The Effect of High Selenite and Selenate Concentrations on Ferric Oxyhydroxides Transformation under Alkaline Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Transformation of Iron Precipitates under Alkaline Conditions
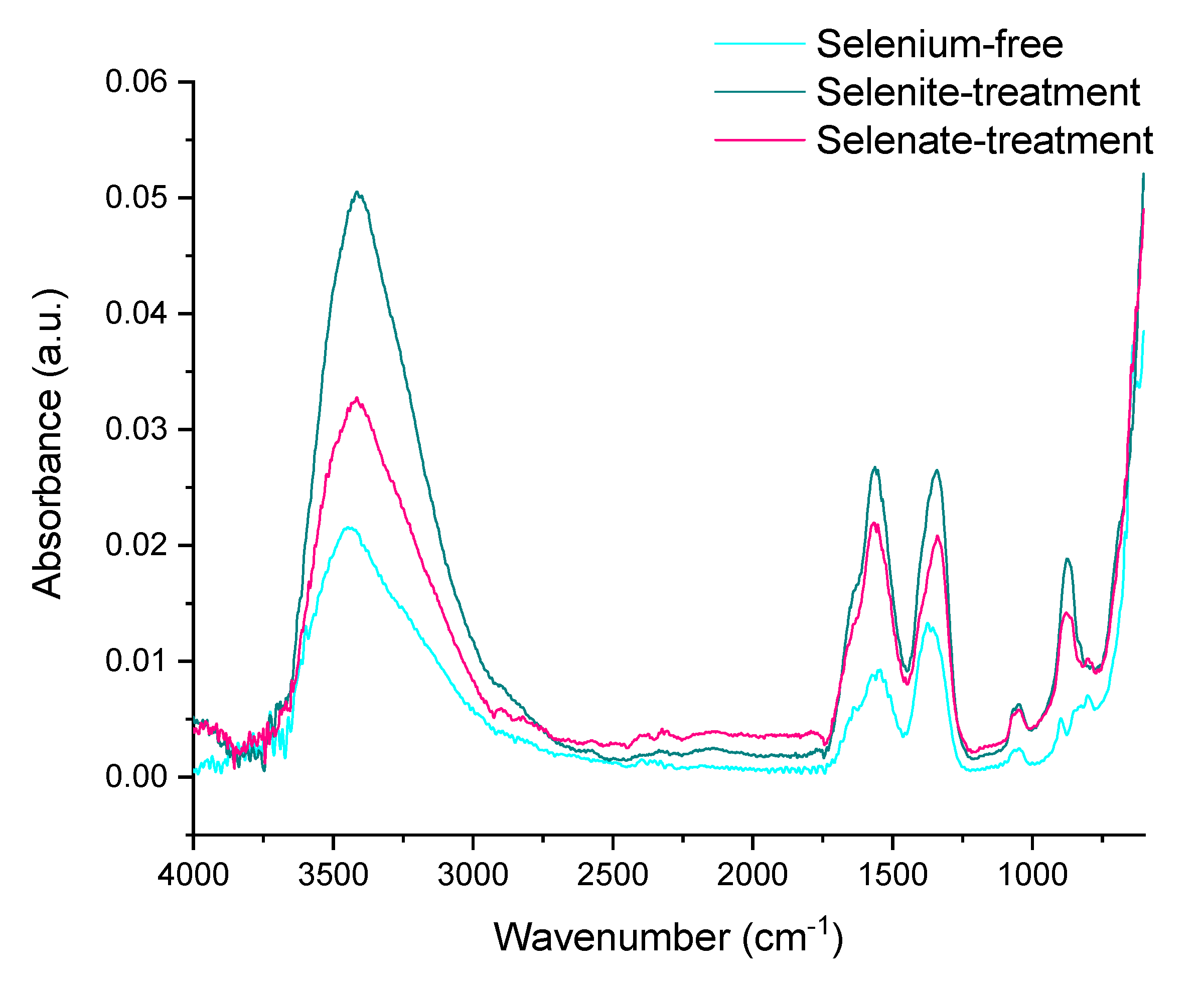
2.2. Effect of Selenium Presence on Ferric Oxyhydroxides Transformation
2.3. Effect of the Selenium on Thermal Transformation of Goethite to Hematite
2.4. Selenite and Selenate Interaction with Ferric Iron Precipitates
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Solid Phase Preparation and Selenium Desorption
3.3. Analytical Procedures
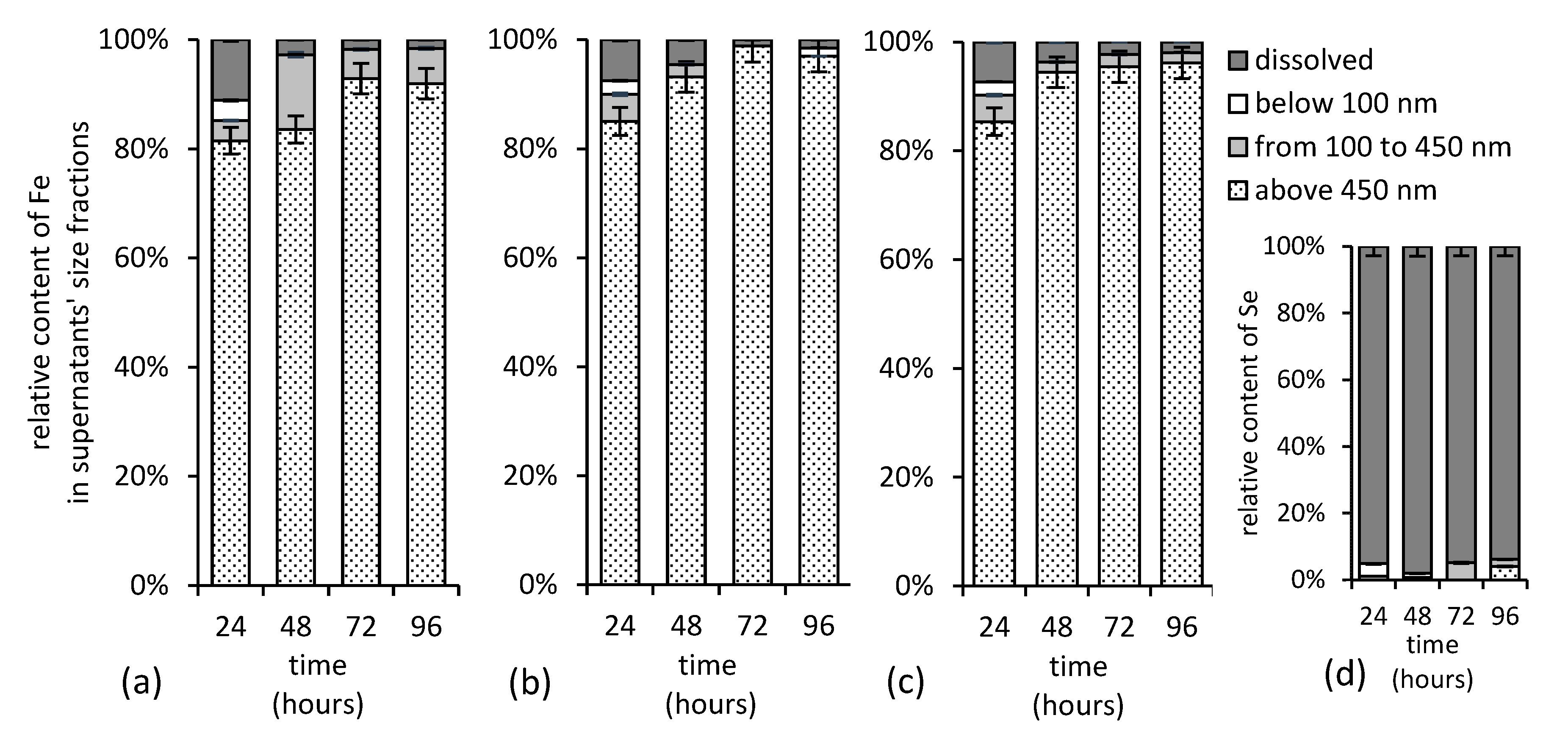
3.4. Iron and Selenium Size Fractionation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bassil, J.; Naveau, A.; Bueno, M.; Razack, M.; Kazpard, V. Leaching behavior of selenium from the karst infillings of the Hydrogeological Experimental Site of Poitiers. Chem. Geol. 2018, 483, 141–150. [Google Scholar] [CrossRef]
- Farkasovska, I.; Zemberyova, M. Determination and speciation by AAS techniques of selenium in environmental and biological samples. Chem. Listy 1999, 93, 633–638. [Google Scholar]
- Kim, S.S.; Min, J.H.; Lee, J.K.; Baik, M.H.; Choi, J.-W.; Shin, H.S. Effects of pH and anions on the sorption of selenium ions onto magnetite. J. Environ. Radioact. 2012, 104, 1–6. [Google Scholar] [CrossRef]
- Hagarova, I.; Nemcek, L. Selenium in Blood Serum of Healthy European Population. Chem. Listy 2020, 114, 329–335. [Google Scholar]
- Žemberyová, M.; Hagarová, I. Determination of selenium in blood serum of children by electrothermal atomic absorption spectrometry. Chem. Listy 2005, 99, 34–39. [Google Scholar]
- Hagarová, I.; Žemberyová, M.; Bajčan, D. Sequential and single step extraction procedures used for fractionation of selenium in soil samples. Chem. Pap. 2005, 59, 93–98. [Google Scholar]
- Zhang, L.; Song, H.; Guo, Y.; Fan, B.; Huang, Y.; Mao, X.; Liang, K.; Hu, Z.; Sun, X.; Fang, Y.; et al. Benefit–risk assessment of dietary selenium and its associated metals intake in China (2017–2019): Is current selenium-rich agro-food safe enough? J. Hazard. Mater. 2020, 398, 123224. [Google Scholar] [CrossRef]
- Dudova, J.; Bujdos, M. Study of Selenium Sorption on Iron Oxide Hydroxides. Chem. Listy 2015, 109, 770–774. [Google Scholar]
