L.O.; Ooki, A.; Pilli, M.; Sadhukhan, P.; Woo, J.; Choi, W.; et al. GULP1 regulates the NRF2-KEAP1 signaling axis in urothelial carcinoma. Sci. Signal. 2020, 13, eaba0443. [Google Scholar] [CrossRef]
- Huang, M.; Long, Y.; Jin, Y.; Ya, W.; Meng, D.; Qin, T.; Su, L.; Zhou, W.; Wu, J.; Huang, C.; et al. Comprehensive analysis of the lncRNA-miRNA-mRNA regulatory network for bladder cancer. Transl. Androl. Urol. 2021, 10, 1286–1301. [Google Scholar] [CrossRef]
- Cui, Z.; Xu, D.; Zhang, F.; Sun, J.; Song, L.; Ye, W.; Zeng, J.; Zhou, M.; Ruan, Z.; Zhang, L.; et al. CD47 blockade enhances therapeutic efficacy of cisplatin against lung carcinoma in a murine model. Exp. Cell Res. 2021, 405, 112677. [Google Scholar] [CrossRef]
- Zhou, H.H.; Chen, L.; Liang, H.F.; Li, G.Z.; Zhang, B.X.; Chen, X.P. Smad3 sensitizes hepatocellular carcinoma cells to cisplatin by repressing phosphorylation of AKT. Int. J. Mol. Sci. 2016, 17, 610. [Google Scholar] [CrossRef] [Green Version]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, Y.; Chen, S.; Wang, J.; Jiang, C.; Hou, W.; Xu, C. JUND-dependent up-regulation of HMOX1 is associated with cisplatin resistance in muscle-invasive bladder cancer. J. Biochem. 2020, 168, 73–82. [Google Scholar] [CrossRef]
- Mirzaei, S.; Mohammadi, A.T.; Gholami, M.H.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Makvandi, P.; Samec, M.; Liskova, A.; et al. Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance. Pharmacol. Res. 2021, 167, 105575. [Google Scholar] [CrossRef]
- Goto, T.; Kashiwagi, E.; Jiang, G.; Nagata, Y.; Teramoto, Y.; Baras, A.S.; Yamashita, S.; Ito, A.; Arai, Y.; Miyamoto, H. Estrogen receptor-β signaling induces cisplatin resistance in bladder cancer. Am. J. Cancer Res. 2020, 10, 2523–2534. [Google Scholar]
- Sikic, D.; Wirtz, R.M.; Wach, S.; Dyskjøt, L.; Erben, P.; Bolenz, C.; Breyer, J.; Otto, W.; Hoadley, K.A.; Lerner, S.P.; et al. Androgen receptor mRNA expression in urothelial carcinoma of the bladder: A retrospective analysis of two independent cohorts. Transl. Oncol. 2019, 12, 661–668. [Google Scholar] [CrossRef]
- Ide, H.; Inoue, S.; Miyamoto, H. Histopathological and prognostic significance of the expression of sex hormone receptors in bladder cancer: A meta-analysis of immunohistochemical studies. PLoS ONE 2017, 12, e0174746. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Yao, J.L.; Chaux, A.; Zheng, Y.; Hsu, I.; Izumi, K.; Chang, C.; Messing, E.M.; Netto, G.J.; Yeh, S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012, 109, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Miyamoto, H. The role of estrogen receptors in urothelial cancer. Front. Endocrinol. 2021, 12, 643870. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Izumi, K.; Yao, J.L.; Miyamoto, H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr. Relat. Cancer 2011, 18, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ishiguro, H.; Kawahara, T.; Miyamoto, Y.; Izumi, K.; Miyamoto, H. GATA3 in the urinary bladder: Suppression of neoplastic transformation and down-regulation by androgens. Am. J. Cancer Res. 2014, 4, 461–473. [Google Scholar] [PubMed]
- Lee, C.; Huang, C.H. LASAGNA-Search 2.0: Integrated transcription factor binding site search and visualization in a browser. Bioinformatics 2014, 30, 1923–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baras, A.S.; Gandhi, N.; Munari, E.; Faraj, S.; Shultz, L.; Marchionni, L.; Schoenberg, M.; Hahn, N.; Hoque, M.O.; Berman, D.; et al. Identification and validation of protein biomarkers of response to neoadjuvant platinum chemotherapy in muscle invasive urothelial carcinoma. PLoS ONE 2015, 10, e0131245. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| n | Expression Levels | P Value | |||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | 0 vs. 1+/2+/3+ | 0/1+ vs. 2+/3+ | ||||
| 0 | 1+ | 2+ | 3+ | ||||
| Tissues | 0.005 | 0.006 | |||||
| Non-neoplastic urothelium | 89 | 9 (10%) | 41 (46%) | 33 (37%) | 6 (7%) | ||
| Urothelial neoplasm | 129 | 33 (26%) | 63 (49%) | 30 (23%) | 3 (2%) | ||
| Tumor Grade | 0.062 | 1.000 | |||||
| PUNLMP + LG | 50 | 8 (16%) | 29 (58%) | 13 (26%) | 0 (0%) | ||
| HG | 79 | 25 (32%) | 34 (43%) | 17 (22%) | 3 (4%) | ||
| Pathologic Stage | 0.007 | 0.014 | |||||
| Non-muscle-invasive | 78 | 13 (17%) | 39 (50%) | 25 (32%) | 1 (1%) | ||
| Muscle-invasive | 51 | 20 (39%) | 24 (47%) | 5 (10%) | 2 (4%) | ||
| Lymph Node Involvement | 0.331 | 1.000 | |||||
| pN0 | 36 | 13 (36%) | 17 (47%) | 4 (11%) | 2 (6%) | ||
| pN1-3 | 13 | 7 (54%) | 4 (31%) | 2 (15%) | 0 (0%) | ||
| GULP1 Expression | P Value | |||
|---|---|---|---|---|
| 0/1+ | 2+/3+ | |||
| Responders | 17 | 8 (47%) | 9 (53%) | 0.044 |
| Non-responders | 26 | 20 (77%) | 6 (23%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teramoto, Y.; Jiang, G.; Goto, T.; Mizushima, T.; Nagata, Y.; Netto, G.J.; Miyamoto, H. Androgen Receptor Signaling Induces Cisplatin Resistance via Down-Regulating GULP1 Expression in Bladder Cancer. Int. J. Mol. Sci. 2021, 22, 10030. https://doi.org/10.3390/ijms221810030
Teramoto Y, Jiang G, Goto T, Mizushima T, Nagata Y, Netto GJ, Miyamoto H. Androgen Receptor Signaling Induces Cisplatin Resistance via Down-Regulating GULP1 Expression in Bladder Cancer. International Journal of Molecular Sciences. 2021; 22(18):10030. https://doi.org/10.3390/ijms221810030
Chicago/Turabian StyleTeramoto, Yuki, Guiyang Jiang, Takuro Goto, Taichi Mizushima, Yujiro Nagata, George J. Netto, and Hiroshi Miyamoto. 2021. "Androgen Receptor Signaling Induces Cisplatin Resistance via Down-Regulating GULP1 Expression in Bladder Cancer" International Journal of Molecular Sciences 22, no. 18: 10030. https://doi.org/10.3390/ijms221810030