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Bio/Technol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Ni, R.; Luo, K.; Tian, X.; Yan, S.; Zhong, J.; Liu, M. Distribution and geological sources of selenium in environmental materials in Taoyuan County, Hunan Province, China. Environ. Geochem. Health 2016, 38, 927–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Tamoto, S.; Tabelin, C.B.; Igarashi, T.; Ito, M.; Hiroyoshi, N. Short and long term release mechanisms of arsenic, selenium and boron from a tunnel-excavated sedimentary rock under in situ conditions. J. Contam. Hydrol. 2015, 175–176, 60–71. [Google Scholar] [CrossRef]
- Etteieb, S.; Magdouli, S.; Zolfaghari, M.; Brar, S. Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci. Total Environ. 2020, 698, 134339. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, X.; Iris, K.; Wang, L.; Zhou, J.; Sun, X.; Rinklebe, J.; Shaheen, S.M.; Ok, Y.S.; Lin, Z. Potentially toxic elements in solid waste streams: Fate and management approaches. Environ. Pollut. 2019, 253, 680–707. [Google Scholar] [CrossRef]
- Li, X.-Q.; Elliott, D.W.; Zhang, W.-X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M.; Aragaw, B.A. Iron-based nanoparticles in wastewater treatment: A review on synthesis methods, applications, and removal mechanisms. J. Saudi Chem. Soc. 2021, 25, 101280. [Google Scholar] [CrossRef]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Ranmadugala, D.; Ebrahiminezhad, A.; Manley-Harris, M.; Ghasemi, Y.; Berenjian, A. Magnetic immobilization of bacteria using iron oxide nanoparticles. Biotechnol. Lett. 2018, 40, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Nur, T.; Loganathan, P.; Ahmed, M.B.; Johir, M.A.H.; Nguyen, T.V.; Vigneswaran, S. Removing arsenic from water by coprecipitation with iron: Effect of arsenic and iron concentrations and adsorbent incorporation. Chemosphere 2019, 226, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.P. Advanced arsenic removal technologies review. In Chemistry of Advanced Environmental Purification Processes of Water; Elsevier: Amsterdam, The Netherlands, 2014; pp. 285–337. [Google Scholar]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Malakootian, M.; Shahamat, Y.D.; Kannan, K.; Mahdizadeh, H. Degradation of p-nitroaniline from aqueous solutions using ozonation/Mg-Al layered double hydroxides integrated with the sequencing batch moving bed biofilm reactor. J. Taiwan Inst. Chem. Eng. 2020, 113, 241–252. [Google Scholar] [CrossRef]
- Malakootian, M.; Kannan, K.; Gharaghani, M.A.; Dehdarirad, A.; Nasiri, A.; Shahamat, Y.D.; Mahdizadeh, H. Removal of metronidazole from wastewater by Fe/charcoal micro electrolysis fluidized bed reactor. J. Environ. Chem. Eng. 2019, 7, 103457. [Google Scholar] [CrossRef]
- Prasad, K.; Rao, K.; Gladis, M.; Naidu, G.R.K.; Prasada Rao, T. Determination of selenium(IV) after co-precipitation with Fe-Ti layered double hydroxides. Chem. Anal. 2006, 51, 613–622. [Google Scholar]
- Mondal, K.; Jegadeesan, G.; Lalvani, S.B. Removal of selenate by Fe and NiFe nanosized particles. Ind. Eng. Chem. Res. 2004, 43, 4922–4934. [Google Scholar] [CrossRef]
- Zhang, Y.; Amrhein, C.; Frankenberger Jr, W.T. Effect of arsenate and molybdate on removal of selenate from an aqueous solution by zero-valent iron. Sci. Total Environ. 2005, 350, 1–11. [Google Scholar] [CrossRef]
- Montgomery, J.M. Water Treatment Principles and Design; Wiley: New York, NY, USA, 1985. [Google Scholar]
- LaGrow, A.P.; Besenhard, M.O.; Hodzic, A.; Sergides, A.; Bogart, L.K.; Gavriilidis, A.; Thanh, N.T.K. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-ray diffraction in solution. Nanoscale 2019, 11, 6620–6628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, B.; Anderson, R.; Bless, D.; Butler, B.; Conway, B.; Dailey, A.; Freed, E.; Gervais, G.; Gill, M.; Grosse, D. Reference Guide to Treatment Technologies for Mining-Influenced Water; EPA 542-R-14-001; Office of Superfund Remediation and Technology Innovation: Washington, DC, USA, 2014. [Google Scholar]
- Merrill, D.T.; Manzione, M.A.; Parker, D.S.; Petersen, J.J.; Chow, W.; Hobbs, A.O. Field evaluation of arsenic and selenium removal by iron coprecipitation. Environ. Prog. 1987, 6, 82–90. [Google Scholar] [CrossRef]
- Okonji, S.O.; Dominic, J.A.; Pernitsky, D.; Achari, G. Removal and recovery of selenium species from wastewater: Adsorption kinetics and co-precipitation mechanisms. J. Water Process. Eng. 2020, 38, 101666. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Chen, Y.; Xu, D. Research on removing selenium from raw water by using Fe/Se co-precipitation system. J. Water Supply Res. Technol.-AQUA 2009, 58, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3050–3056. [Google Scholar] [CrossRef]
- Matulová, M.; Urík, M.; Bujdoš, M.; Duborská, E.; Cesnek, M.; Miglierini, M.B. Selenite sorption onto goethite: Isotherm and ion-competitive studies, and effect of pH on sorption kinetics. Chem. Pap. 2019, 73, 2975–2985. [Google Scholar] [CrossRef]
- Matulová, M.; Bujdoš, M.; Miglierini, M.B.; Mitróová, Z.; Kubovčíková, M.; Urík, M. The effects of selenate on goethite synthesis and selenate sorption kinetics onto a goethite surface—A three-step process with an unexpected desorption phase. Chem. Geol. 2020, 556, 119852. [Google Scholar] [CrossRef]
- Ahmad, A.; Rutten, S.; Eikelboom, M.; de Waal, L.; Bruning, H.; Bhattacharya, P.; van der Wal, A. Impact of phosphate, silicate and natural organic matter on the size of Fe(III) precipitates and arsenate co-precipitation efficiency in calcium containing water. Sep. Purif. Technol. 2020, 235, 116117. [Google Scholar] [CrossRef]
- Francisco, P.C.M.; Sato, T.; Otake, T.; Kasama, T.; Suzuki, S.; Shiwaku, H.; Yaita, T. Mechanisms of Se(IV) Co-precipitation with Ferrihydrite at Acidic and Alkaline Conditions and Its Behavior during Aging. Environ. Sci. Technol. 2018, 52, 4817–4826. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Schwertmann, U.; Fischer, W.R. Natural “amorphous” ferric hydroxide. Geoderma 1973, 10, 237–247. [Google Scholar] [CrossRef]
- Börsig, N.; Scheinost, A.C.; Shaw, S.; Schild, D.; Neumann, T. Uptake mechanisms of selenium oxyanions during the ferrihydrite-hematite recrystallization. Geochim. Cosmochim. Acta 2017, 206, 236–253. [Google Scholar] [CrossRef] [Green Version]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Smith, S.C.; Dohnalkova, A.C.; Russell, C.K. Transformation of 2-line ferrihydrite to 6-line ferrihydrite under oxic and anoxic conditions. Am. Mineral. 2003, 88, 1903–1914. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, S.; Wang, Y.; Gomez, M.A.; Duan, Y.; Jia, Y. The Transformation of Two-Line Ferrihydrite into Crystalline Products: Effect of pH and Media (Sulfate versus Nitrate). ACS Earth Space Chem. 2018, 2, 577–587. [Google Scholar] [CrossRef]
- Cui, H.; Ren, W.; Lin, P.; Liu, Y. Structure control synthesis of iron oxide polymorph nanoparticles through an epoxide precipitation route. J. Exp. Nanosci. 2013, 8, 869–875. [Google Scholar] [CrossRef]
- Čanecká, L.; Bujdoš, M.; Gregor, M.; Hudec, P.; Boriová, K.; Dudová, J. Sorption of P(V), As(V), and Sb(V) Oxyanions on Goethite and Hematite During their Thermal Transformation. Sep. Sci. Technol. 2014, 49, 721–726. [Google Scholar] [CrossRef]
- Busca, G.; Cotena, N.; Rossi, P.F. Infrared spectroscopic study of micronised geothite. Mater. Chem. 1978, 3, 271–283. [Google Scholar] [CrossRef]
- Amrani, M.A.; Ghaleb, A.M.; Ragab, A.E.; Ramadan, M.Z.; Khalaf, T.M. Low-Cost Goethite Nanorods for As (III) and Se (VI) Removal from Water. Appl. Sci. 2020, 10, 7237. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochim. Acta Part A 2002, 58, 967–981. [Google Scholar] [CrossRef]
- Rochester, C.H.; Topham, S.A. Infrared study of surface hydroxyl groups on haematite. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 1073–1088. [Google Scholar] [CrossRef]
- Walter, D.; Buxbaum, G.; Laqua, W. The mechanism of the thermal transformation from goethite to hematite. J. Therm. Anal. Calorim. 2001, 63, 733–748. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J. Changes of crystal morphology of aged goethite over a range of pH (2–13) at 100 °C. Appl. Clay Sci. 2011, 51, 192–197. [Google Scholar] [CrossRef]
- Cornell, R.; Giovanoli, R.; Schindler, P. Effect of silicate species on the transformation of ferrihydrite into goethite and hematite in alkaline media. Clays Clay Miner. 1987, 35, 21–28. [Google Scholar] [CrossRef]
- Gálvez, N.; Barrón, V.; Torrent, J. Effect of Phosphate on the Crystallization of Hematite, Goethite, and Lepidocrocite from Ferrihydrite. Clays Clay Miner. 1999, 47, 304–311. [Google Scholar] [CrossRef]
- Wang, S.; Lei, L.; Zhang, D.; Zhang, G.; Cao, R.; Wang, X.; Lin, J.; Jia, Y. Stabilization and transformation of selenium during the Fe(II)-induced transformation of Se(IV)-adsorbed ferrihydrite under anaerobic conditions. J. Hazard. Mater. 2020, 384, 121365. [Google Scholar] [CrossRef]
- Ye, C.; Ariya, P.A.; Fu, F.; Yu, G.; Tang, B. Influence of Al(III) and Sb(V) on the transformation of ferrihydrite nanoparticles: Interaction among ferrihydrite, coprecipitated Al(III) and Sb(V). J. Hazard. Mater. 2021, 408, 124423. [Google Scholar] [CrossRef]
- Zhao, J.; Huggins, F.E.; Feng, Z.; Huffman, G.P. Ferrihydrite: Surface Structure and Its Effects on Phase Transformation. Clays Clay Miner. 1994, 42, 737–746. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Effects of Adsorbed Arsenate on the Rate of Transformation of 2-Line Ferrihydrite at pH 10. Environ. Sci. Technol. 2011, 45, 5557–5563. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, Y.; Wang, Z.; Yu, Z. A study of the morphology of the goethite crystallization process. Chem. Eng. J. 1998, 69, 1–5. [Google Scholar] [CrossRef]
- Gialanella, S.; Girardi, F.; Ischia, G.; Lonardelli, I.; Mattarelli, M.; Montagna, M. On the goethite to hematite phase transformation. J. Therm. Anal. Calorim. 2010, 102, 867–873. [Google Scholar] [CrossRef]
- Ruan, H.D.; Gilkes, R.J. Kinetics of thermal dehydroxylation of aluminous goethite. J. Therm. Anal. 1996, 46, 1223–1238. [Google Scholar] [CrossRef]
- Mackenzie, R.C. Thermal Analysis. In Physicochemical Methods of Mineral Analysis; Nicol, A.W., Ed.; Springer: Boston, MA, USA, 1975; pp. 389–420. [Google Scholar]
- Balistrieri, L.S.; Chao, T.T. Adsorption of selenium by amorphous iron oxyhydroxide and manganese dioxide. Geochim. Cosmochim. Acta 1990, 54, 739–751. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Selenium removal from water and its recovery using iron (Fe3+) oxide/hydroxide-based nanoparticles sol (NanoFe) as an adsorbent. Sep. Purif. Technol. 2013, 103, 167–172. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Surface area of ferrihydrite consistently related to primary surface charge, ion pair formation, and specific ion adsorption. Chem. Geol. 2020, 532, 119304. [Google Scholar] [CrossRef]
- Cristiano, E.; Hu, Y.J.; Siegfried, M.; Kaplan, D.; Nitsche, H. A comparison of point of zero charge measurement methodology. Clays Clay Miner. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Zhang, P.; Sparks, D.L. Kinetics of selenate and selenite adsorption/desorption at the goethite/water interface. Environ. Sci. Technol. 1990, 24, 1848–1856. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Sample | 2nd Day of Precipitation | 5th Day of Precipitation | |||||
|---|---|---|---|---|---|---|---|
| Spectral Component | Dist | S1 | S2 | Dist | S1 | S2 | |
| Parameter | |||||||
| Selenium-free | Arel (%) | 51.7 | 29.4 | 18.9 | 47.6 | 30.9 | 21.5 |
| Bhf (T) | 28.6 | 38.4 | 37.4 | 31.2 | 38.1 | 37.2 | |
| δ (mm/s) | 0.35 | 0.37 | 0.24 | 0.35 | 0.37 | 0.24 | |
| εΔ (mm/s) | −0.28 | −0.28 | −0.28 | −0.28 | −0.27 | −0.27 | |
| Selenite-treated | Arel (%) | 60.0 | 22.2 | 17.8 | 51.5 | 27.6 | 20.9 |
| Bhf (T) | 29.4 | 38.0 | 37.0 | 31.1 | 38.0 | 37.1 | |
| δ (mm/s) | 0.35 | 0.37 | 0.24 | 0.35 | 0.37 | 0.24 | |
| εΔ (mm/s) | −0.29 | −0.27 | −0.27 | −0.28 | −0.27 | −0.27 | |
| Selenate-treated | Arel (%) | 56.2 | 27.2 | 16.6 | 47.5 | 31.9 | 20.6 |
| Bhf (T) | 28.9 | 38.1 | 37.1 | 30.7 | 38.1 | 37.2 | |
| δ (mm/s) | 0.35 | 0.37 | 0.24 | 0.35 | 0.37 | 0.24 | |
| εΔ (mm/s) | −0.29 | −0.28 | −0.28 | −0.28 | −0.27 | −0.27 | |
| Sample | Thermal | Transformation | |||||
|---|---|---|---|---|---|---|---|
| Spectral Component | S1 | S2 | S3 | S4 | S5 | S6 | |
| Parameter | |||||||
| Selenium-free | Arel (%) | 31.3 | 27.3 | 12.1 | 5.4 | 18.4 | 5.5 |
| Bhf (T) | 51.3 | 50.5 | 49.6 | 48.5 | 47.1 | 38.6 | |
| δ (mm/s) | 0.38 | 0.39 | 0.37 | 0.37 | 0.32 | 0.36 | |
| εΔ (mm/s) | −0.21 | −0.21 | −0.23 | −0.27 | −0.29 | −0.30 | |
| Selenite-treated | Arel (%) | 36.3 | 27.0 | 11.6 | 5.6 | 19.5 | - |
| Bhf (T) | 51.2 | 50.3 | 49.2 | 47.9 | 46.6 | - | |
| δ (mm/s) | 0.38 | 0.38 | 0.38 | 0.35 | 0.31 | - | |
| εΔ (mm/s) | −0.21 | −0.21 | −0.25 | −0.25 | −0.30 | - | |
| Selenate-treated | Arel (%) | 31.5 | 28.8 | 12.7 | 6.0 | 16.4 | 4.6 |
| Bhf (T) | 51.3 | 50.5 | 49.5 | 48.4 | 46.8 | 38.9 | |
| δ (mm/s) | 0.38 | 0.39 | 0.37 | 0.36 | 0.32 | 0.34 | |
| εΔ (mm/s) | −0.21 | −0.21 | −0.24 | −0.27 | −0.30 | −0.35 | |
| Sample | Element (Weight %) | 2nd Day of Precipitation | 5th Day of Precipitation | Thermal Transformation |
|---|---|---|---|---|
| Selenium-free | Fe | 26.8 ± 3.2 | 26.2 ± 2.3 | 33.2 ± 1.3 * |
| Selenite-treated | Fe | 25.7 ± 1.9 | 25.2 ± 1.9 | 25.5 ± 2.5 |
| Se | 2.3 ± 0.9 | 1.5 ± 0.1 | 0.93 ± 0.08 ** | |
| Selenate-treated | Fe | 25.4 ± 2.5 | 26.1 ± 0.37 | 36.01 ± 2.04 * |
| Se | 2.5 ± 1.0 | 1.1 ± 0.20 | 0.95 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulová, M.; Bujdoš, M.; Miglierini, M.B.; Cesnek, M.; Duborská, E.; Mosnáčková, K.; Vojtková, H.; Kmječ, T.; Dekan, J.; Matúš, P.; et al. The Effect of High Selenite and Selenate Concentrations on Ferric Oxyhydroxides Transformation under Alkaline Conditions. Int. J. Mol. Sci. 2021, 22, 9955. https://doi.org/10.3390/ijms22189955
Matulová M, Bujdoš M, Miglierini MB, Cesnek M, Duborská E, Mosnáčková K, Vojtková H, Kmječ T, Dekan J, Matúš P, et al. The Effect of High Selenite and Selenate Concentrations on Ferric Oxyhydroxides Transformation under Alkaline Conditions. International Journal of Molecular Sciences. 2021; 22(18):9955. https://doi.org/10.3390/ijms22189955
Chicago/Turabian StyleMatulová, Michaela, Marek Bujdoš, Marcel B. Miglierini, Martin Cesnek, Eva Duborská, Katarína Mosnáčková, Hana Vojtková, Tomáš Kmječ, Július Dekan, Peter Matúš, and et al. 2021. "The Effect of High Selenite and Selenate Concentrations on Ferric Oxyhydroxides Transformation under Alkaline Conditions" International Journal of Molecular Sciences 22, no. 18: 9955. https://doi.org/10.3390/ijms22189955






